Enhanced cold active lipase production by metagenomic library recombinant clone CALIP3 with a step-wise temperature and dissolved oxygen levelcontrolstrategy☆
2016-05-30ZhuhuaChanRunpingWangFanYangRunyingZeng
Zhuhua Chan ,Runping Wang ,Fan Yang ,Runying Zeng ,2,*
1 State Key Laboratory Breeding Base ofMarine Genetic Resource,Third Institute of Oceanography,SOA,Xiamen 361005,China
2 South China Sea Bio-Resource Exploitation and Utilization Collaborative Innovation Center,Guangzhou 510000,China
1.Introduction
Cold active lipases are active at cold temperatures in comparison to the lipases from mesophiles or thermopiles[1].These lipases have evolved a range ofstructuralfeatures thatconfer a high levelof flexibility.Notably,the active site of cold active lipases could adapt to low temperatures by adopting a unique conformation with low activation enthalpy,low-substrate af finity,and high speci fic activity.Cold active lipases may serve as a novel source for biotechnological enzymes because of their high catalytic activity atlow temperatures and unusual substrate speci ficities[2].These properties offer potential economic bene fits in detergent,textile,and food industries[3],and in bioremediation of polluted water and soils[1].Indeed,cold active lipases have emerged as an important biocatalyst in biomedicalapplications,such as medicaland pharmaceuticalapplication[4,5],fine chemicalsynthesis[6,7],food industry[8],and environmentalapplications[9,10].
Cold active lipases are primarily distributed in microorganisms living at low temperatures near 5°C.Most of these bacterial strains were isolated from deep-sea or Polar regions,which are permanently cold.Many cold active lipases have been isolated from marine psychrophilic and psychrotolerant bacteria,such as Stenotrophomonas[11],Aeromonas[12],Moritella[13],Photobacterium[14],Pseudoalteromonas[15],Pseudomonas[16],and Psychrobacter[17].Most of Cold active lipases are extracellular enzymes thatare highly sensitive to nutritional and physicochemical factors such as temperature,agitation,pH,nitrogen source,carbon source,inducers,inorganic sources,and dissolved oxygen.Submerged fermentation is the most common method used for cold active lipase production[17,18].The production ofcold active lipase is considered temperature dependentand thermolabile[19].As reported,Aspergillus nidulans WG312 produced cold active lipase by utilizing olive oilas an inducer at30°C[20].An isolate of Pseudoalteromonas sp.wp27 produced lipases at 25°C in 14 days with yeast extract as carbon source and olive oil and Tween 80 as inducers[21].A two-stage oxygen supply strategy was developed for enhanced lipase production by Bacillus subtilis[22].Enhancement of lipopeptide production was proposed with a two-temperature-stage process[23].High celldensity fed-batch fermentation was developed for the production ofa microbiallipase[24].
However,98.0%to 99.8%ofmicroorganisms in environmentalsample are not readily cultivable using currently available techniques[25].The latest trend in lipase research is the search ofnoveland improved lipases through molecular approaches,such as the metagenomic approach which is developed to direct explore naturalcommunities[26].The metagenome overcomes the bottleneck of traditionalculture approach[27]and provides opportunities to utilize the resources from non-cultivable microorganisms.A novel lipase was isolated from a metagenomic library derived from Baltic Sea sediment[28].A novel psychrophilic esterase was obtained directly from the metagenomic DNA isolated from the activated sludge[29].Thermostable lipolytic enzymes were obtained from the metagenomic DNA[30,31].Deep-sea environments feature permanent cold,thus may provide suitable growth conditions for microorganism harboring cold active enzyme.However,to this date cold active lipases derived from metagenomic library of deep sea sediment are rarely reported.
In this work,a cold active lipase-producing clone CALIP3,which was generated by transforming Escherichia coli with deep-sea sediment metagenomic library,was used for lipase production.The effects of both temperature and DO on cold active lipase production by batch culture were investigated.Based on the kinetic analysis oflipase production under different temperatures and DO conditions,a step-wise temperature and DO controlstrategy was proposed to improve lipase production.With the proposed step-wise temperature and DO control strategy,the activity and production ofcold active lipase were enhanced signi ficantly.The results obtained here may be usefulfor the production ofother cold active enzymes from recombinant clone of metagenomic library.
2.Materials and Methods
2.1.Microorganism and culture conditions
The cold active lipase-producing clone CALIP3(preserved at-80°C)was generated by transforming E.coli cells with deep-sea sediment(157°24′E,19°30′N)metagenomic library in our previous study.
The seed culture medium consisted of glucose 10 g·L-1,NaCl 10 g · L-1,yeast extract powder 2.5 g·L-1,and peptone 5 g·L-1.The pH of the medium was adjusted to 7.0.Culture medium was sterilized at 121°C for 20 min,subsequently chloramphenicolwas added to the final concentration of 12.5 g·ml-1.The seed culture medium was incubated at 37 °C with shaking at 200 r·min-1for 12 h to 14 h in an Erlenmeyer flask.The fermentation medium(pH 7.0)contained the following components:glucose 30 g·L-1,yeast extract powder 10 g·L-1,peptone 10 g·L-1,(NH4)2SO44 g·L-1,K2HPO45 g·L-1and NaCl10 g·L-1.The medium was sterilized at 121°C for 20 min,followed by chloramphenicol addition with the finalconcentration of12.5 g·ml-1.The fermentation medium was inoculated with 5%inoculum(v/v)(OD600=1.1).Erlenmeyer flasks(250 ml)containing 50 ml medium were used for both seeding and fermentation.The fermentation medium was maintained at the same conditions for 40 h to 48 h.
Batch fermentation was carried out in a stirred fermenter(Biostat 5,Sartorius,Germany)with a 5 L working volume.The seed culture(5%,v/v)was inoculated into the fermentation medium.Agitation was provided by two Rushton impellers and varied from 200 to 800 r·min-1,and aeration was provided by a ring sparger with a range of1.0–10 L·L-1·min-1,so thatitcould controlDOlevelatapproximately 20%which was monitored with a DO electrode(Mettler Toledo).The pH change was detected by a pH electrode(Mettler Toledo)during cultivation.The fermentation temperature was maintained at different temperatures by re-circulating water.
2.2.Effects oftemperature on cellgrowth and cold active lipase production
In fluences of temperature on cell growth,acetic acid formation,residualglucose concentration and cold active lipase production were investigated.The temperature was respectively controlled at 25°C,30 °C,34 °C,and 37 °C by automatic controlofre-circulating water.
2.3.Effects of DO levels on cellgrowth and cold active lipase production
In fluences ofDOlevels on cellgrowth,acetic acid formation,residual glucose concentration,and cold active lipase production were investigated.The DO levels were controlled at 10%,20%,30%,and 40%by automatic control of agitation speed and aeration rate.The cold active lipase production without DO controlwas also conducted and the agitation speed and aeration rate were set as 200 r·min-1and 5 L·L-1·min-1,respectively.
2.4.Lipase activity assay
Lipase activity was assayed according to the method described by Gupta[32].In brief,1 ml of isopropanol containing 3 mg of pnitrophenylpalmitate(p-NPP)was mixed with 9 mlof 0.05 mol·L-1Tris–HCl(pH 8.0)containing 40 mg Triton X-100 and 10 mg gumarabic.The mixture was stirred until all the constituents were dissolved completely.To initiate hydrolysis,2.4 ml of the freshly prepared substrate solution was transferred into each test tube and 0.1 mlof the enzyme solution was added.The mixture was incubated for 15 min at 35°C.The opticaldensity at 410 nm was measured against an enzyme-free control.One lipase unit(U)was de fined as the amount ofenzyme needed to release 1μmol p-nitrophenolperminute underthe standard assay conditions.All enzyme assays were performed in triplicates,and the average values were calculated.
2.5.Cellconcentration measurement
The sample from the fermentation broth was centrifuged at4°C and 9000 g for 20 min.The pellet was re-suspended in reverse osmosis water.The concentration of cells was monitored by optical density measurement at 600 nm(OD600).
2.6.Analyticalmethods
Samples were withdrawn from fermenter for analysis at regular intervals.The biomass was determined for at least three 50 ml cell suspensions that were harvested by centrifugation(6000 g,5 min),washed with distilled water,and then dried at 60°C for 24 h to a constant mass(dry cellmass,DCM).Glucose and acetic acid concentration were measured with a Biopro file 300A biochemicalanalyzer(Nova Biomedical,Waltham,MA).The pH was measured automatically with electrodes attached to the fermenters.
2.7.Calculation ofkinetic parameters
The speci fic cellgrowth rate(μ/h-1),speci fic glucose consumption rate(qs/h-1),and speci fic rhamsan gum formation rate(qp/h-1)were estimated from the experimentalor fitted data ofcellgrowth(Cx/g·L-1),residualglucose concentration(Cs/g·L-1),and lipase production(Cp/g·L-1)using Eqs.(1)to(3),respectively.The fitted data were obtained by interposing the experimentaldata ofcellgrowth,residualglucose concentration,or lipase production at a de finite time(d t=0.1 h)with the approximation method ofcubic spline interpolation in Grapher software(Version 4,Golden Software,Inc.,USA)

3.Results and Discussion
3.1.Cold active lipase production at different temperatures
Temperature is one of the well-documented factors in fluencing fermentation.Our previous study had showed thatthe lipase produced by CALIP3 was a cold active lipase.The lipase was active and stable at the temperatures ranging from-20 °C to 30 °C,and the activity declined rapidly above 40°C(Fig.1).
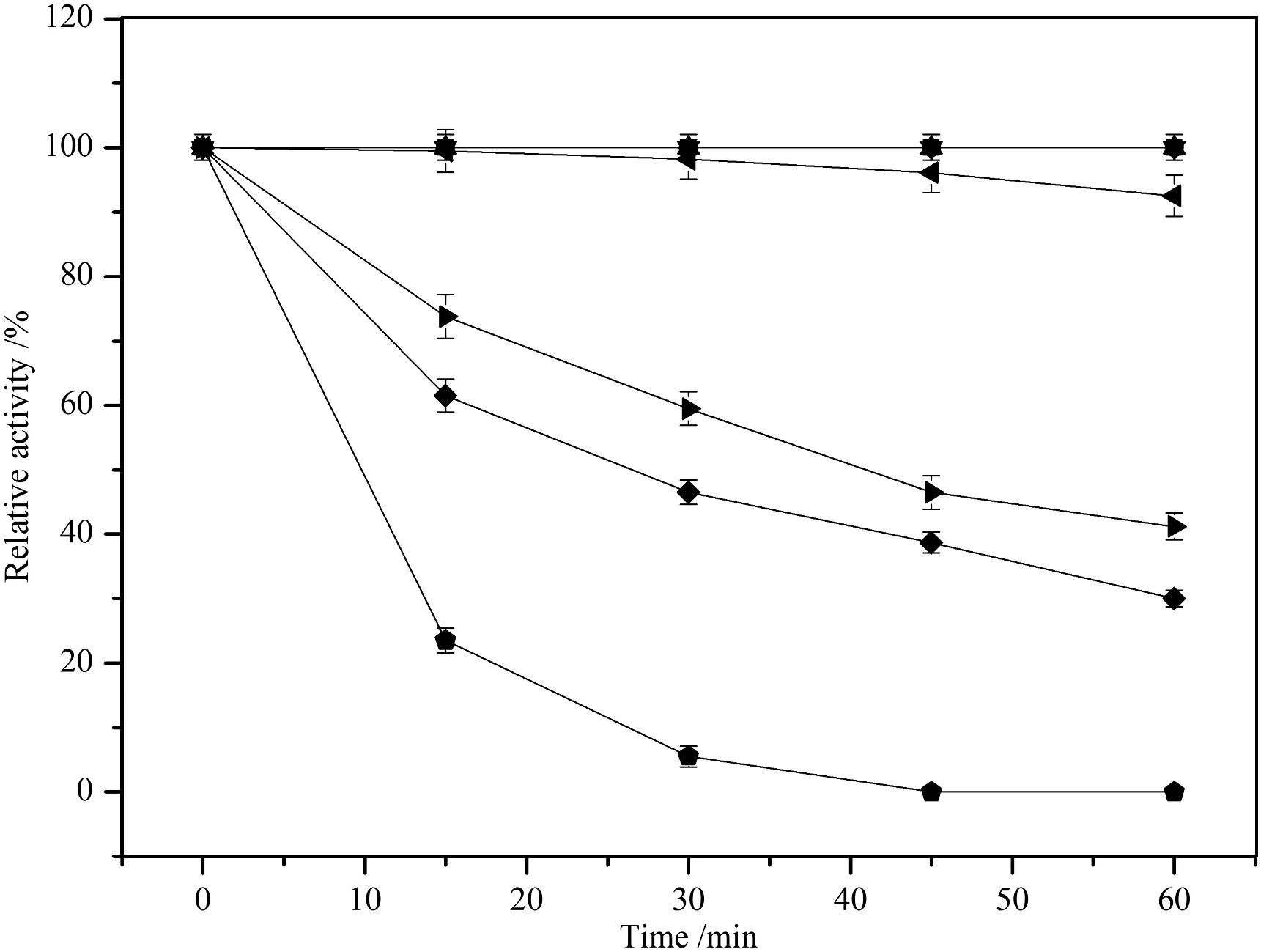
Fig.1.Effectoftemperature on lipase stability.–■– -20 °C,–●– 4 °C,–▲– 10 °C,–▼– 20 °C,–◀– 30 °C,–▶– 40 °C,–◆– 50 °C,60°C.
The effect oftemperature on cellgrowth and cold active lipase production by CALIP3 is shown in Fig.2.The maximum DCMreached 7.93,9.23,10.03,and 10.85 g·L-1when temperature was controlled at 25,30,34,and 37°C,respectively,indicating that 37°C was the optimum temperature for cell growth.With the increase of temperature,the time to reach stationary phase shortened and cell concentration increased.The maximum cold active lipase activity reached 207.1,275.3,202.1,and 167.7 U·ml-1when temperature was controlled at 25,30,34,and 37 °C,respectively.The results showed that 30 °C was the optimum temperature for lipase production.With the increase of temperature,the time to reach maximum lipase activity shortened,while the lipase activity was lower at high temperatures(34°C and 37°C).This suggested that the production oflipase using CALIP3 was affected by temperature,and neither high(37 °C)nor too low(25 °C)temperature was bene ficialfor lipase production.This indicated that the lipase production was regulated by temperature,rather than by the cell growth rate.According to the study of cold active enzyme,culture temperature in fluenced the secretion ofenzyme and the activity of enzyme.As reported,low temperature is conducive to enzyme production,and enzyme production period was usually from the beginning of the exponentialphase to stationary phase ofgrowth[33,34].The cold active lipase gene was from cold deep-sea environments,and maybe controlled by the upstream cold activated regulatory elements.In the construction oflarge fragmentlibraries,these regulatory elements may be cloned to the recombinant E.coli cells together with lipase gene,which provides the possible reason for why the temperature can affectlipase production.

Fig.2.In fluence oftemperature on cellgrowth(a),glucose consumption(b),cold-active lipase activity(c).25 °C(–▼–),30 °C(–▲–),34 °C(–●–),37 °C(–■–).
To further understand the characteristics offermentation process at different temperatures,various parameters were evaluated and the results are shown in Table 1.The glucose consumption increased from 23.67 to 27.73 g·L-1,and cell growth increased from 7.93 to 10.85 g·L-1when temperature increased from 25 °C to 37 °C.However,the maximum acetic acid concentration of1.65 g·L-1was achieved at 37°C,indicating that the cells cultured at high temperature produced more acetic acid.The maximal productivity of lipase reached 7.06 U·ml-1·h-1at 30 °C(Table 1).According to the results in Fig.2 and Table 1,itcan be inferred that there was an optimum temperature for cellgrowth and lipase synthesis,and itwas hard to achieve the goal of high production,high substrate consumption rate and high productivity at the same time by only controlling one temperature in the fermentation process.

Table 1 Comparison ofparameters in the batch fermentation using different temperature controlstrategies

Fig.3.Speci fic lipase production rate(q p)atdifferenttemperatures.25 °C(curve 1),30 °C(curve 2),34 °C(curve 3),37 °C(curve 4).The curves are calculated from Eq.(3)with the kinetic data listed in Table 1.

Fig.4.Time-course effects oftwo-stage temperature controlstrategy on cellgrowth(–♦–),glucose consumption(–■–),acetic acid concentration(–▼–),cold–active lipase activity(–▲–),and dissolved oxygen concentration(–●–)during batch fermentation.
3.2.Kinetic analysis of cold active lipase production at different temperatures
To analyze the kinetic characteristics of the effectoftemperature on cold active lipase production,speci fic lipase production rate(qp/h-1)was calculated based on the data in Fig.3.Though the temperature for the high qpis 34 °C during early fermentation period(0–15 h),qpdecreased quickly at the later period when temperature was kept at 34 °C.However,the highest qpcould be achieved at 34 °C atthe initial stage of fermentation.The qpwas higher at the later period at 30°C(Fig.3)and the average speci fic growth rate was also relatively high at30°C(Table 1).Therefore,to improve the ef ficiency oflipase activity,it is presumed that lipase accumulation in fermentative system by CALIP3 was separately regulated:The temperature of 34°C was preferred atthe early stage ofcultivation(before 15 h)to ensure higher qp,and 30°C was then maintained after24 h to ensure lipase formation.
3.3.Batch fermentation for cold active lipase production with two-stage temperature controlstrategy by CALIP3
Based on the effectoftemperature on cold active lipase production,a two-stage temperature controlstrategy was developed,in which the temperature was kept at 34°C for the first15 h,and then switched to 30°C,to enhance the production ofcold active lipase(Fig.4).The results are shown in Fig.3 and summarized in Table 1.As expected,cold active lipase activity(315.2 U·ml-1)and productivity(8.08 U·ml-1·h-1)in the two-stage temperature controlled fermentation process were further enhanced by both 14.5%,compared with those offermentation with temperature controlled at30°C(Table 1).
3.4.Cold active lipase activity atdifferent DO levels
DO is another important environmental factor in the process of microbialfermentation.It plays a very important role in cellgrowth,product synthesis and maintenance of cell metabolism.Too high or too low DO levelis not good for cellgrowth and product synthesis.To understand the effects of DO levels on cold active lipase production,fermentations at different DO levels(10%,20%,30%,and 40%)were investigated along with the previous two-stage temperature control strategy.Fig.4 also illustrates the pro files ofDOin batch culture without DOcontrol.DOlevelcontinually decreased from 0 to 30 h,and reached a minimalvalue of8.0%at 30 h,and then DO levelincreased slowly,and reached 18%at 42 h.The maximum DCM,acetic acid concentrations and cold active lipase activity reached 10.68,1.13 g·L-1and 315.2 U·ml-1,respectively,and glucose concentration continually decreased and reached 2.74 g·L-1at39 h.
The effects of DO on cell growth and cold active lipase activity by CALIP3 were further investigated by keeping DO levelat 10%,20%,30%and 40%constantly throughout fermentations.As shown in Fig.5,the maximal DCM reached 7.53,9.07,10.25,and 8.26 g·L-1when DO levelwas maintained at10%,20%,30%and 40%,respectively,indicating that 30%was the optimum DO levelfor cellgrowth.The maximalcold active lipase activity reached 224.1,307.8,321.5 and 257.3 U·ml-1when DO was maintained at 10%,20%,30%and 40%,respectively.The average speci fic glucose consumption rate increased from 0.0742 to 0.101 h-1when DO increased from 10%to 40%.The maximalproductivity of lipase reached 8.24 U·ml-1·h-1at a DO levelof 30%(Table 2).Fig.5 shows that the DCM and maximum cold active lipase at a DO level of 30%were higher than those at the other three DO levels.However,the highest acetic acid concentration of 2.04 g·L-1was achieved at a DO levelof40%,indicating that the cells cultured at high DO levelproduced more acetic acid.The acetic acid of high concentration conversely exerted inhibitory effects on cellgrowth,and this may account for the fact that DCMwas low at excessive oxygen supply and low oxygen supply.
3.5.Kinetic analysis ofcold active lipase production at different DO levels
When the cells grew under conditions oflow oxygen supply,the oxygen demand of the cells was not satis fied,which willresult in a lower yield of product(the maximum lipase activity was 224.1 U·ml-1at a DO levelof10%).On the other hand,an excessive oxygen supply may cause a decrease in productivity because of the resulting losses ofsubstrate by direct oxidation during the fermentation process.In addition,DO levelis controlled by manipulating the air flow or the stirrer speed applied to large–scale industrialreactors,so that an excessive oxygen supply often leads to unacceptable power consumption[35].Therefore,itis necessary to develop an optimaloxygen supply strategy to achieve the maximallipase production.
Fig.6 shows the kinetics of qprate at different DO levels of10%,20%,30%and 40%.The results indicated that the highest qpwas obtained at different DOlevels atdifferentculture stages.During 0–18 h,the highest qpwas obtained at the DO level of 30%,while during 18–42 h,the highest qpwas obtained at the DO levelof20%.
3.6.Enhanced cold active lipase production using a step-wise temperature and DO controlstrategy

Fig.5.In fluence of dissolved oxygen concentration(DO)on cell growth(a),glucose consumption(b),cold-active lipase activity(c).Different DO levels of 10%(–▼–),20%(–▲–),30%(–●–),40%(–■–)were investigated.
Based on above results,a regulation ofDO levelwas proposed as follows to enhance lipase production:0–15 h,DO 30%and temperature 34 °C;15–18 h,DO 30%and temperature 34 °C;18–39 h,DO 20%and temperature 34°C.The results showed thatDCMreached the maximum value of 10.45 g·L-1at 27 h.The maximum lipase activity reached 354.6 U·ml-1at 39 h,and the maximum acetic acid concentration was only 0.85 g·L-1,which was far below the values gained by single oxygen supplying approach(Fig.7).The maximum productivity of lipase reached 9.09 U·ml-1·h-1,which was higher than the values achieved without temperature and DO controlby 28.8%(Table 2).

Table 2 Comparison ofparameters in the batch fermentation using different dissolved oxygen controlstrategies

Fig.6.Speci fic lipase production rate(q p)atdifferentdissolved oxygen levels.Different DO levels of10%(curve 1),20%(curve 2),30%(curve 3),40%(curve 4)were investigated.The curves are calculated from Eq.3 with the kinetic data listed in Table 2.
4.Conclusions
For the cold active lipase production by CALIP3,a step-wise DO and temperature controlstrategy was proposed to improve lipase production.In this strategy,temperature and DO were controlled at 34°C,30%during 0–15 h;30 °C,30%during 15–18 h and 30 °C,20%during 18–39 h,respectively.With this strategy,the maximum lipase activity reached 354.6 U·ml-1at 39 h,which was 28.8%higher than that achieved without temperature and DO control(275.3 U·ml-1).The proposed step-wise temperature and DO control strategy may be suitable for the production of the other cold active enzymes by recombinant clone ofmetagenomic library.
Metagenomics have a profound positive effect on the expression and production of greater and greater amounts of recombinant proteins,which means more competitive prices,by introducing new or tailored catalytic activities of these proteins at low temperature.Thus,efforts have to be made in order to achieve economical overproduction of cold active lipase in heterologous hosts.Further investigations should consider optimizing fermentation conditions of the cold active lipase.

Fig.7.Time-course effects of the step–wise temperature and DO controlstrategy on cellgrowth(–♦–),glucose consumption(–■–),acetic acid concentration(–▼–),cold–active lipase activity(–▲–),and dissolved oxygen concentration(–●–)during batch fermentation.
[1]B.Joseph,P.W.Ramteke,G.Thomas,Cold active microbial lipases:Some hot issues and recent developments,Biotechnol.Adv.26(2008)457–470.
[2]B.Joseph,P.W.Ramteke,G.Thomas,N.Shrivastava,Standard review cold-active microbial lipases:A versatile tool for industrial applications,Biotechnol.Mol.Biol.Rev.2(2007)39–48.
[3]M.Salameh,J.Wiegel,Lipases from extremophiles and potential for industrial applications,Adv.Appl.Microbiol.61(2007)253–283.
[4]Y.Shimada,Y.Watanabe,A.Sugihara,T.Baba,T.Ooguri,S.Moriyama,T.Terai,Y.Tominaga,Ethylesteri fication of docosahexaenoic acid in an organic solvent-free system with immobilized Candida antarctica lipase,J.Biosci.Bioeng.92(2001)19–23.
[5]Y.Ota,T.Sawamoto,M.Hasuo,Tributyrin speci fically induces a lipase with a preference for the sn-2 position of triglyceride in Geotrichum sp.FO401B,Biosci.Biotechnol.Biochem.64(2000)2497–2499.
[6]N.Zhang,W.C.Suen,W.Windsor,L.Xiao,V.Madison,A.Zaks,Improving tolerance of Candida antarctica lipase B towards irreversible thermal inactivation through directed evolution,Protein Eng.16(2003)599–605.
[7]W.F.Slotema,G.Sandoval,D.Guieysse,A.J.Straathof,A.Marty,Economically pertinent continuous amide formation by direct lipase-catalyzed amidation with ammonia,Biotechnol.Bioeng.82(2003)664–669.
[8]K.E.Jaeger,T.Eggert,Lipases for biotechnology,Curr.Opin.Biotechnol.13(2002)390–397.
[9]T.Suzuki,T.Nakayama,T.Kurihara,T.Nishino,N.Esaki,Cold-active lipolytic activity ofpsychrotrophic Acinetobacter sp.strain no.6,J.Biosci.Bioeng.92(2001)144–148.
[10]C.Gerday,M.Aittaleb,M.Bentahir,J.P.Chessa,P.Claverie,T.Collins,S.D'Amico,J.Dumont,G.Garsoux,D.Georlette,A.Hoyoux,T.Lonhienne,M.A.Meuwis,G.Feller,Cold-adapted enzymes:from fundamentals to biotechnology,Trends Biotechnol.18(2000)103–107.
[11]M.Li,L.R.Yang,G.Xu,J.P.Wu,Screening,puri fication and characterization ofa novel cold-active and organic solvent-tolerant lipase from Stenotrophomonas maltophilia CGMCC 4254,Bioresour.Technol.148(2013)114–120.
[12]H.Lee,M.Ahn,S.Kwak,W.Song,B.Jeong,Puri fication and characterization of cold active lipase from psychrotrophic Aeromonas sp.LPB 4,J.Mocrobiol.Seoul41(2003)22–27.
[13]X.Yang,X.Lin,T.Fan,J.Bian,X.Huang,Cloning and expression of lipP,a gene encoding a cold-adapted lipase from Moritella sp.2-5-10-1,Curr.Microbiol.56(2008)194–198.
[14]H.S.Ryu,H.K.Kim,W.C.Choi,M.H.Kim,S.Y.Park,N.S.Han,T.K.Oh,J.K.Lee,New cold-adapted lipase from Photobacterium lipolyticum sp.nov.that is closely related to filamentous fungallipases,Appl.Microbiol.Biotechnol.70(2006)321–326.
[15]D.de Pascale,A.M.Cusano,F.Autore,E.Parrilli,G.di Prisco,G.Marino,M.L.Tutino,The cold–active Lip1 lipase from the Antarctic bacterium Pseudoalteromonas haloplanktis TAC125 is a member of a new bacterial lipolytic enzyme family,Extremophiles 12(2008)311–323.
[16]J.W.Zhang,R.Y.Zeng,Molecular cloning and expression of a cold-adapted lipase gene from an Antarctic deep sea psychrotrophic bacterium Pseudomonas sp.7323,Mar.Biotechnol.10(2008)612–621.
[17]I.Yumoto,K.Hirota,Y.Sogabe,Y.Nodasaka,Y.Yokota,T.Hoshino,Psychrobacter okhotskensis sp.nov.,a lipase-producing facultative psychrophile isolated from the coast of the Okhotsk Sea,Int.J.Syst.Evol.Microbiol.53(2003)1985–1989.
[18]M.Dieckelmann,L.A.Johnson,I.R.Beacham,The diversity of lipases from psychrotrophic strains of Pseudomonas:A novellipase from a highly lipolytic strain of Pseudomonas fluorescens,J.Appl.Microbiol.85(1998)527–536.
[19]N.Rashid,Y.Shimada,S.Ezaki,H.Atomi,T.Imanaka,Low-temperature lipase from psychrotrophic Pseudomonas sp.strain KB700A,Appl.Environ.Microbiol.67(2001)4064–4069.
[20]I.Mayordomo,F.Randez–Gil,J.A.Prieto,Isolation,puri fication,and characterization of a cold-active lipase from Aspergillus nidulans,J.Agric.Food.Chem.48(2000)105–109.
[21]X.Zeng,X.Xiao,P.Wang,F.Wang,Screening and characterization of psychrotrophic,lipolytic bacteria from deep-sea sediments,J.Microbiol.Biotechnol.14(2004)952–958.
[22]P.Song,C.Chen,Q.Tian,M.Lin,H.Huang,S.Li,Two–stage oxygen supply strategy for enhanced lipase production by Bacillus subtilis based on metabolic flux analysis,Biochem.Eng.J.71(2013)1–10.
[23]Y.Zhu,J.Li,H.Cai,H.Ni,A.Xiao,L.Hou,Characterization ofa new and thermostable esterase from a metagenomic library,Microbiol.Res.168(2013)589–597.
[24]M.N.I.Salehmin,M.S.M.Annuar,Y.Chisti,High celldensity fed-batch fermentation for the production ofa microbiallipase,Biochem.Eng.J.85(2014)8–14.
[25]W.R.Streit,R.A.Schmitz,Metagenomics—The key to the uncultured microbes,Curr.Opin.Microbiol.7(2004)492–498.
[26]R.Gupta,N.Gupta,P.Rathi,Bacteriallipases:An overview of production,puri fication and biochemicalproperties,Appl.Microbiol.Biotechnol.64(2004)763–781.
[27]P.Gupta,J.Vakhlu,Metagenomics:A quantum jump from bacterialgenomics,Indian J.Microbiol.51(2011)539–541.
[28]F.Hardeman,S.Sjoling,Metagenomic approach for the isolation of a novel lowtemperature-active lipase from uncultured bacteria of marine sediment,FEMS Microbiol.Ecol.59(2007)524–534.
[29]C.Roh,F.Villatte,Isolation of a low-temperature adapted lipolytic enzyme from uncultivated micro-organism,J.Appl.Microbiol.105(2008)116–123.
[30]Z.Zhu,R.Li,G.Yu,W.Ran,Q.Shen,Enhancement of lipopeptides production in a two-temperature-stage process under SSF conditions and its bioprocess in the fermenter,Bioresour.Technol.127(2013)209–215.
[31]P.Tirawongsaroj,R.Sriprang,P.Harnpicharnchai,T.Thongaram,V.Champreda,S.Tanapongpipat,K.Pootanakit,L.Eurwilaichitr,Novelthermophilic and thermostable lipolytic enzymes from a Thailand hot spring metagenomic library,J.Biotechnol.133(2008)42–49.
[32]N.Gupta,P.Rathi,R.Gupta,Simpli fied para-nitrophenylpalmitate assay for lipases and esterases,Anal.Biochem.311(2002)98–99.
[33]P.Secades,B.Alvarez,J.A.Guijarro,Puri fication and characterization of a psychrophilic,calcium-induced,growth-phase-dependent metalloprotease from the fish pathogen Flavobacterium psychrophilum,Appl.Environ.Microbiol.67(2001)2436–2444.
[34]V.Berchet,D.Boulanger,A.M.Gounot,Use of gel electrophoresis for the study of enzymatic activities of cold-adapted bacteria,J.Microbiol.Methods 40(2000)105–110.
[35]Z.J.Wang,H.Y.Wang,Y.L.Li,J.Chu,M.Z.Huang,Y.P.Zhuang,S.L.Zhang,Improved vitamin B(12)production by step-wise reduction of oxygen uptake rate under dissolved oxygen limiting level during fermentation process,Bioresour.Technol.101(2010)2845–2852.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- In situ synthesis ofhydrophobic magnesium hydroxide nanoparticles in a novelimpinging stream-rotating packed bed reactor☆
- Enhancing the hydration reactivity ofhemi-hydrate phosphogypsum through a morphology-controlled preparation technology☆
- Synthesis and characterization ofcopolymers ofpoly(m-xylylene adipamide)and poly(ethylene terephthalate)oligomers by melt copolycondensation
- Improvement of CO2 capture performance ofcalcium-based absorbent modi fied with palygorskite☆
- Adsorption behavior ofcarbon dioxide and methane in bituminous coal:A molecular simulation study☆
- Characterization of the adsorption behavior ofaqueous cadmium on nanozero-valent iron based on orthogonalexperiment and surface complexation modeling☆
