Controlled release and enhanced antibacterial activity of salicylic acid by hydrogen bonding with chitosan☆
2016-06-01ZujinYangYanxiongFangHongbingJi
Zujin Yang ,Yanxiong Fang ,Hongbing Ji,*
1 School of Chemistry and Chemical Engineering,The Key Laboratory of Low-carbon Chemistry&Energy Conservation of Guangdong Province,Sun Yat-sen University,Guangzhou 510275,China
2 Huizhou Research Institute,Huizhou 516216,China
3 Faculty of Chemical Engineering and Light Industry,Guangdong University of Technology,Guangzhou 510006,China
1.Introduction
Skin is one of the most important organs of human body which provides an overlaying protective barrier against environment.However,54%of the world population is affected by skin disease each year[1].Acne is a very common skin disease,and it affects more than 96%of teenagers.A study indicated that acne can cause some serious psychological problemse.g.depression,suicidal ideation and anxiety[2].Medicinal plants containing salicylic acid have been used to treat various skin disorders[3].Salicylic acid(SA)is a phenolic compound widely distributed in many plants,especially white willow,meadowsweet and wintergreen[4,5].SA has been used to relieve pain,reduce fever and prevent heart attack and stroke[6].SA is also a key ingredient in many skin-care products for the treatment of acne,psoriasis,calluses,corns,keratosis pilaris and warts[7,8].However,application of SA has been limited due to its low solubility in water(2 g·L-1at 20 °C)and strong irradiation on skin.Incorporation of SA into polymer matrix has been regarded as the most efficient method.
Microencapsulation is a way in which liquid droplets,solid particles or gaseous compounds are entrapped.The technology has been widely applied in pharmaceutical,food,pesticide,cosmetic,textile and other related fields[9-11].It provides an effective method for controlled release and protection of active substances.In addition,SA is an analgesic and antipyretic drug.Drug released from microcapsules or hydrogels is usually trigged or controlled by environment and pores of the matrix materials[12,13].Therefore,selection of proper wall materials is important for improving performance of active compounds.
Chitosan is a natural polysaccharide derived from alkaline deacetylation of chitin.It is the second most abundant biopolymer after cellulose,and is an environmentally-friendly material with many superior propertiese.g.biocompatibility,biodegradability and non-toxicity and can be used in many areas,such as wastewater treatment,food processing,pharmaceuticals,biomaterials,and agriculture[14-17].In addition,it is a very promising compound as natural antioxidants in biological systems[18].Therefore,chitosan is suitable as the wall material.
The aim of this work is to study encapsulation of SA and improvement of its release effectively with chitosan as wall material.Structure of the microcapsule has been characterized with various physico-chemical techniques.Release performance,mechanism,and Minimum Inhibitory Concentration(MIC)of the encapsulated SA were investigated with both experiments and quantum chemical calculations systematically.The results indicate that the method has great potential applications for functional materials in cosmetics industry.
2.Experimental
2.1.Materials
SA(A.R.)was purchased from Aladdin Industrial Corporation(Shanghai),China.Chitosan(degree of deacetylation≥90.0%,Mw=1.43×105Da)was purchased from Zhejiang Aoxing Biotechnology Co.Ltd.,China.All other reagents and solvents are of analytical grade and used without further purification unless indicated.Distilled water was used throughout.
2.2.Preparation and characterization of SA/chitosan microcapsules
Chitosan solution was prepared by dissolving 3.0 g chitosan in 100 ml acetic acid(3.0%,by mass)under vigorous stirring.Different amounts of SA dissolved in 10 ml 95%ethanol were slowly added to the chitosan solution and refluxed at60°C for 4 h.An aqueous emulsion could be obtained from this mixture with a Fluke homogenizer at 18000 r·min-1for 60 min.The emulsion was continuously stirred with a magnetic bar throughout the drying process.Spray drying was used for preparing SA/chitosan microcapsules due to its relatively low cost,rapid,reproducible and easy scale-up[19].The operation conditions for a Lab Plant SD-06 atomizer were:diameter of the atomizer nozzle(0.5 mm),liquid flow rate(8 ml·min-1),and inlet and outlet air temperatures were 170 and 85-90°C,respectively.The dried microcapsules collected in a cyclone were stored in plastic bottles at 4°C for further analysis.
The particle size distribution of SA/chitosan microcapsules was measured using a laser particle size analyzer(Mastersizer 2000,Malvern Instruments Limited,Malvern,UK).Particle size was measured using the dry sample adapter and the volume mean diameter(Vd)was recorded.IR spectra of the microcapsules was recorded on a Bruker TENSOR 37 FTIR spectrometer.SEM was used to observe the morphology of the microcapsules(FE-SEM,JSM-6330F).Samples were coated with thin layer of gold prior to examination under the electron beam.An operating voltage of 25 kV was used.
2.3.Determination of SA content in microcapsules
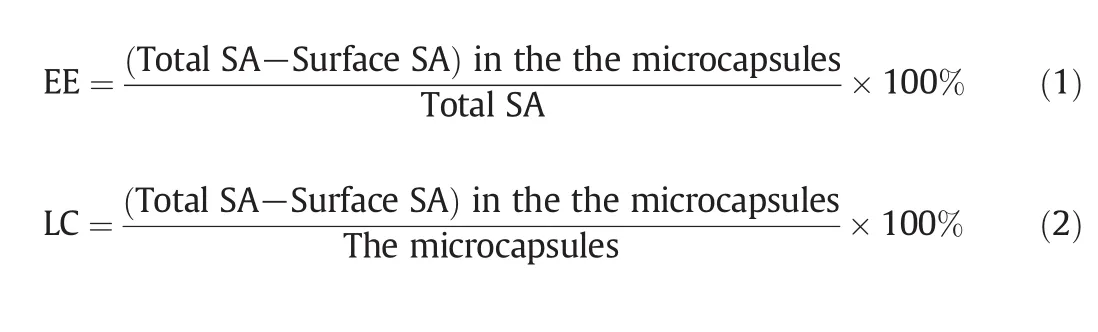
SA/chitosan microcapsules were washed with ethanol solution twice.The amount of surface SA in the solution was measured on UV-2450 spectrophotometer(Hitachi,Japan).The absorbance at 297 nm as the absorption maximum of SA was measured at 298 K.The microcapsules were further suspended in phosphate buffer saline(PBS)pH 7.2(USP XXIII),and treated with ultrasonic irradiation twice at 15 min intervals.The amount of incorporated SA was measured with the same method.Encapsulation efficiency(EE)and loading capacity(LC)of the microcapsules were calculated as:
2.4.Microbiological assay
Microbiological assay was performed for blank microspheres,SA,and SA/chitosan microcapsules.MIC(μg·ml-1)against selected strains ofStaphylococcus aureus,Staphylococcus epidermidis,andPropionibacterium acneswas determined using the agar dilution method in Mueller-Hinton agar medium,as previously reported[20].
2.5.In vitro release study
In vitrorelease study was conducted for all prepared SA/chitosan microcapsules.Microcapsules containing 20 mg of SA and a reference sample with the same amount of SA were kept in 100 ml phosphate buffer pH 7.4(USP XXIII)at 37°C under a constant rotation of 150 r·min-1.An aliquot of the release medium(2 ml)was sampled and its absorbance at 297 nm was measured for quantitation of SA.Another 2 ml phosphate buffer was then added to keep the volume of the release media constant.
The release data were evaluated with the release kinetics theories[21-23]:
Zero order kinetics:

hereFtrepresents the fraction of drug released in timetandK0is the apparent release constant for zero order kinetics.
First order kinetics:

hereFrepresents the fraction of drug released in timetandK1is the release constant for first order kinetics.
Higuchi model:

hereFrepresents the fraction of drug released in timetandK2is the
Higuchi dissolution constant.
For the Korsmeyer-Peppas model,the drug release mechanism was often derived from Fick's law,and the anomalous behavior was described by the following equation:

Here Mtis the drug released at timet,M∞is the quantity of drug released at infinite time,kis the kinetic constant andnis the release exponent.When ln(Mt/M∞)was plotted against lnt,its slope givesnand the intercept corresponds tok.nobtained from the Korsmeyer-Peppas model indicates that drug release was dominated by the diffusion process.nis used to characterize different release mechanisms.n≤0.45 indicates Fickian diffusion,in which the rate of diffusion is less than that of relaxation.nin the range of0.45<n<0.89 indicates that the mechanism is neither Fickian diffusion nor anomalous diffusion,where the diffusion and relaxation rates are comparable.Whenn>0.89,the major mechanism for drug release is Case II diffusion(relaxation-controlled transport)in which diffusion is very rapid compared to the relaxation process of polymer[21].
2.6.Quantum-chemical calculations
Quantum-chemical calculations were used to access the formation of these molecular complexes at molecular level,which can provide some information regarding the energy and structure of inclusion complex.In the present investigation,chitosan is a polysaccharide with high molecular weight.It composed mainly of 2-deoxy-2-amino-D-glucopyranose units,and partially of 2-deoxy-2-acetamido-D-glucopyranose linkedvia(1→4)-β-glycosidic bonds.In orderto explore the host-guest interaction mechanism,a basic assumption is that the binding sites are 2-deoxy-2-amino-D-glucopyranose and 2-deoxy-2-acetamido-D-glucopyranose units in chitosan,and the global properties of these sites are not significantly different from the properties of chitosan.On the basis of theoretical analysis,hydrophobic interactions are postulated to be the main driving forces in the inclusion complexation of SA with 2-deoxy-2-amino-D-glucopyranose and 2-deoxy-2-acetamido-D-glucopyranose units in chitosan.
All calculations described here were performed with the program package DMol3 in Materials Studio(Version 6.0)(Accelrys Inc.,USA).It has been proved to be feasible[24].Geometries of host,guest and host-guest inclusion complex were optimized using the local density approximation(LDA)in the Perden-Wang(PWC)form at the Double Numerical plus d-functions(DND)basis set level[25,26].
Each complex was minimized in energy under vacuum.The binding energy(BE)can be expressed as:

HereECis the total energy of the inclusion complex,EGis the sum of total energy of guest,andEHis the total energy of host.2-Deoxy-2-amino-D-glucopyranose and 2-deoxy-2-acetamido-D-glucopyranose units were used as the host,and SA was selected as the model guest.The more negative the binding energy is,the more thermodynamically favorable the inclusion complex is.
3.Results and Discussion
3.1.Microcapsule preparation
Chitosan has been used to prepare microcapsules for slow release of active compounds[14].Recently,spray drying method has been employed for the preparation of microcapsules by some authors[19,27].In the present study,the effect of SA/chitosan ratios on the microcapsule compositions and encapsulation efficiencies has been investigated.The results indicated that the encapsulation efficiencies of microcapsules decreased with the increase of core/wall material ratios(Table 1).

Table 1Expected and actual microcapsule compositions and encapsulation efficiencies
Table 1 listed the theoretical compositions and the actual SA contents for different ratios.Encapsulation efficiency in the range of 80%-92%demonstrated that spray-drying is a good way for preparation of SA microcapsules.The ratio of SA and chitosan of 1:3(by mass)was optimal while considering both costand performance.The size distribution of the microcapsules is normal.The microcapsules are mostly in the size range of 2-20 μm in diameter,and the mean particle size is 7 μm(Fig.1).
3.2.FTIR analysis
FTIR is a very powerful technique to investigate the inter-molecular interactions between SA and chitosan because the specific interaction affects the local electronic density and the corresponding absorption frequency[28].The FTIR spectra of SA(Curve a),chitosan(Curve b),and SA/chitosan microcapsules(Curve c)were shown in Fig.2.

Fig.1.Size distribution of the microcapsules measured by a laser particle size analyzer.
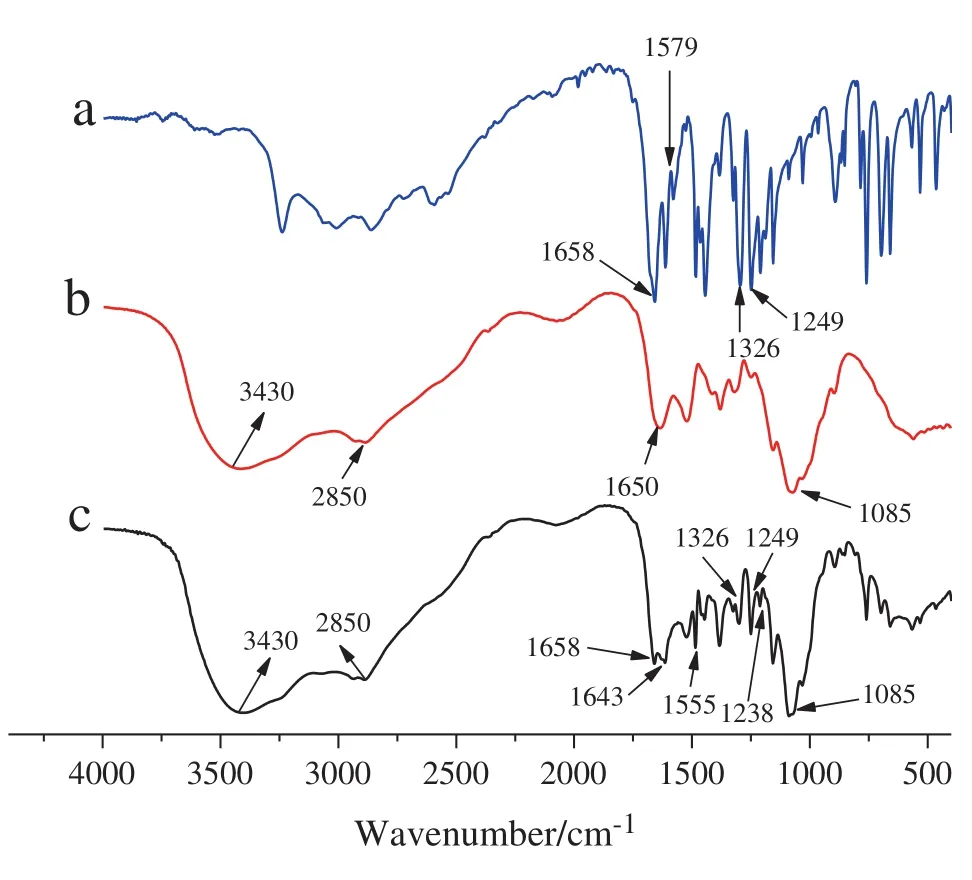
Fig.2.FTIR spectra of SA(a),chitosan(b),and SA/chitosan microcapsules(c).
The IR spectrum of SA(Curve a)exhibits a very strong band at 1658 cm-1,which is attributed to C═O stretching vibrations of the carboxylic acid group.The bands at 1579,1326,and 1249 cm-1are assigned to the asymmetrical and symmetrical stretching of the deprotonated carboxylate group and the phenolic Ph-O stretching vibration,respectively.The bending band of Ph-O-H is at 1384-1326 cm-1[29,30].The characteristic absorption bands of chitosan(Curve b)reveals distinctive and characteristic bands of O-H and N-H stretching vibrations of amine groups at 3430 cm-1,C-H stretching vibrations at 2850 cm-1,and N-H bending vibrations at 1598 cm-1.The band around 1085 cm-1is assigned to the unsymmetric stretching of C-O-C[31].The IR spectrum of SA/chitosan microcapsule(Curve c)is similar to that of chitosan(Curve a),indicating that the main structure of the microcapsule and chitosan is similar.The absorption bands of SA at 1658,1326 and 1249 cm-1were observed for the microcapsule,which indicated that SA is present in the microcapsule.In addition,it should be noted that the characteristic peak of amide I(NH2bending)shifted from 1650 to 1643 cm-1,and new peaks appeared at 1238(C-O-C stretch)and 1555 cm-1(N-H…O═C).Furthermore,the absorption of C-O-H in microcapsule at 1384 cm-1was much stronger than that of SA.Based on the above discussion,it is reasonable that the inter-molecular interactions between SA and chitosan were formed.
3.3.SEM analysis
The morphology of the microcapsules(SA/chitosan mass ratio 1:3)were investigated by SEM and demonstrated in Fig.3.
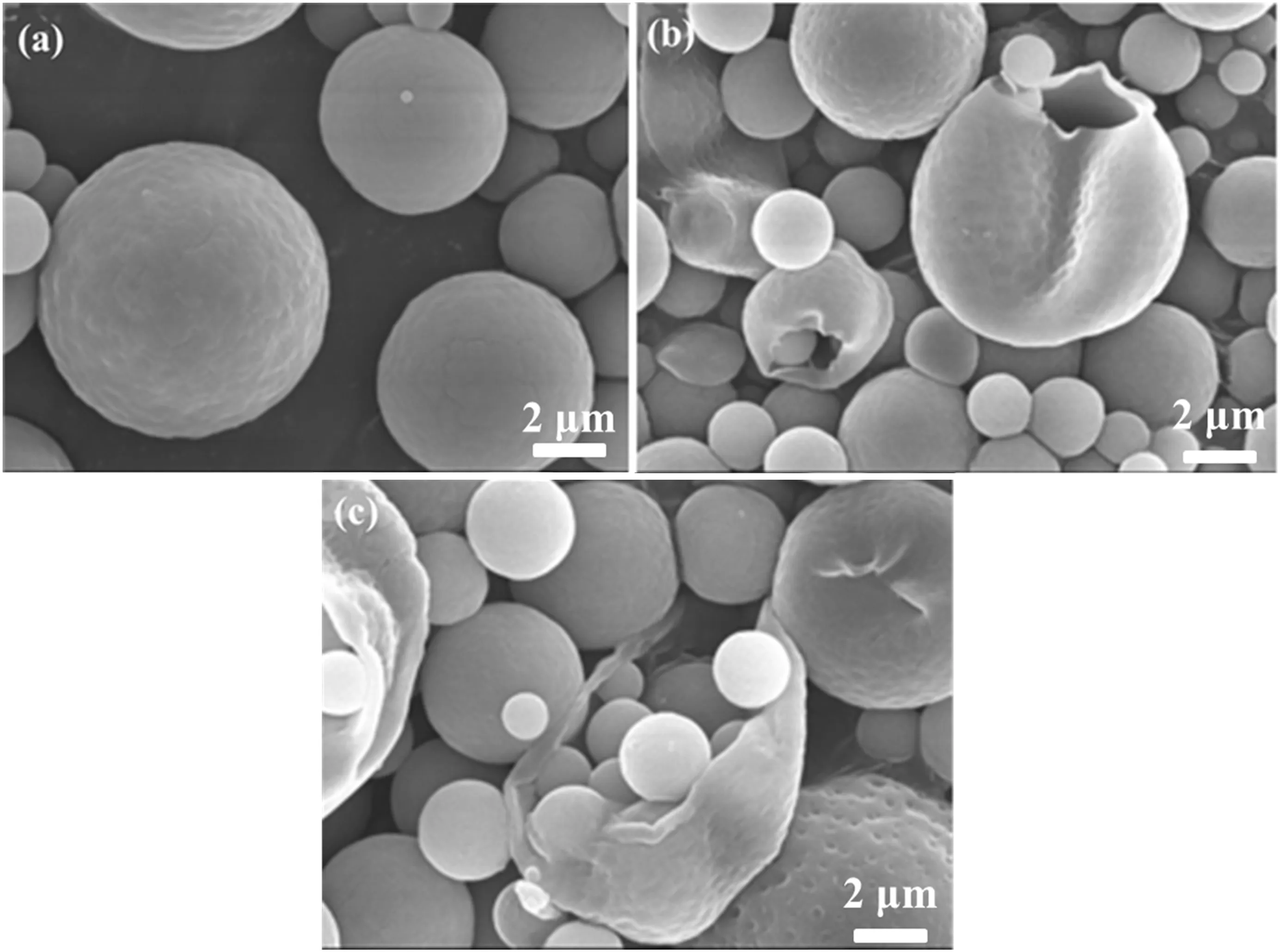
Fig.3.SEM of SA/chitosan microcapsules.
It could be found that the microcapsules have regularly spherical shape,smooth surface,and even dispersion,with different sizes(Fig.3).The microcapsules are distributed mostly in the size range of 2-20 μm in diameter,which was similar to that measured by laser diffraction particle size analyzer.Some broken microcapsules can be observed in Fig.3(c).It could be clearly found that cores indeed were wrapped inside the microcapsules.
3.4.Minimum Inhibitory Concentration(MIC)
Table 2 listed the results of microbiological tests.MICs of pure SA againstS.aureus,S.epidermidis,andP.acneswere 25,25,and 12 μg·ml-1,respectively.Chitosan microcapsules demonstrated higher MIC values with respect to pure SA.However,taking into account the actual SA content of these microcapsules(23.3%),a significant improvement of drug activity could be observed.The enhancement could be explained by the interaction of the hydroxyl or amino group of chitosan with SA to form the intermolecular hydrogen bond C═O…H-O and N…H-O,which eliminated the intra-molecular hydrogen bond of SA and increased its antibacterial capacity.It has been reported before that the formation of SA complexes could improve its antioxidant activity according to the fact that several phenolic compounds can form intermolecular hydrogen bonds rather than intra-molecular hydrogen bonds after inclusion[32].
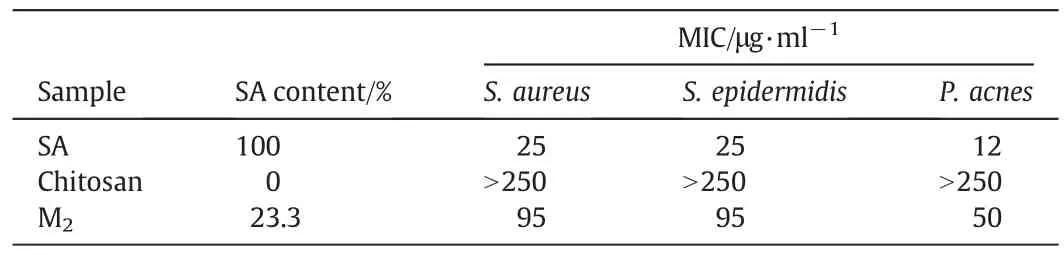
Table 2Antimicrobial activities of SA and microspheres expressed as MIC(μg·ml-1)
3.5.Release profile of SA microcapsules
Fig.4 showed thein vitroSA release profiles of chitosan microcapsules,compared with the dissolution profiles of pure SA.SA was completely dissolved in about48 min.However,chitosan microcapsules modulated SA release in the range of 1 h to 4 h.Cumulative release rate for M1,M2,and M3increased rapidly,and gradually reach equilibrium.The slowest release rates for M3(40.4%SA)could be attributed to the formation of microcapsule aggregates after contact with the dissolution medium.However,the active ingredient might not be released from the microcapsules through 6 h,indicating that interactions occurred between SA and chitosan.
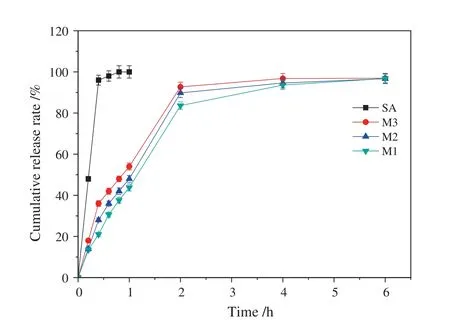
Fig.4.In vitro SA release profile from chitosan microcapsules.
In order to investigate the release mechanism of SA from the chitosan microcapsules,four different release modelse.g.zero order kinetics,first order kinetics,Higuchi model and Korsmeyer-Peppas model were used to fit the experimental data obtained from the release experiments.The determination coefficients(R2)of the four models and the corresponding characteristic parameters were summarized in Table 3.
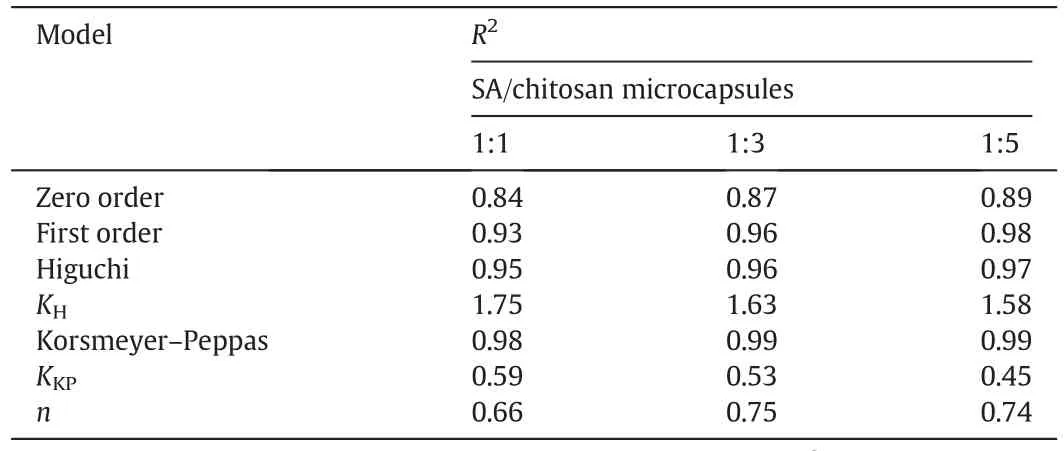
Table 3Kinetic models for SA/chitosan microcapsules release
As shown in Table 3,theR2values for Higuchimodeland Korsmeyer-Peppas model exceeded 0.95,and Korsmeyer-Peppas model was the most suitable model for describing SA release kinetics from the chitosan microcapsules,because of the correlation coefficient approaching 0.99.For Korsmeyer-Peppas model,ngave an indication of the release mechanism[21].As shown in Table 3,the exponentnvalues for the release of SA from chitosan microspheres were between 0.43 and 0.85,suggesting a non-Fickian diffusion or anomalous diffusion release behavior,thus the diffusion and relaxation rates were the main factor controlling SA release.
3.6.The role of hydrogen bonding
Geometry and energy of the most stable inclusion complex of 2-deoxy-2-amino-D-glucopyranose and 2-deoxy-2-acetamido-D-glucopyranose units with SA were determined by the program package DMol3 method and shown in Fig.5.
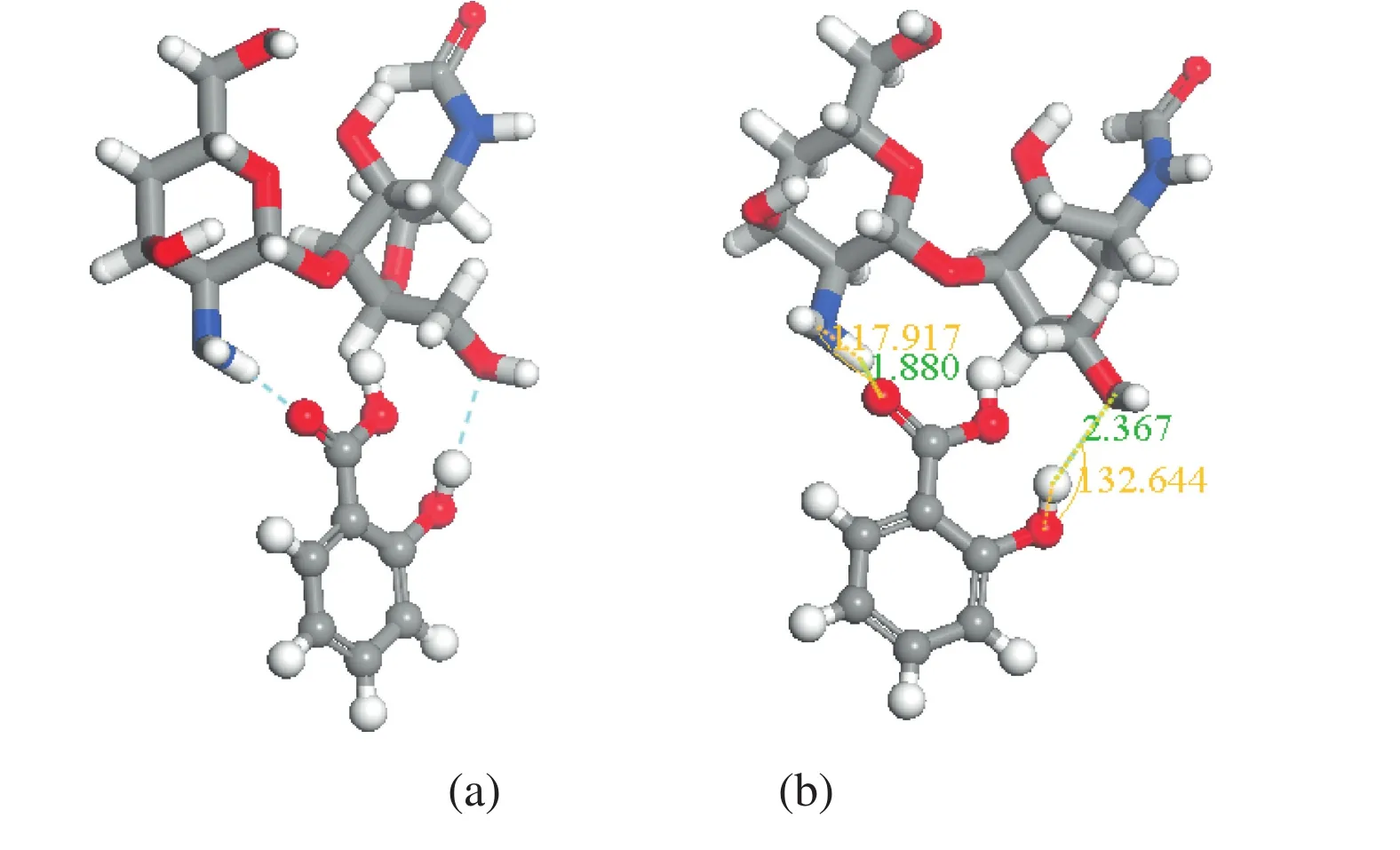
Fig.5.Optimized structure of the 2-deoxy-2-amino-D-glucopyranose and 2-deoxy-2-acetamido-D-glucopyranose unit/salicylic acid obtained by DMol3 method.(a)Host-guest C═O…H-O and N…H-O interactions in the complex;(b)bond distance(nm)for the hydrogen bonding interactions with the cutoff criteria for hydrogen-bond C═O…H-O and N…H-O with H…O distances equal or less than 0.31 nm and angles at H larger than 90°between the 2-deoxy-2-amino-D-glucopyranose and 2-deoxy-2-acetamido-D-glucopyranose units/salicylic acid.
Two types of hydrogen bonding interactions,e.g.N…H-O and C═O…H-O,have been considered.For the hydrogen bond interactions of N…H-O and C═O…H-O,the cut-off criteria was the N…O distance(≤0.31 nm),the O…O distance(≤0.31 nm),and the bond angle(≥90°),respectively[25,33].The hydrogen bond lengths of N…H-O and C═O…H-O are,in turn,0.1880 and 0.2236 nm,and the corresponding bond angles are 117.917°and 132.664°,which demonstrated that the hydrogen bonding between chitosan and SA played vital roles on the formation of the stable inclusion complexes.As previously reported[33],the more negative the binding energy,the more stable the corresponding complex.The negative BE(-27.28 kJ·mol-1)further demonstrated that chitosan can form stable complex with SA.Based on the DFT results,the stable complexes of chitosan with SA significantly eliminated the intra-molecular hydrogening bond of SA so as to improve its release performance and the antibacterial capacity.
4.Conclusions
The present study described preparation of SA microcapsules by spray drying for the purpose of controlling SA release and improving its antibacterial activity.FTIR study of the microcapsules indicated the formation of the weak interactions between SA and chitosan.SEM morphology confirmed the spherical shape and smooth surface of the microcapsules with diameterin the range of2 and 20μm.Theinvitrorelease profile revealed that SA could be released within 6 h after microcapsulation.Kinetic studies demonstrated that the release of SA from microcapsules followed the diffusion and relaxation rates.The MIC values of SA microcapsules againstS.aureus,S.epidermidis,and P.acnesranged from 95 to 50 μg·ml-1.Enhanced antibacterial activity was attributed to the intermolecular hydrogen bonding between SA and chitosan.Quantum chemical calculation results further demonstrated the hydrogen bonding interactions.
[1]L.K.Andersen,M.D.P.Davis,The epidemiology of skin and skin-related diseases:A review of population-based studies performed by using the Rochester Epidemiology Project,Mayo Clin.Proc.88(2013)1462-1467.
[2]L.Misery,Consequences of psychological distress in adolescents with acne,J.Invest.Dermatol.131(2011)290-292.
[3]N.Lall,N.Kishore,Are plants used for skin care in South Africa fully explored?J.Ethnopharmacol.153(2014)61-84.
[4]C.M.J.Pieterse,L.C.van Loon,Salicylic acid-independent plant defence pathways,Trends Plant Sci.4(1999)52-58.
[5]D.Kumar,Salicylic acid signaling in disease resistance,Plant Sci.228(2014)127-134.
[6]S.C.Gupta,B.Sung,S.Prasad,L.J.Webb,B.B.Aggarwal,Cancer drug discovery by repurposing:Teaching new tricks to old dogs,Trends Pharmacol.Sci.34(2013)508-517.
[7]A.Swanson,K.Canty,Common pediatric skin conditions with protracted courses:A therapeutic update,Dermatol.Clin.31(2013)239-249.
[8]R.S.Mueller,K.Bergvall,E.Bensignor,R.Bond,A review of topical therapy for skin infections with bacteria and yeast,Vet.Dermatol.23(2012)330-362.
[9]P.L.Lam,R.Gambari,Advanced progress of microencapsulation technologies:In vivo and in vitro models for studying oral and transdermal drug deliveries,J.Control.Release178(2014)25-45.
[10]A.Jamekhorshid,S.M.Sadrameli,M.Farid,A review of microencapsulation methods of phase change materials(PCMs)as a thermal energy storage(TES)medium,Renew.Sustain.Energy Rev.31(2014)531-542.
[11]B.Peña,C.Panisello,G.Aresté,R.Garcia-Valls,T.Gumí,Preparation and characterization of polysulfone microcapsules for perfume release,Chem.Eng.J.179(2012)394-403.
[12]Z.Cai,J.T.Zhang,F.Xue,Z.Hong,D.Punihaole,S.A.Asher,2D photonic crystal protein hydrogel coulometer for sensing serum albumin ligand binding,Anal.Chem.86(2014)4840-4847.
[13]Z.Cai,N.L.Smith,J.T.Zhang,S.A.Asher,Two-dimensional photonic crystal chemical and biomolecular sensors,Anal.Chem.87(2015)5013-5025.
[14]Q.X.Wu,D.Q.Li,S.J.Yao,Design of chitosan and its water soluble derivatives-based drug carriers with polyelectrolyte complexes,Mar.Drugs12(2014)6236-6253.
[15]M.N.V.Ravi Kumar,A review of chitin and chitosan applications,React.Funct.Polym.46(2000)1-27.
[16]M.B.Vásconez,S.K.Flores,C.A.Campos,J.Alvarado,L.N.Gerschenson,Antimicrobial activity and physical properties of chitosan-tapioca starch based edible films and coatings,Food Res.Int.42(2009)762-769.
[17]M.Z.Elsabee,E.S.Abdou,Chitosan based edible films and coatings:A review,Mater.Sci.Eng.33(2013)1819-1841.
[18]M.Mengíbar,I.Mateos-Aparicio,B.Miralles,Á.Heras,Influence of the physicochemical characteristics of chito-oligosaccharides(COS)on antioxidant activity,Carbohydr.Polym.97(2013)776-782.
[19]B.N.Estevinho,F.Rocha,L.Santos,A.Alves,Microencapsulation with chitosan by spray drying forindustry applications—Areview,TrendsFoodSci.Technol.31(2013)138-155.
[20]C.Coutant,D.Olden,J.Bell,J.D.Turnidge,Disk diffusion interpretive criteria for fusidic acid susceptibility testing ofStaphylococciby the National Committee for Clinical Laboratory Standards method,Diagn.Microbiol.Infect.Dis.25(1996)9-13.
[21]T.Hayashi,H.Kanbe,M.Okada,M.Suzuki,Y.Ikeda,Y.Onuki,T.Kaneko,T.Sonobe,Formulation study and drug release mechanism of a new theophylline sustainedrelease preparation,Int.J.Pharm.304(2005)91-101.
[22]R.W.Korsmeyer,R.Gurny,E.Doelker,P.Buri,N.A.Peppas,Mechanisms of solute release from porous hydrophilic polymers,Int.J.Pharm.15(1983)25-35.
[23]M.R.Brophy,P.B.Deasy,Application of the Higuchi model for drug release from dispersed matrices to particles of general shape,Int.J.Pharm.37(1987)41-47.
[24]B.Delley,An all-electron numerical method for solving the local density functional for polyatomic molecules,J.Chem.Phys.92(1990)508-517.
[25]Z.J.Yang,H.B.Ji,2-Hydroxypropyl-β-cyclodextrin polymer as a mimetic enzyme for mediated synthesis of benzaldehyde in water,ACS Sustainable Chem.Eng.1(2013)1172-1179.
[26]Z.J.Yang,H.G.Jiang,X.T.Zhou,Y.X.Fang,H.B.Ji,β-Cyclodextrin polymer promoted green synthesis of cinnamaldehyde to natural benzaldehyde in aqueous solution,Supramol.Chem.24(2012)379-384.
[27]C.Muzzarelli,V.Stanic,L.Gobbi,G.Tosi,R.A.A.Muzzarelli,Spray-drying of solutions containing chitosan together with polyuronans and characterisation of the microspheres,Carbohydr.Polym.57(2004)73-82.
[28]N.Pongali,S.Prabu,V.N.Vijayakumar,M.L.N.Madhu Mohan,Characterization of a hydrogen bonded liquid crystal homologous series:Detailed FTIR studies in various mesophases,J.Mol.Struct.994(2011)387-391.
[29]E.C.Yost,M.I.Tejedor-Tejedor,M.A.Anderson,In situ CIR-FTIR characterization of salicylate complexes at the goethite/aqueous solution interface,Environ.Sci.Technol.24(1990)822-828.
[30]B.Unal,Z.Durmus,H.Kavas,A.Baykal,M.S.Toprak,Synthesis,conductivity and dielectric characterization of salicylic acid-Fe3O4nanocomposite,Mater.Chem.Phys.123(2010)184-190.
[31]S.F.Hosseini,M.Zandi,M.Rezaei,F.Farahmandghavi,Two-step method for encapsulation oforegano essential oilin chitosan nanoparticles:Preparation,characterization and in vitro release study,Carbohydr.Polym.95(2013)50-56.
[32]Y.Tsai,H.H.Tsai,C.P.Wu,F.J.Tsai,Preparation,characterisation and activity of the inclusion complex of paeonol with β-cyclodextrin,Food Chem.120(2010)837-841.
[33]H.B.Ji,Q.P.Long,H.Y.Chen,X.T.Hu,X.F.Zhou,β-Cyclodextrin inclusive interaction driven separation of organic compounds,AIChE J.57(2011)2341-2352.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Influence of synthesis parameters on the properties of LiFePO4/C cathode material☆
- The structure,tensile properties and water resistance of hydrolyzed feather keratin-based bioplastics☆
- Preparation of dendritic bismuth film electrodes and their application for detection of trace Pb(II)and Cd(II)☆
- Wheat straw pretreatment with KOH for enhancing biomethane production and fertilizer value in anaerobic digestion☆
- Performance evaluation of a modified step-feed anaerobic/anoxic/oxic process for organic and nutrient removal
- Biodiesel synthesis via metal oxides and metal chlorides catalysis from marine alga Melanothamnus afaqhusainii
