Characterization of the adsorption behavior ofaqueous cadmium on nanozero-valent iron based on orthogonalexperiment and surface complexation modeling☆
2016-05-30DongmeiLiuHuanTangYingZhaoFuyiCuiJingLu
DongmeiLiu,Huan Tang,Ying Zhao*,FuyiCui*,Jing Lu
1 State Key Laboratory ofUrban Water Resource and Environment,Harbin Institute ofTechnology,Harbin 150090,China
2 Schoolof Municipaland EnvironmentalEngineering,Harbin Institute ofTechnology,Harbin 150090,China
1.Introduction
Cadmium(Cd)is a major environmental contaminant which is known to accumulate in rice plants[1].Cd can be introduced into the environment through a combination of natural processes(volcanic eruptions and forest fires)as well as anthropogenic activities(nonferrous metals production,electroplating,manufacturing of Ni–Cd batteries and pigments,application ofphosphatic fertilizers,and burning of fossilfuels)[2,3].The naturaloccurrence of Cd in groundwater is ofgreatconcern due to the toxicity ofCd and the potentialfor chronic exposure[4].To address this problem,the World Health Organization(WHO)had set a maximum guideline concentration of0.003 mg·L-1for Cd in drinking water[5].On this occasion many public watersystems have to adopt cadmium removal processes in their water treatment systems in order to meet the stringent drinking water standards.
Various technologies are currently available to remove cadmium from water,such as chemicalmethods[6–8],membrane separation technique[9],ion exchange technique[10]and adsorption technique[11–13].Adsorption is a common practice for Cd removalfrom drinking water due to technologicaland cost advantages.
Nano zero-valentiron(nZVI)has been developed as an ef ficientabsorbent material to treat various heavy metals from contaminated water[14].The speci fic removalmechanisms involved depend on the standard redox potential(E0)of the target metal.The E0of Fe0is-0.44 V.Metals with E0s that are more negative or similar to-0.44 V willbe removed by adsorption,others with E0s that are more positive than-0.44 will be removed by reduction and precipitation.Metals with slightly more positive E0than Fe0willbe removed by reduction,adsorption,oxidation or co-precipitation which depend upon the prevailing geochemicalconditions[15,16].
As shown by several studies,factors such as aqueous phase pH,concentrations of contaminants and the presence of competitive ions have a signi ficant effect on nZVI's reactivity with metals[17–21].Although researches had investigated the factors that may have effect on the ef ficiency of nZVI,comparatively little research had studied which factor is the most signi ficant one.Figuring out this factor will mean a lot on the application ofnZVI.
Among allthe nZVIsynthesis methods,chemicalreduction is widely used due to its simplicity and chemicalhomogeneity[22].During the nZVIpreparation,mechanicalagitation needs to be employed to insure the size of ZVI at nanoscale[23].Aggregation of nanoparticles was reported to be caused by the large surface area and magnetic dipole–dipole interactions of the individual particles[24].If the solution is stirred vigorously,the aggregation would be weakened to some extent.However,this mechanical stir method brings some questions(1)the waste ofelectricity,(2)some of the nanoparticles may stick to the agitator which are hard to clean.
The main objective ofthis study is to find outthe mostsigni ficantfactor that affects the ef ficiency ofnZVI.First,nZVIwillbe prepared by an improved liquid-phase reduction and characterized to verify this new method.Then an orthogonalexperiment and simulation willbe performed underthe same conditions to find outthe mostsigni ficantfactor.
2.Materials and Methods
2.1.Synthesis ofnZVIparticles
The nanoparticles were synthesized via the classical reaction as follows:
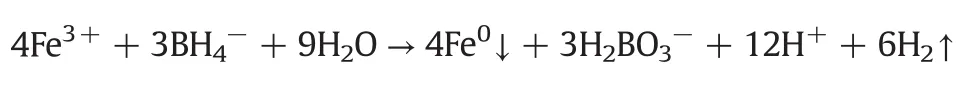
Allthe aqueous solutions were prepared with deionized deoxygenated water.Before the reaction begins,the FeCl3·6H2O(1 mol·L-1)was mixed with PVP(1 g·L-1).Then the NaBH4(1.6 mol·L-1)solution was added to the mixture mentioned above.The nitrogen was sparged into the solution during the whole process to maintain an anaerobic atmosphere.
2.2.Characterization ofnZVI
Morphologicalanalysis of the samples was performed by transmission electron microscopy(TEM)using a FEITecnai G2 F30 operated at 300 kV.Samples for TEMobservation were suspended in ethanoland then supported on a double carbon membrane.
The speci fic surface area(SBET)ofnZVIwas calculated by Brunauer–Emmett–Teller(BET)N2method,and the pore size was measured by the BJH(Barrett,Joyner,and Halenda)N2adsorption/desorption isotherm method at 77 K using the automatic analyzer(ASAP 2020,Micromeritics,USA).
2.3.Orthogonalexperiment
The experiments were based on an orthogonalarray experiment design and the following three variables were analyzed:initialpH of the solution,dosage ofnZVIand the initialconcentration ofcadmium.These three factors were identi fied to have signi ficant impact on the ef ficiency ofnZVI[25].
Other factors such as temperature and existence ofother metalions maybe as importantas the parameters picked.But the effectoftemperature had been studied by Boparai et al.completely so we didn't take this factor into account[26].As for other metalions,such as Pb(II)and As(III),although they may have signi ficant effect on the adsorption of Cd2+by nZVI,it is not appropriate to consider this factor in orthogonal experiment.Therefore,the effects ofmetalions were excluded in our orthogonal experiment.Li et al.had studied the effect of pH in the range of 4–9[27],and Zhang et al.had studied the effect of pH in the range of3–8.6[28].Butin actualproject,the pHofef fluents from chemicalfactory may beyond this range,so itis necessary to study the pHin a larger range.On this occasion,the range ofpH used in our experiment was 2–10.Based on the waste water ofmetallurgicalindustry,the concentration of Cd(II)used in our experiment was 10,20 and 40 mg·L-1.And the dose ofnZVIwas based on ourearlierexperiments.Above all,an L9(33)matrix,which is an orthogonalarray of three factors and three levels,was employed to assign the considered factors and levels as listed in Table 1.
Data analysis would be carried out through the range analysis to re flect the magnitudes of the three factors.
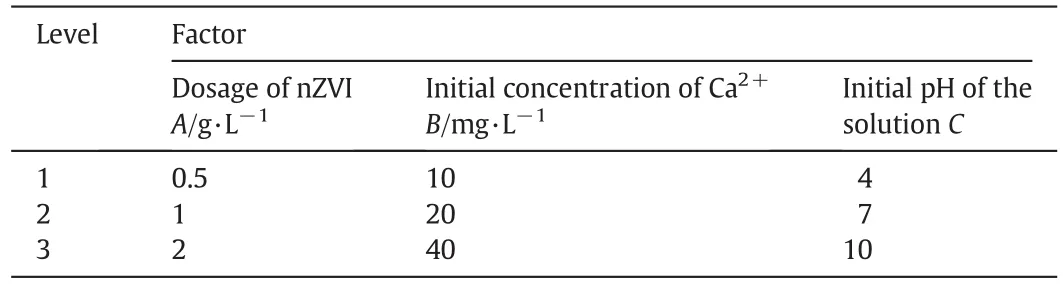
Table 1 Levels and factors in orthogonalexperiment design
Allthe experiments were performed at ambient temperature and pressure using a 250.0 mlserum bottle.The pH values of the solutions were controlled and adjusted with the 0.02 Mbuffer as Boparai et al.had used[29].
The contents were mixed on a thermostatic oscillator operated at 200 r·min-1.After 1 h an aliquot of supernatant was sampled and filtered using a 0.22μm filter(Millipore),the samples were then transferred into clean falcon tubes and diluted using nitric acid(5%).The remaining concentration of cadmium in the filtrate was determined by means of inductively coupled plasma-optical emission spectroscopy(ICP-OES,AA 200,Agilent Technologies).Blank experiments with and without nZVIand without filtering were also performed to obtain the initialcadmium concentration prior to adsorption,as well as to rule out filtration as a reason forthe decrease on the concentration ofcadmium in the supernatant.
2.4.Surface complexation modeling
In aqueous solution,transition metals are known to assume different chemicalforms that the formations depend on the pH of the solution.The speciation analysis ofaqueous Cd2+ions was performed at various initialconcentration,temperature,pH,and ionic strength values using visualMINTEQ software.
In actualwater,bare nZVIparticles willreactwith water and oxygen to form outer iron(hydr)oxide layer[30].On this occasion,the essence of the adsorption is the interaction between Cd and the outer layer.Based on this,a surface complexation modeling was carried out by VisualMINTEQ 3.0.
The iron oxide/hydroxide–water interface was represented by,for example,≡FeOH and ≡FeO,where the“≡Fe”represents the solid–surface interface.Adsorbed materials are written as complexes of these surface oxides,for example,≡FeOCd+.And the possible reactions are as follows:

Modeling was performed using the Diffuse Layer Model(DLM).This surface precipitation modelcan simulate the distribution of ions between adsorbed and dissolved phases based on the reactions as is mentioned above.Since the DLM model has been successfully used to describe metalion adsorption on pure mineralmaterials[31],it is unnecessary to go into details about the theory.Parameters used in the modeling were based on experiment data and/or previous reports[29].
3.Result and Discussion
3.1.Characterization ofnZVI
The microimages of the synthesized nZVIare shown in Fig.1.As shown(Fig.1(a)and(b)),the aggregates can reach to severalmicrometers in length but less than 100 nm in diameter(Fig.1(c))
The resultdemonstrated thatwe can use N2bubbling and PVP to replace mechanicalstirring in the preparation ofnZVI.PVP,which is a kind ofsurfactantthathas been widely used in the field ofsynthesizing nanosilver,can modify the surface properties of the particles[32].The added PVP can effectively preventthe aggregation ofZVIparticlesby(1)reducing the speci fic surface energy ofnZVIparticles and(2)increasing the steric hindrance between nZVIparticles.
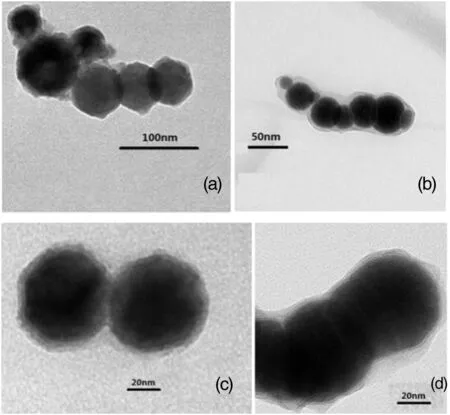
Fig.1.TEMimage of the nZVI.
The morphologies of nZVIs showed in Fig.1(a)and(c)were fresh and those in Fig.1(b)and(d)were placed for two months after preparation.As is shown in Fig.1(b)and(d),there is a layer ofsemi-transparent film less than 10 nmthick around the black particle.Itwas reported thatthe exposure ofnZVIto oxygen can lead the formation ofiron oxide layer which contains Fe2O3,Fe3O4and FeOOH[33].The iron nanoparticles possessed a core–shellstructure.The shellrepresented the oxidized part that surrounded the Fe0core and it can prevent the nZVIagainst further oxidation[34].The addition ofnZVIto aqueous systems usually generates OH-by the reduction ofwater,resulting in the immobilization ofmetals by precipitation as hydroxides[35].
BET analysis indicated a speci fic surface area of 20.3159 m2·g-1.This data was used in the simulation later.Fig.2 is the adsorption isotherm line,the trend of the plot indicated that nZVIis a typicalmaterial without holes or with macroporous.
The TEMand BET results showed that nZVIprepared with this new approach possessed excellent surface characters.With the presence of PVP,the size ofZVIcan be insured to be in nanosize withoutmechanical stirring.
Compared to traditionalmethods(Fig.3),this improved method has severaladvantages as follows.
·Compared with the three-necked flask,the serum bottle used as reactor is easier to clean and the solid–liquid separation is easier to achieve.
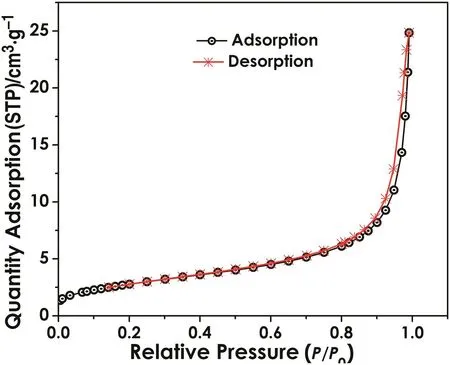
Fig.2.BJH adsorption/desorption isotherm.

Fig.3.Diagrammatic sketch ofcomparison between traditionalmethod and new method in the synthesis ofnZVI.
·In conventional approaches,negative pressure would be formed induced by rapid stirring,air will be introduced into the reaction mixture and the nZVIwould be oxidized more easily.
3.2.Orthogonalexperiment
An L9(33)orthogonalexperimentwas performed and the results are listed in Table 2.All the experiments were aimed at increasing the removalrate ofcadmium by nZVI.

Table 2 Results of the orthogonalexperiment
In order to figure out which factor is the most important,a range analysis is necessary.There are two parameters in a range analysis:Kjiand Rj.Kjiis de fined as the sum of the evaluation indexes of alllevels(i,i=1,2,3)in each factor(j,j=A,B,C)andis the mean value of Kji.Rjis de fined as the range between the maximumand minimumvalue ofand is used for evaluating the importance of the factors,i.e.Larger Rjvalue means a greater importance of the factor[36].The calculation is displayed below(for the factor of A):
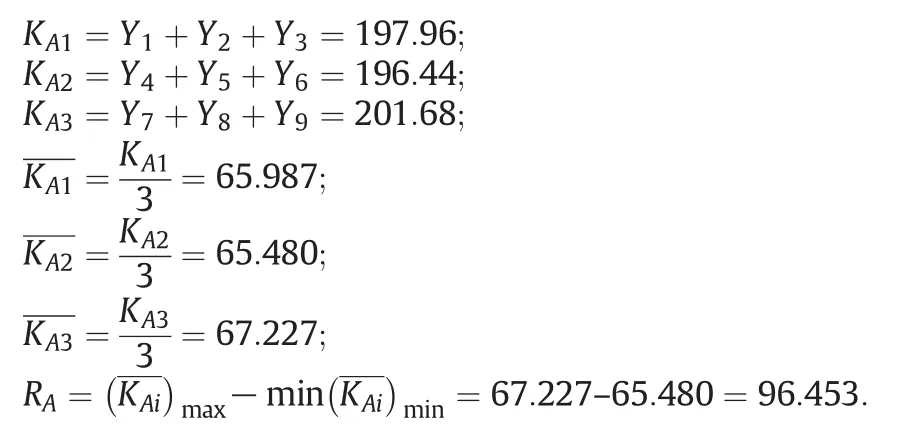
where KAiis the K value of the i levelof the factor A;and Yiis the value of the result of the trial number.Other K values of the factors can be determined by the same calculation steps.The mean values of Kfor different factors at different levels in the range analysis were listed in Table 3.
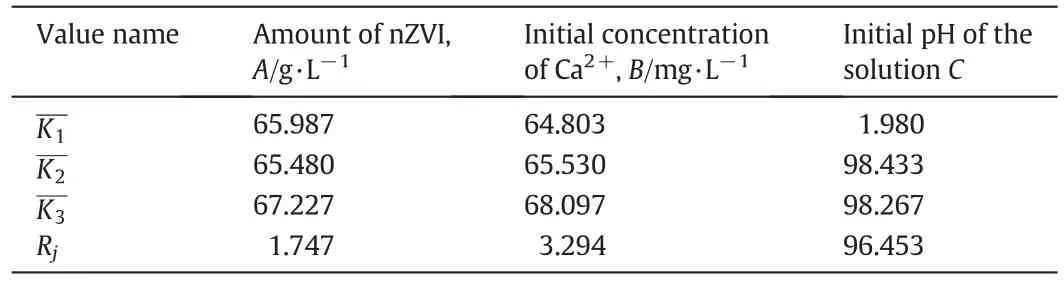
Table 3 Range analysis data of the removalrate
As is mentioned above,Rjindicates the signi ficance ofa factor and a larger Rjmeans that this factor has a more signi ficant impacton the removalrate.Based on the comparation of Rj,the prominence order of the factors was:initialpHof the solution(96.453)>initialconcentration of cadmium(3.294)>amount of nZVI(1.747).RCis the largest,which means a smallchange in the initialpH willhave a signi ficant effect on the removalrate ofcadmium.
The mean value ofeach factor was displayed in Fig.4.Based on the changes in the mean value ofeach factor,it can be speculated that the removalrate sharply increased from 1.98%to 98.267%with the initial pH increased from 4 to 10.When the amount ofnZVIincreased from 1 g·L-1to 4 g·L-1,the removalrate increased steadily from 65.987%to 67.213%,which suggested that the amount ofnZVIhad a little effect on the removal ef ficiency of nZVI.Similarly,it can be speculated that the effect of the cadmium concentration ion is very small.

Fig.4.Relationship between mean value ofeach factor and the removalrate.
Based on the orthogonalexperimentand the range analysis,itcan be speculated that the initial pH of the solution is the most signi ficant factor that affects the adsorption performance of Cd2+onto nZVI.
3.3.Surface precipitation modeling
In order to con firm that initialpH is the most signi ficant factor,a simulation was performed.Parameters used in the modeling are as follows.The speci fic surface area of the iron(hydr)oxide shell was 20.3159 m2·g-1which was obtained from the BET analysis.The site density oftype 1 sites(weak sorption sites)was 2.26 sites nm-2,and of type 2 sites(strong sites)was 0.056 sites nm-2as recommended.The electrolyte was 0.01 mol·L-1NaNO3.After the adsorption equilibrium achieved,the result of the distribution of ions between adsorbed and dissolved phases is shown in Fig.5.The tendency of the curve showed that in acidic condition most of the cadmium was dissolved and Cd2+was found to be the dominant species.If the solution pH was higher,more cadmium would be adsorbed onto the nZVI and(≡FeO)2Cd was found to be the dominant form.
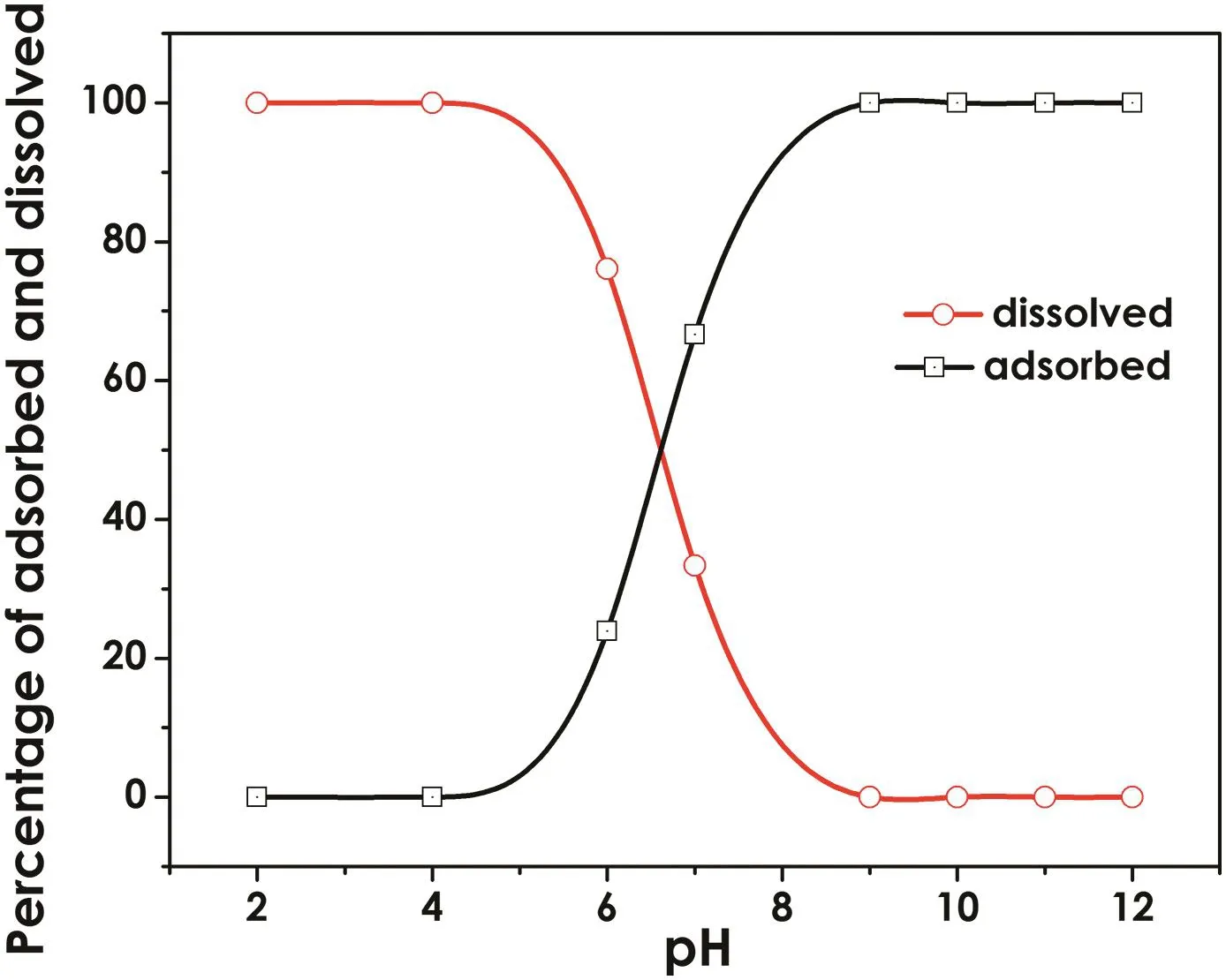
Fig.5.Distribution ofions between adsorbed and dissolved phases in different pH.
Simulations were performed under the same condition and the range ofeach factor is illustrated in Fig.6.The result showed that the range of factor C(99.99)is much larger than that of factor A(7.15)and factor B(7.16).The result is consistent with the conclusion we drew upon the orthogonalexperiment:the initialpH is the most signi ficant factor.
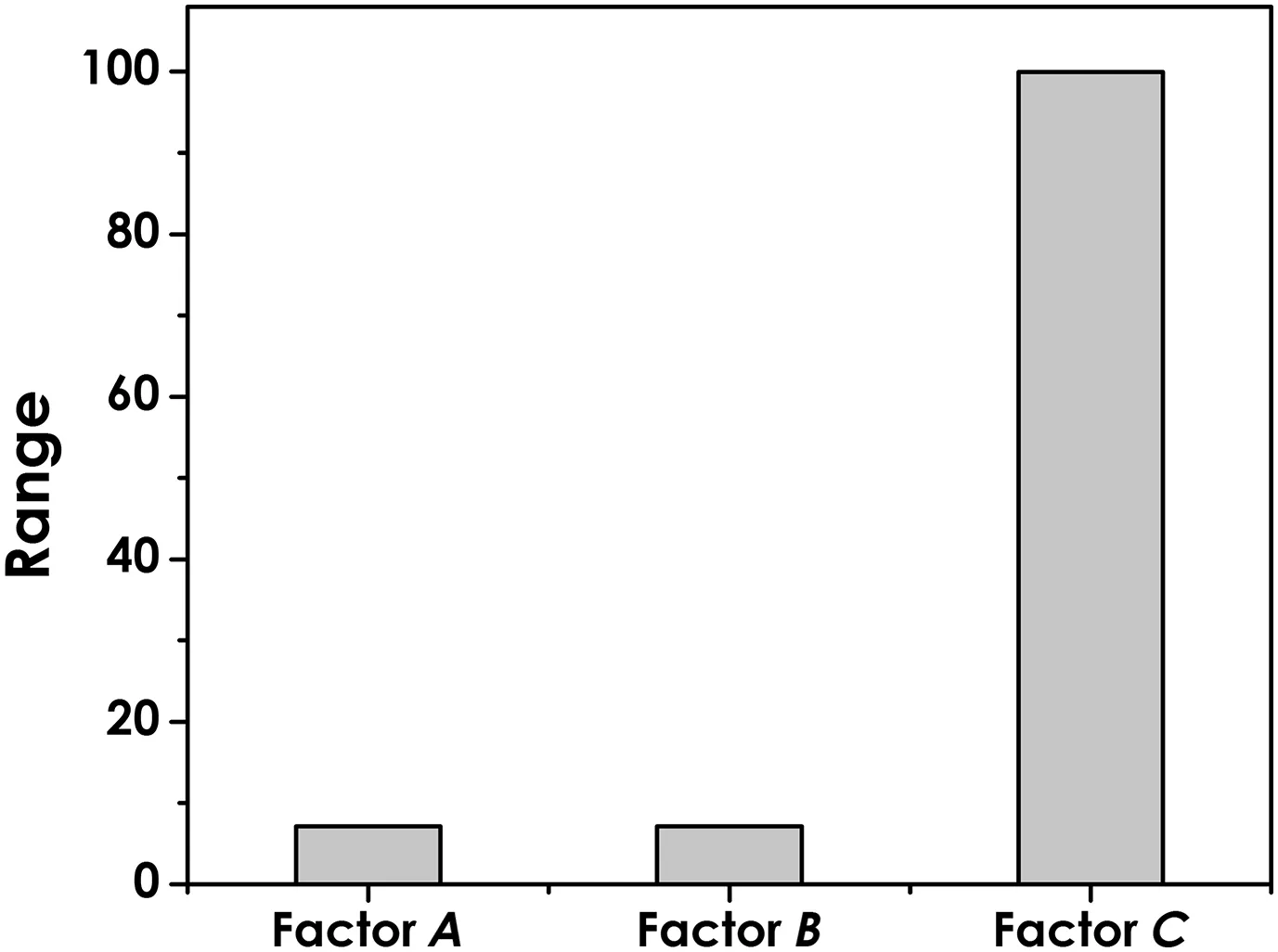
Fig.6.Range ofeach factor calculated based on simulation.
Both the experiments and the simulations showed that pH has the most signi ficant effect on the adsorption ef ficiency of the nZVI.In the range of4–10,the higher the pH,the more cadmium is adsorbed.This conclusion is important to maximize the adsorption rates and adsorption capacity ofnZVIfor cadmium.
As the E0of cadmium is-0.40 V,which is close to that of Fe0(-0.44 V),Cd will be removed by sorption and/or precipitation.The dominant adsorption mechanism and the existing form of cadmium in aqueous solutions are both related to the solution pH.At lower pH,Cd2+is found to be the dominant species(Fig.7).Since the isoelectric point(IEP)ofnZVIwas around 8.1–8.2[37].At pH values below the IEP,the surface is expected to possess a positive potential,this willdecrease the adsorption ofmetalanions due to electrostatic repulsion.At higher pH,the abundant hydroxylwillresult in the immobilization ofmetals in the form ofhydroxides and precipitation is the dominantremovalmechanism.Fig.7 is the species distribution ofcadmium overa pHrange of1 to 14.Itcan be seen thatatpH 10,Cd2+existsdominantas Cd(OH)2,and the percentage of Cd(OH)2is about 85%,this means that the Cd2+willbe removed from water by precipitation without nZVI.But in the presence of nZVI,the removalrate of Cd2+at pH 12 is 97.1–99.2 in our experiment,which means that nZVIcan promote the removalofcadmium.In acidic conditions,the lowerthe pH,the strongerthe repulsion.In alkaline conditions,the higher the pH,the easier the precipitation is to form.

Fig.7.The speciation of cadmium over a pH range of1 to 14.
4.Conclusions
In this study,traditionalliquid-phase reduction for preparing nZVI was improved by the introduction of PVP.TEMand BET analyses indicated that the improve method was better for nZVI preparation.An L9(33)orthogonalexperiment was employed to find out the dominant factor that affects the removalrate of cadmium by nZVI.Three factors were examined:initialpH of the solution,concentration of cadmium and the dosage of nZVI,and initial pH was found to be the most signi ficant factor.Surface precipitation modeling showed in different conditions,the Ca2+was removed by nZVIin different mechanisms.
[1]S.Ishikawa,N.Ae,M.Sugiyama,M.Murakami,T.Arao,Genotypic variation in shoot cadmium concentration in rice and soybean in soils with different levels of cadmium contamination,Soil Sci.Plant Nutr.51(2005)101–108.
[2]M.D.L.Dinis,A.Fiúza,Exposure assessment to heavy metals in the environment:Measures to eliminate or reduce the exposure to criticalreceptors,environmental heavy metal pollution and effects on child mental development,Springer,Netherlands,2011 27–50.
[3]T.Kikuchi,M.Okazaki,K.Toyota,T.Motobayashi,M.Kato,The input–outputbalance of cadmium in a paddy field of Tokyo,Chemosphere 67(2007)920–927.
[4]G.F.Nordberg,Historical perspectives on cadmium toxicology,Toxicol.Appl.Pharmacol.238(2009)192–200.
[5]WHO,Guidelines for drinking water quality,second ed.,Recommendations,Vol.1,WHO,Geneva,1993.
[6]O.Tunay,T.Ozkok,T.O.Hanci,I.Kabdasli,Applicability of phosphonic acid complexes as a means of cadmium removal from aqueous solutions,J.Selcuk Univ.Nat.Appl.Sci.(2013)70–77.
[7]L.Charerntanyarak,Heavy metals removal by chemicalcoagulation and precipitation,Water Sci.Technol.39(1999)135–138.
[8]S.A.Bayar,E.Yilmaz,R.Boncukcuoğlu,B.A.Fìla,M.M.Kocakerìm,Effects of operationalparameters on cadmium removalfrom aqueous solutions by electrochemical coagulation,Desalin.Water Treat.51(2013)2635–2643.
[9]B.Pospiech,W.Kujawski,Ionic liquids as selective extractants and ion carriers of heavy metal ions from aqueous solutions utilized in extraction and membrane separation,Rev.Chem.Eng.31(2015)179–191.
[10]A.Akcil,C.Erust,S.Ozdemiroglu,A review of approaches and techniques used in aquatic contaminated sediments:Metalremovaland stabilization by chemicaland biotechnological processes,J.Clean.Prod.86(2015)24–36.
[11]J.A.Arcibar-Orozco,J.R.Rangel-Mendez,P.E.Diaz-Flores,Simultaneous adsorption of Pb(II)–Cd(II),Pb(II)–phenol,and Cd(II)–phenolby activated carbon cloth in aqueous solution,Water Air Soil Pollut.226(2015)1–10.
[12]R.Liu,F.Liu,C.Hu,Simultaneous removalofCd(II)and Sb(V)by Fe–Mn binary oxide:Positive effects of Cd(II)on Sb(V)adsorption,J.Hazard.Mater.300(2015)847–854.
[13]L.Yan,Y.Huang,J.Cui,Simultaneous As(III)and Cd removalfrom copper smelting wastewater using granular TiO2columns,Water Res.68(2015)572–579.
[14]S.M.Ponder,J.G.Darab,T.E.Mallouk,Remediation of Cr(VI)and Pb(II)aqueous solutions using supported,nanoscale zero-valent iron,Environ.Sci.Technol.34(2000)2564–2569.
[15]X.Q.Li,W.X.Zhang,Sequestration ofmetalcations with zero valentiron nanoparticles—A study with high resolution X-ray photoelectron spectroscopy,J.Phys.Chem.C 111(2007)6939–6946.
[16]D.O'Carroll,B.Sleep,M.Krol,H.Boparai,C.Kocur,Nanoscale zero valent iron and bimetallic particles for contaminated site remediation,Adv.Water Resour.51(2013)104–122.
[17]T.Tosco,M.P.Rapini,C.C.Viggi,R.Sethi,Nanoscale zerovalent iron particles for groundwater remediation:A review,J.Clean.Prod.44(2014)10–21.
[18]M.Rivero-Huguet,W.D.Marshall,Reduction ofhexavalent chromium mediated by micro-and nano-sized mixed metallic particles,J.Hazard Mater.169(2009)1081–1087.
[19]T.Phenrat,Y.Liu,R.D.Tilton,G.V.Lowry,Adsorbed polyelectrolyte coatings decrease Fe0nanoparticle reactivity with TCE in water:Conceptualmodeland mechanisms,Environ.Sci.Technol.43(2009)150–154.
[20]Y.Liu,S.A.Majetich,R.D.Tilton,D.S.Sholl,G.V.Lowry,TCE dechlorination rates,pathways,and ef ficiency of nanoscale iron particles with different properties,Environ.Sci.Technol.39(2005)1338–1345.
[21]F.He,D.Zhao,C.Paul,Field assessment of carboxymethyl cellulose stabilized iron nanoparticles for in situ destruction of chlorinated solvents in source zones,Water Res.44(2010)2360–2370.
[22]W.Zhang,Nanoscale iron particles for environmental remediation:An overview,J.Nanopart.Res.5(2003)323–332.
[23]Y.H.Hwang,D.G.Kim,H.S.Shin,Effects ofsynthesis conditions on the characteristics and reactivity of nano scale zero valent iron,Appl.Catal.B Environ.105(2011)144–150.
[24]T.Phenrat,N.Saleh,K.Sirk,R.D.Tilton,G.V.Lowry,Aggregation and sedimentation of aqueous nanoscale zerovalent iron dispersions,Environ.Sci.Technol.41(2007)284–290.
[25]N.Efecan,T.Shahwan,A.E.Eroglu,Characterization of the adsorption behavior of aqueous Cd(II)and Ni(II)ions on nanoparticles of zero-valent ironM.S.Thesis İzmir Institute of Technology,2008.
[26]H.K.Boparai,M.Joseph,D.M.O'Carroll,Kinetics and thermodynamics of cadmium ion removal by adsorption onto nano zerovalent iron particles,J.Hazard.Mater.186(2011)458–465.
[27]Y.Li,H.Ma,B.Ren,T.Li,Simultaneous adsorption and degradation of Cr(VI)and Cd(II)ions from aqueous solution by silica-coated Fe0nanoparticles,J.Anal.Methods Chem.2013(2013).
[28]Y.Zhang,Y.Li,C.Dai,H.Zhou,W.Zhang,Sequestration of Cd(II)with nanoscale zero-valent iron(nZVI):Characterization and test in a two-stage system,Chem.Eng.J.244(2014)218–226.
[29]H.K.Boparai,M.Joseph,D.M.O'Carroll,Cadmium(Cd2+)removal by nano zerovalent iron:Surface analysis,effects ofsolution chemistry and surface complexation modeling,Environ.Sci.Pollut.Res.20(2013)6210–6221.
[30]Y.P.Sun,X.Q.Li,J.S.Cao,W.X.Zhang,H.P.Wang,Characterization ofzero-valent iron nanoparticles,Adv.Colloid Interf.120(2006)47–56.
[31]X.Wen,Q.Du,H.Tang,Surface complexation modelfor the heavy metaladsorption on naturalsediment,Environ.Sci.Technol.32(1998)870–875.
[32]K.A.K.Huynh,L.Chen,Aggregation kinetics of citrate and polyvinylpyrrolidone coated silver nanoparticles in monovalent and divalent electrolyte solutions,Environ.Sci.Technol.45(2011)5564–5571.
[33]J.T.Nurmi,P.G.Tratnyek,V.Sarathy,D.R.Baer,J.E.Amonette,K.Pecher,C.Wang,J.C.Linehan,D.W.Matson,R.L.Penn,M.D.Driessen,Characterization and properties of metallic iron nanoparticles:Spectroscopy,electrochemistry,and kinetics,Environ.Sci.Technol.39(2005)122–130.
[34]X.Li,W.Zhang,Sequestration ofmetalcations with zerovalent iron nanoparticles—A study with high resolution X-ray photoelectron spectroscopy(HR-XPS),J.Phys.Chem.C 111(2007)6939–6946.
[35]W.Yan,A.A.Herzing,C.J.Kiely,W.Zhang,Nanoscale zero-valent iron(nZVI):Aspects of the core–shellstructure and reactions with inorganic species in water,J.Contam.Hydrol.118(2010)96–104.
[36]X.Wu,D.Y.C.Leung,Optimization of biodiesel production from camelina oil using orthogonalexperiment,Appl.Energy 88(2011)3615–3624.
[37] Ç.Üzüm,T.Shahwan,A.E.Eroğlu,I.Lieberwirth,T.B.Scott,K.R.Hallam,Application of zero-valent iron nanoparticles for the removal of aqueous Co2+ions under various experimentalconditions,Chem.Eng.J.144(2008)213–220.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- In situ synthesis ofhydrophobic magnesium hydroxide nanoparticles in a novelimpinging stream-rotating packed bed reactor☆
- Enhancing the hydration reactivity ofhemi-hydrate phosphogypsum through a morphology-controlled preparation technology☆
- Synthesis and characterization ofcopolymers ofpoly(m-xylylene adipamide)and poly(ethylene terephthalate)oligomers by melt copolycondensation
- Improvement of CO2 capture performance ofcalcium-based absorbent modi fied with palygorskite☆
- Adsorption behavior ofcarbon dioxide and methane in bituminous coal:A molecular simulation study☆
- Enhanced cold active lipase production by metagenomic library recombinant clone CALIP3 with a step-wise temperature and dissolved oxygen levelcontrolstrategy☆
