亚急性汞暴露对小鼠肾汞含量及肾转运体表达的影响
2016-05-30陆远富周少玉石京山
王 洋,陆远富,周少玉,石京山,刘 杰
(遵义医学院 药理学教研室暨基础药理省部共建教育部重点实验室, 贵州 遵义 563099)
基础医学研究
亚急性汞暴露对小鼠肾汞含量及肾转运体表达的影响
王洋,陆远富,周少玉,石京山,刘杰
(遵义医学院 药理学教研室暨基础药理省部共建教育部重点实验室, 贵州 遵义563099)
[摘要]目的 探讨亚急性汞暴露对小鼠肾汞含量及肾转运体表达的影响。方法 选取成年昆明种小鼠35只, 随机分成5组, 每组7只, 分别用蒸馏水、氯化汞(32 mg/kg)、甲基汞(2.6 mg/kg)、朱砂(300 mg/kg)和安宫牛黄丸(3 000 mg/kg)灌胃给药, 1天1次, 44 d后收集肾组织, 采用原子荧光分光光度计检测肾汞含量, 用RT-PCR法检测肾转运体mRNA的表达。结果 与空白组比较, 氯化汞组的肾汞蓄积含量是空白组的300倍, 甲基汞组是空白组的40倍, 而朱砂和安宫牛黄丸组没有明显变化。进一步研究发现: 氯化汞诱导有机阴离子转运体Oat1增加6倍, Oat3增加60倍, Oatp4c1增加8倍; 诱导外排转运体Mdr1b增加6倍, Mate2-k增加11倍; 抑制转运体Oat2降低95%, Oatp1a降低85%, 而Urat1增加10倍。甲基汞诱导转运体Oat3增加17倍, Mate2-k增加10倍, Urat1增加7倍, 抑制转运体Oat2降低50%, Mrp6降低60%。朱砂诱导Oat3增加20倍。结论 氯化汞、甲基汞、朱砂和安宫牛黄丸肾汞蓄积程度不同, 其主要原因是对肾转运体的影响不同。
[关键词]氯化汞; 甲基汞; 硫化汞;朱砂; 安宫牛黄丸; 汞蓄积; 肾转运体
Renal transporters mediate the renal secretion and reabsorption of exogenous and endogenous substances and are sensitive to toxic insults. Alterations in renal transporters can result in unexpected toxic effects and altered pharmacokinetics of drugs and other chemicals[1-5]. At least 37 transporters have been identified in mouse kidneys[6-7], and 29 transporters in brush-border and basolateral membrane of rat kidney have been quantified by proteomics recently. Renal transporters are categorized into four classes: (1)Basolateral uptake transporters such as organic anion transporterOat1 andOat3, organic cation transporterOct1,Oct2 and organic anion transporting polypeptideOatp4c1; (2)Apical brush-border membrane efflux transporters such as multidrug resistance-associated proteinMrp2,Mrp4, multidrug and toxin extrusionMate1,Mate2-k, breast cancer resistance proteinBcrp, and multidrug resistance proteinMdr1b; (3)Apical uptake transporters such as uric acid transporterUrat1andOatp1a1;(4) Basolateral transporter to efflux chemicals from tubule cells to blood such as the organic solute transporterOstαandOstβ[8].Transporters in the kidney play critical roles in detoxification of xenobiotics, and are sensitive to a wide variety of drugs and toxicants[1,9]. For example, Oats transport metals, drugs, and other toxicants into renal cells and result in higher concentration of toxicants in the kidney leading to nephrotoxicity[10-12]. Transporters, such asOcts,OctnsandMates, mediate the uptake, elimination and distribution of cationic drugs, toxicants, and environmental waste products[7,13]. TheMrpsplay a critical role in the tubular efflux of a wide variety of toxicants[14]. Kidney is the primary target organ that takes up and accumulates HgCl2and MeHg from the circulation[15]. It is known thatOat1 andOat3 mediate Hg uptake into kidneys[12,16], and deletion ofOat1 protects against HgCl2nephrotoxicity[17].Mrp2 plays a role in renal Hg elimination into the urine[3,18-19]. The acute effects of HgCl2and MeHg on renal transporters have been documente, however, little is known about mercury sulfide accumulation in the kidney and interaction with renal transporters, especially following repeated exposures. Mercury sulfide, either in the form of cinnabar (α-HgS) or metacinnabar (β-HgS), are included in some traditional medicines. The inclusion of heavy metals in herbal medicines may pose a potential risk to public health that has receiving growing attention worldwide. We have recently reported the differential nephrotoxicity of HgCl2, MeHg from HgS and HgS-containing traditional medicines following repeated exposures to mice[20], with marked differences in renal Hg accumulation. Thus, we hypothesize that HgS has less effects on renal transporters because of the low accumulation of Hg in kidneys. The present study was aimed to examine this hypothesis and to determine whether there is a correlation between the effects of repeated Hg exposure on renal transporters and their renal accumulation.
1Materials and methods
1.1Chemicals and animalsHgCl2and MeHg were purchased from Sigma Chemical Company (St. Louis, MO). Cinnabar (96% HgS) and AGNH (containing 10% HgS) were purchased from Guiyang De-Chang-Xiang Drug Company (Guizhou, China). Other reagents were reagent grade.
Adult Kunming mice, (25±2) g, male and female, were purchased from the Laboratory Animal Center of the Third Military Medical University (Chongqing, China). Mice were maintained in a room at (22±2)℃ with a 12 h light-dark cycle, and had free access to standard rodent chow and water. They were allowed to acclimate for at least seven days prior to the experiment. All experiments were carried out in full compliance with the WHO Guidance of Humane Care and Use of Laboratory Animals.
1.2Experimental designAdult mice were divided randomly into five groups, 7 mice per group. Mice were administrated by gavage distilled water (Control), HgCl2(32 mg/kg), MeHg (2.6 mg/kg), cinnabar (HgS, 300 mg/kg) and cinnabar (10%)-containing An-Gong-Niu-Huang Wan (AGNH,3 000 mg/kg, equivalent to 300 mg/kg HgS). Mice were gavaged daily for 44 days. The dose selection was based on the literature[21-22]and our previous publications[20,23].
1.3Determination of Hg accumulationA portion of kidney from each mouse, weighing about 100 mg, was digested 2 h in 65% nitric acid at 163 ℃ and brought to 25 ml with distilled water. Aliquots of 5 ml were incubated 30 min with 5% sulfourea and ascorbic acid solution, and Hg contents were determined with Atomic Fluorescence Spectrometry (Kechuang Haiguan Instrument Co. Ltd, Beijing, China). These assays were performed by Guizhou Chemical Analysis Center of the Chinese Academia of Sciences[20,23].
1.4RNA isolation and Real-time PCR analysisApproximately 50-100 mg of kidney tissue was homogenized in 1 ml of TRIzol (Invitrogen, Carlsbad, CA), and total RNA was extracted according to themanufacturer’s instructions, followed by purification with RNeasy kits (Qiagen, Valencia, CA). The quality of RNA was determined by 260/280 ratios. Purified RNA was reverse transcribed with Oligo-dT primers and MuLV reverse transcriptase. The Power SYBR Green Master Mix (Applied Biosystems, Foster City, CA) was used for real-time RT-PCR analysis. The primers were designed by Primer3 software and are listed in Table 1. The expression of genes of interest was first normalized with the 18S mRNA of the same sample, and the relative transcript level was calculated setting the control of 100%.
1.5Statistical analysisData are expressed as mean and standard error. SPSS 16 software was used for statistical analysis. Data were analyzed using a one-way analysis of variance (ANOVA), followed by Duncan’s multiple range test.P<0.05 was considered statistically significant.
Tab 1Sequences of the primers
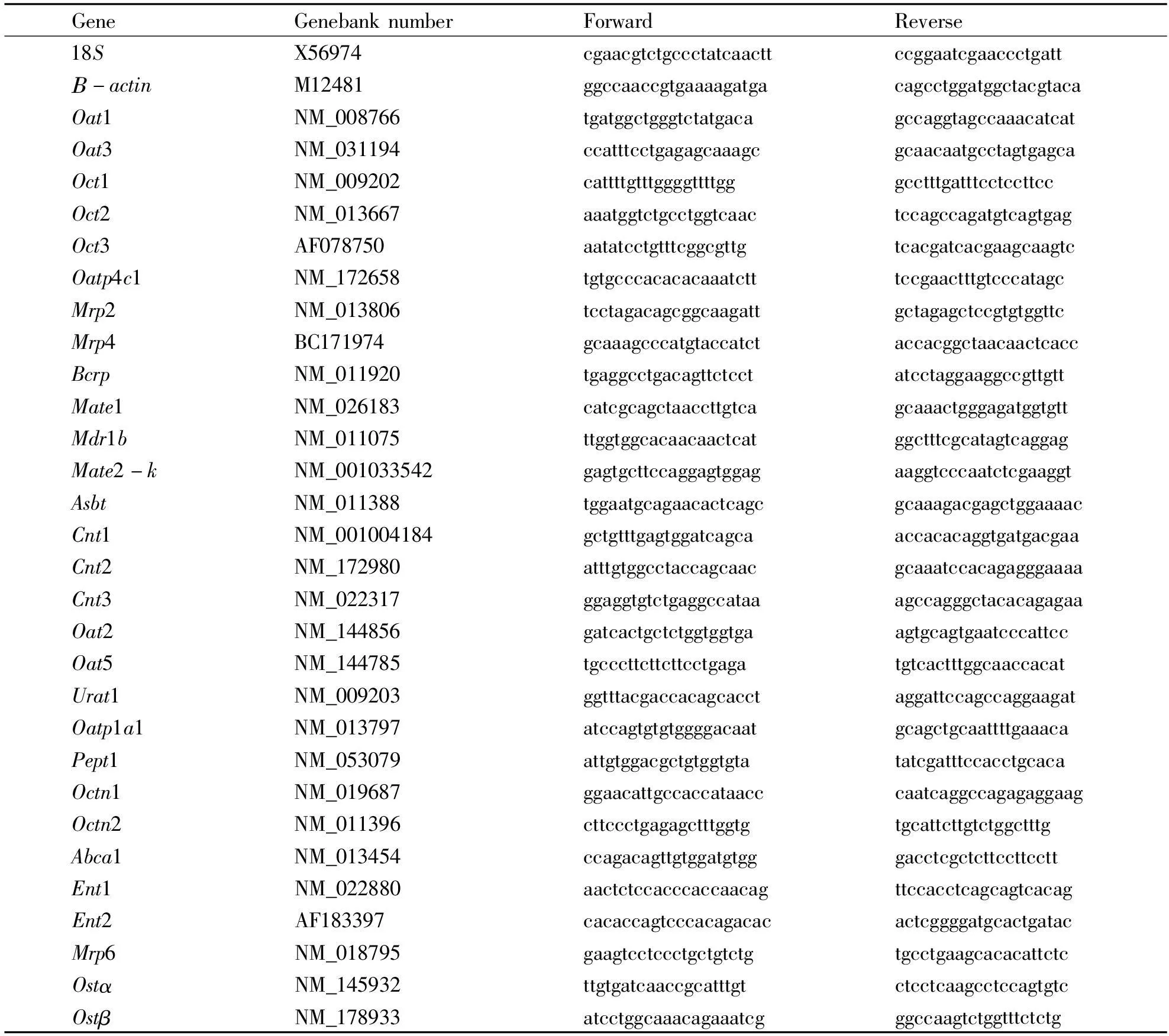
1.3DeterminationofHgaccumulationAportionofkidneyfromeachmouse,weighingabout100mg,wasdigested2hin65%nitricacidat163℃andbroughtto25mlwithdistilledwater.Aliquotsof5mlwerein-cubated30minwith5%sulfoureaandascorbicacidsolution,andHgcontentsweredeterminedwithAtom-qualityofRNAwasdeterminedby260/280ratios.PurifiedRNAwasreversetranscribedwithOligo-dTprimersandMuLVreversetranscriptase.ThePowerSYBRGreenMasterMix(AppliedBiosystems,FosterCity,CA)wasusedforreal-timeRT-PCRanaly-sis.TheprimersweredesignedbyPrimer3softwareGeneGenebanknumberForwardReverse18SX56974cgaacgtctgccctatcaacttccggaatcgaaccctgattΒ-actinM12481ggccaaccgtgaaaagatgacagcctggatggctacgtacaOat1NM_008766tgatggctgggtctatgacagccaggtagccaaacatcatOat3NM_031194ccatttcctgagagcaaagcgcaacaatgcctagtgagcaOct1NM_009202cattttgtttggggttttgggcctttgatttcctccttccOct2NM_013667aaatggtctgcctggtcaactccagccagatgtcagtgagOct3AF078750aatatcctgtttcggcgttgtcacgatcacgaagcaagtcOatp4c1NM_172658tgtgcccacacacaaatctttccgaactttgtcccatagcMrp2NM_013806tcctagacagcggcaagattgctagagctccgtgtggttcMrp4BC171974gcaaagcccatgtaccatctaccacggctaacaactcaccBcrpNM_011920tgaggcctgacagttctcctatcctaggaaggccgttgttMate1NM_026183catcgcagctaaccttgtcagcaaactgggagatggtgttMdr1bNM_011075ttggtggcacaacaactcatggctttcgcatagtcaggagMate2-kNM_001033542gagtgcttccaggagtggagaaggtcccaatctcgaaggtAsbtNM_011388tggaatgcagaacactcagcgcaaagacgagctggaaaacCnt1NM_001004184gctgtttgagtggatcagcaaccacacaggtgatgacgaaCnt2NM_172980atttgtggcctaccagcaacgcaaatccacagagggaaaaCnt3NM_022317ggaggtgtctgaggccataaagccagggctacacagagaaOat2NM_144856gatcactgctctggtggtgaagtgcagtgaatcccattccOat5NM_144785tgcccttcttcttcctgagatgtcactttggcaaccacatUrat1NM_009203ggtttacgaccacagcacctaggattccagccaggaagatOatp1a1NM_013797atccagtgtgtggggacaatgcagctgcaattttgaaacaPept1NM_053079attgtggacgctgtggtgtatatcgatttccacctgcacaOctn1NM_019687ggaacattgccaccataacccaatcaggccagagaggaagOctn2NM_011396cttccctgagagctttggtgtgcattcttgtctggctttgAbca1NM_013454ccagacagttgtggatgtgggacctcgctcttccttccttEnt1NM_022880aactctccacccaccaacagttccacctcagcagtcacagEnt2AF183397cacaccagtcccacagacacactcggggatgcactgatacMrp6NM_018795gaagtcctccctgctgtctgtgcctgaagcacacattctcOstαNM_145932ttgtgatcaaccgcatttgtctcctcaagcctccagtgtcOstβNM_178933atcctggcaaacagaaatcgggccaagtctggtttctctg遵义医学院学报39卷
2Results
2.1Renal Hg accumulationAs shown in Figure 1, the concentration of Hg in mouse kidneys after treatment with HgCl2and MeHg was increased 300- fold (121 μg/g) and 80-fold (35 μg/g) over controls (0.4 μg/g), respectively. The Hg accumulation after HgS (3.7 μg/g) and AGNH (0.9 μg/g) administration was much lower than that after administration of HgCl2or MeHg (Fig 1).

Mice were orally administrated HgCl2 (32 mg/kg), MeHg (2.6 mg/kg), Cinnabar (300 mg/kg), and AGNH (3 000 mg/kg), daily for 44 days. Tissues were digested in HNO3, followed by analysis with Atomic Fluorescence Spectrometry. Data are the mean±SD of 6 mice and expressed as μg(Hg)/g wet tissue. *: Significantly different from controls,P<0.05.Fig 1 Accumulation of Hg in mouse kidneys
2.2Expression of renal basolateral uptake transportersWe determined whether the repeated administration of mice with Hg had a differential effects on renal basolateral uptake transporters. It is known that on the basolateral membrane of renal proximal tubules,Oat1,Oat3, andOatp4c1 are the primary anion transporters, whereasOct1 andOct2 are the primary cation transporters[8]. As shown in Figure 2, the expression ofOat1 was increased 6-fold by HgCl2but was not altered by MeHg, HgS, or AGNH when compared to the control. The expression ofOat3 was increased 60-fold by HgCl2, 25-fold by MeHg and 17-fold by HgS. The expression ofOatp4c1 was increased 8-fold by HgCl2and 3-fold by MeHg, whereas the expressions ofOct1,Oct2 andOct3 were not altered by any of the Hg treatments (Fig 2A).
2.3Expression of renal apical efflux transportersBrush-border efflux transportersMrp2,Mrp4,Bcrp,Mate1,Mate2-k, andMdr1bare responsible for the renal secretion of chemicals into the glomerular filtrate[8]. As shown in Figure 3, HgCl2and MeHg increased the expression ofMrp4 by 3-fold andMdr1bby 3-6 fold, respectively, but these transporters were not changed by HgS or AGNH.Mate2-kexpression was increased 3-11 fold after the various Hg treatments. The expression ofMrp2,Bcrp, andMate1 were not altered by any of the Hg treatments (Fig 2B).

Mice were orally administrated with HgCl2 (32 mg/kg), MeHg (2.6 mg/kg), Cinnabar (300 mg/kg), and AGNH (3 000 mg/kg), daily for 44 days. Total RNA was isolated for RT-PCR analysis. Data are the mean±SD of 6 mice. *: Significantly different from controls,P<0.05.Fig 2 Expression of basolateral uptake transporters (A) and apical efflux transporters (B) in kidneys
2.4Expression of renal apical uptake transportersThe apical uptake transporters in kidneys are involved in the reabsorption of chemicals from the glomerular filtrate. HgCl2decreased the expression ofAsbtby 60%,Oat2 94%, andOatp1a1 85%, whereas MeHg decreasedAsbtby 60% andOat2 50%. The expression ofUrat1 was increased 10-fold by HgCl2, 7-fold by MeHg, and 5-fold by HgS, respectively, when compared to the controls. The expression of the apical uptake transportersCnt1,Cnt2,Cnt3,Oat5,Pept1,Octn1 andOctn2 were not changed by any of the treatments (Fig 3A).
2.5Expression of renal basolateral efflux transportersEfflux pumps on the basolateral membrane of renal proximal tubules participate in the transport of chemicals from the kidneys back into the blood. We therefore determined the effect of repeated Hg exposure on the renal basolateral efflux transportersEnt2,Ostα,Mrp6, andAbca1. It was found that HgCl2increased the expression ofEnt2 by 200%, but decreased the expression ofOstbby 95%. MeHg decreased the expression ofMrp6 by 60% andOstbby 50%. Cinnabar and AGNH had no effect on the expression of basolateral efflux transporters. The expression ofAbca1 was increased 2-fold by MeHg (Fig 3B).

Mice were orally administrated with HgCl2 (32 mg/kg), MeHg (2.6 mg/kg), Cinnabar (300 mg/kg), and AGNH (3 000 mg/kg), daily for 44 days. Total RNA was isolated for RT-PCR analysis. Data are the mean±SD of 6 mice.*:Significantly different from controls, P < 0.05.Fig 3 Expression of apical uptake transporters (A) and basolateral efflux transporters (B) in kidneys
3Discussion
The present study clearly demonstrates that repeated exposure to various forms of Hg results in significant renal Hg accumulation in the rank order of HgCl2>MeHg>HgS>AGNH. More importantly, we found profound changes in the expression of some transporters in the kidney after repeated Hg exposure, which could play important roles in Hg accumulation in the kidney and nephrotoxicity. For example, HgCl2increased the expression ofOat1,Oat3,Oatp4c1,Mrp4,Mdr1b,Mate2-k,Urat1 andEnt2, whereas it decreasedOatp1a1,Asbt,Oat2 andOstα. To our knowledge, this is the first systematic examination of the effects of repeated Hg exposures on the expression of renal transporters.
Basolateral transporters participate in Hg uptake. BothOat1 andOat3 are located in the basolateral membranes of renal proximal tubule cells, and are responsible for the uptake of some drugs and toxicants from the blood into renal cells[24].Oat1 andOat3 mediate the uptake of thiol-Hg2+conjugates[25]into Madin-Darby Canine Kidney (MDCK) cells. It has been reported that in MDCK cells stably transfected with humanOat1, an increase in the accumulation of both HgCl2and MeHg occurs, resulting in increased toxicity[26-27]. Following acute HgCl2exposure (5 mg/kg, sc 18 h),Oat1 protein expression was increased in renal homogenates, but decreased in renal basolateral membranes, whereasOat3 protein expression was decreased in both kidney homogenates and basolateral membranes. The authors suggested this might be an adaptive mechanism to prevent Hg uptake into cells[16]. In the present study, however, after repeated exposure (44 days) to HgCl2(32 mg/kg, po) and MeHg (2.6 mg/kg, po), bothOat1 (6-fold) andOat3 (17 to 60-fold) were markedly increased. This indicates a significant shift in the expression ofOat3 after repeated treatment of Hg (18 h vs 44 days), which may be associated with the nephrotoxicity of repeated Hg exposure. However, one cannot rule out the possibility that the route of absorption (sc vs po) of the mercurial may be an important factor contributing to the difference in the expression ofOat3. HgS did not increaseOat1, but increasedOat3 by17-fold.Oatp4c1 is predominately expressed in kidneys[6]and its known substrates are digoxin and ouabain, as well as estrone3-sufate[22,28-29].Oatp4c1 expression was increased by HgCl2and MeHg. Whether Hg is a substrate forOatp4c1, and the role of Oatp4c1 upregulation by Hg compounds has yet to be investigated[22,29].
Apical efflux transporters are important for the renal secretion of chemicals and toxicants into the urine and are important in protecting against Hg accumulation and Hg nephrotoxicity[18].Mrptransporters contribute to the efflux of Hg[30]. It has been demonstrated that in cisplatin-induced acute renal injury, the expression of renal efflux transportersMdr1b,Mrp4 andMrp2 was significantgly increased[31]. Furthermore, inMrp2-deficient TR(-) rats receiving MeHg injection, the amount of Hg excreted in urine and feces was reduced, supporting an important role ofMrp2 for the elimination of MeHg[18]. Similarly, HgCl2elimination inMrp2-deficient TR(-) rats was also decreased[32]. In the present study, we found repeated exposure of HgCl2and MeHg increased the expression ofMrp4 (3-fold) andMdr1b(3-6 fold), suggesting that nephrotoxicants can up-regulate these efflux transporters, possibly in an attempt to eliminate their cellular burden[29].
Mate2-k,the main multidrug and toxin extrusion efflux transporter in humans, andMate1 are responsible for the detoxification of xenobiotics by mediating the tubular secretion of compounds across the bush-border membranes of the kidney[33]. RodentMate2 is predominantly expressed in the testis, but there are no isoforms corresponding tohMATE2-K[34]. Because functional protein is derived directly from theMate2 gene in mice, and not from a splice variant as the humanhMATE2 gene. MouseMate2 mRNA is expressed at high levels in the testis, whereas its constitutive expression is low in most other tissues[35]. The present study confirms that the constitutive expression ofMate2 in mouse kidney is low, but its expression increased 11-fold by MeHg.Mate2 was the only transpoter whose mRNA was increased by all mercurials tested in the present study.
Apical uptake transporters in kidneys play a critical role in the reabsorption of toxicants from the tubule lumen. Inhibition of apical transporters could reduce reabsorption and enhance urinary clearance of Hg. The expression ofOat2 andOatp1a1 mRNA are decreased by cisplatin[9,31]. In the present study, it was found that bothOatp1a1 andOat2 were decreased by HgCl2and MeHg, supporting that the reduced apical transporters could be an adaptive mechanism to reduce toxicant reabsorption.
Urat1 is the renal apical urate/anion exchanger (Slc22a12), and plays an important role in uric acid reabsorption[36]and its dysregulation may lead to hyperuricaemia[37]. During acute HgCl2exposure, uric acid may play a beneficial role against HgCl2toxicity by preventing renal oxidative stress and tissue damage[7,38]. In the present study,Urat1 was found to increase 10-, 7- and 5-fold after repeated treatment with HgCl2, MeHg, or HgS, respectively. However, whether the markedinduction ofUrat1 is beneficial or harmful response to repeated Hg exposure remains to be determined.
Basolateral efflux transporters in kidney participate in the transport of chemicals back into the blood.Mrp6 is involved in the transport of glutathione (GSH) conjugates back into blood[39]. Mercuric ions have a very high affinity for thiol-containing biomolecules, such as glutathione[15]. In the present study,Mrp6 was decreased by MeHg, which may result in a decrease in the transport of Hg-thiol back to the blood.Ostβis a novel heteromeric bile acid and sterol transporter[40]. In the present study, the expression ofOstβwas decreased following repeated Hg exposure.Abca1 mediates cholesterol efflux to apolipoprotein A-I (apoA-I) and generates HDL[41], whileEnt2 participatesin the renal disposition of nucleosides[42]. In the present study, the expression ofEnt2 was increased by HgCl2, whereasAbca1 was slightly increased by MeHg, but the significance of such alterations is not immediately clear.
The effect of mercurials on renal transporter is dependent of the chemical forms of Hg. As shown in Figure 1, HgCl2produced the highest renal Hg accumulation (121 μg/g), while AGNH resulted in the least accumulation of Hg, which is consistent with their effects on the renal transporters[7].
Repeated HgCl2exposures significantly increasedOat1,Oat3 andOatp4c1. The marked increase of these uptake transporters could well contribute to renal Hg accumulation, whereas the increase of apical efflux transpotersMrp4,Mdr1b,Mate2 and apical uptake transportersOatp1a1,Asbt,Oat2, could be considered as cellular adaptive mechanisms to reduce Hg burden in the cells.
MeHg mainly affects the brain, but also targets the kidney[21,23]. Results in the present study demonstrate that MeHg significantly accumulates in the kidney (35 μg/g), even at 10% of the HgCl2dose,and significantly influences the expression of the basolateral uptake transpoters,Oat3 andOatp4c1. Similarly, the expression of apical efflux transporterMate2-k,Mrp4,andMdr1bwere increased, and apical uptake transporterAsbtandOat2 expression were decreased. Notably, it was observed that the induction ofMate2 (11-fold) by MeHg was significantly higher than that by HgCl2(5.5-fold).
In comparison, HgS and AGNH administration resulted in much less renal Hg accumulation (3.7 μg/g and 0.9 μg/g, respectively), even at a 10-fold higher dose over HgCl2,HgS only increased the basolateral uptake transporterOat3 (17-fold) and the apical efflux transporterMate2-k(4-fold), whereas AGNH only had a mild effect onMate2-k, suggesting thatMate2-kcould be an important apical efflux transporter for cellular elimination of Hg compounds. In addition, AGNH differs from HgS in Hg accumulation and effectson transporters, suggesting HgS-herb interactions in the AGNH formula.
In summary, the present study clearly demonstrates that some kidney transporters are markedly altered by repeated Hg exposure. The alterations in renal transporters are dependent on the chemical form of the mercurials and are correlated with renal Hg accumulation and nephrotoxicity.
[References]
[1] Endres C J, Hsiao P, Chung F S, et al. The role of transporters in drug interactions[J]. Eur J Pharm Sci, 2006, 27(5): 501-517.
[2] Saito H.Pathophysiological regulation of renal SLC22A organic ion transporters in acute kidney injury:pharmacological and toxicological implications[J]. Pharmacol Therapeut,2010, 125(1): 79-91.
[3] Bridges C C, Joshee L, Zalups R K. MRP2 and the handling of mercuric ions in rats exposed acutely to inorganic and organic species of mercury[J]. Toxicol Appl Pharm, 2011, 251(1): 50-58.
[4] Bridges C C, Joshee L, Zalups R K. Aging and the disposition and toxicity of mercury in rats[J]. Exp Gerontol, 2014, 53(2): 31-39.
[5] Jia W, Du F, Liu X, et al. Renal tubular secretion of tanshinol: molecular mechanisms, impact on its systemic exposure, and propensity for dose-related nephrotoxicity and for renal herb-drug interactions[J]. Drug Metab Dispos, 2015, 43(5): 669-678.
[6] Cheng X, Klaassen C D. Tissue distribution, ontogeny, and hormonal regulation of xenobiotic transporters in mouse kidneys[J]. Drug Metab Dispos, 2009, 37(11): 2178-2185.
[7] Eraly S A, Liu H C, Jamshidi N, et al. Transcriptome-based reconstructions from the murine knockout suggest involvement of the urate transporter, URAT1 (slc22a12), in novel metabolic pathways[J]. Biochemistry and Biophysics Reports, 2015, 3(5): 51-61.
[8] Klaassen C D, Aleksunes L M. Xenobiotic, bile acid, and cholesterol transporters: function and regulation[J]. Pharmacol Rev, 2010, 62(1): 1-96.
[9] Henjakovic M, Hagos Y, Krick W, et al. The human organic anion transporter 2 (OAT2) is distinct from OAT1 and OAT3 with respect to transport function[J]. Am J Physiol-Renal, 2015,309(10):843-851.
[10] Nigam S K, Bush K T,Bhatnagar V. Drug and toxicant handling by the OAT organic anion transporters in the kidney and other tissues[J]. Nat Clin Pract Nephr, 2007, 3(8): 443-448.
[11] Sweet D H. Organic anion transporter (Slc22a) family members as mediators of toxicity[J]. Toxicol Appl Pharm, 2005, 204(3): 198-215.
[12] Uchida Y, Toyohara T, Ohtsuki S, et al. Quantitative Targeted Absolute Proteomics for 28 Transporters in Brush-Border and Basolateral Membrane Fractions of Rat Kidney[J]. J Pharm Sci-US, 2016, 105(2): 1011-1016.
[13] Koepsell H, Lips K,Volk C. Polyspecific organic cation transporters: structure, function, physiological roles, and biopharmaceutical implications[J]. Pharm Res-Dordr, 2007, 24(7): 1227-1251.
[14] van de Water F M, Masereeuw R, Russel F G. Function and regulation of multidrug resistance proteins (MRPs) in the renal elimination of organic anions[J]. Drug Mtab Rev, 2005, 37(3): 443-471.
[15] Bridges C C, Zalups R K. Molecular and ionic mimicry and the transport of toxic metals[J]. Toxicol Appl Pharm, 2005, 204(3): 274-308.
[16] Di Giusto G, Anzai N, Ruiz M L, et al. Expression and function of Oat1 and Oat3 in rat kidney exposed to mercuric chloride[J]. Arch Toxicol, 2009, 83(10): 887-897.
[17] Torres A M, Dnyanmote A V, Bush K T, et al. Deletion of multispecific organic anion transporter Oat1/Slc22a6 protects against mercury-induced kidney injury[J]. J Biol Chem, 2011, 286(30): 26391-26395.
[18] Zalups R K, Bridges C C. MRP2 involvement in renal proximal tubular elimination of methylmercury mediated by DMPS or DMSA[J]. Toxicol Appl Pharm, 2009, 235(1): 10-17.
[19] Zalups R K, Joshee L, Bridges C C. Novel Hg2+-induced nephropathy in rats and mice lacking Mrp2: evidence of axial heterogeneity in the handling of Hg2+along the proximal tubule[J]. Toxicol Sci, 2014, 142(1): 250-260.
[20] Lu Y F, Wu Q, Yan J W, et al. Realgar, cinnabar and An-Gong-Niu-Huang Wan are much less chronically nephrotoxic than common arsenicals and mercurials[J]. Exp Biol Med, 2011, 236(2): 233-239.
[21] Shi J Z, Kang F, Wu Q, et al. Nephrotoxicity of mercuric chloride, methylmercury and cinnabar-containing Zhu-Sha-An-Shen-Wan in rats[J]. Toxicol Lett, 2011, 200(3): 194-200.
[22] Sui Y, Yang H, Tian X, et al. Effect of Zhusha Anshen pill, cinnabar, HgS, HgCl2and MeHg on gene expression of renal transporters in mice[J]. China Journal of Chinese Materia Medica, 2015, 40(3): 506-510.
[23] Lu Y F, Wu Q, Liang S X, et al. Evaluation of hepatotoxicity potential of cinnabar-containing An-Gong-Niu-Huang Wan, a patent traditional Chinese medicine[J]. Regul Toxicol Pharm, 2011, 60(2): 206-211.
[24] Burckhardt G, Burckhardt B C. In vitro and in vivo evidence of the importance of organic anion transporters (OATs) in drug therapy[J]. Drug Transporters, 2010,201(12): 29-104.
[25] Aslamkhan A G, Han Y H, Yang X P, et al. Human renal organic anion transporter 1-dependent uptake and toxicity of mercuric-thiol conjugates in Madin-Darby canine kidney cells[J]. Mol Pharmacol, 2003, 63(3): 590-596.
[26] Zalups R K, Ahmad S. Homocysteine and the renal epithelial transport and toxicity of inorganic mercury: role of basolateral transporter organic anion transporter 1[J]. J Am Soc Nephrol, 2004, 15(8): 2023-2031.
[27] Zalups R K, Ahmad S. Transport of N-acetylcysteine S-conjugates of methylmercury in Madin-Darby canine kidney cells stably transfected with human isoform of organic anion transporter 1[J]. J Pharmacol Exp Ther, 2005, 314(3): 1158-1168.
[28] Yamaguchi H, Sugie M, Okada M, et al. Transport of estrone 3-sulfate mediated by organic anion transporter OATP4C1: estrone 3-sulfate binds to the different recognition site for digoxin in OATP4C1 [J]. Drug Metab Pharmacok, 2010, 25(3): 314-317.
[29] Zhu Q, Lu Y, Shi J, et al. Distinct effect of Wansheng Huafeng Dan containing ardisia crenata on renal transporters, mercury accumulation and Kim-1 expression from mercuric chloride[J].China Journal of Chinese Materia Medica,2014,39(10):1892-1896.
[30] Aleo M F, Morandini F, Bettoni F, et al. Endogenous thiols and MRP transporters contribute to Hg2+efflux in HgCl2-treated tubular MDCK cells[J]. Toxicology, 2005, 206(1): 137-151.
[31] Aleksunes L M, Augustine L M, Scheffer G L, et al. Renal xenobiotic transporters are differentially expressed in mice following cisplatin treatment[J]. Toxicology, 2008, 250(2): 82-88.
[32] Bridges C C, Joshee L, Zalups R K. MRP2 and the DMPS- and DMSA-mediated elimination of mercury in TR(-) and control rats exposed to thiol S-conjugates of inorganic mercury[J]. Toxicol Sci, 2008, 105(1): 211-220.
[33] Tanihara Y, Masuda S, Sato T, et al. Substrate specificity of MATE1 and MATE2-K, human multidrug and toxin extrusions/H+-organic cation antiporters[J]. Biochem Pharmacol, 2007, 74(2): 359-371.
[34] Terada T, Inui K I.Physiological and pharmacokinetic roles of H+/organic cation antiporters (MATE/SLC47A) [J]. Biochem Pharmacol, 2008, 75(9): 1689-1696.
[35] Lickteig A J, Cheng X, Augustine L M, et al. Tissue distribution, ontogeny and induction of the transporters multidrug and toxin extrusion (MATE) 1 and MATE2 mRNA expression levels in mice[J]. Life Sci, 2008, 83(1): 59-64.
[36] Anzai N, Kanai Y, Endou H. New insights into renal transport of urate[J]. Curr Opin Rheumatol, 2007, 19(2): 151-157.
[37] Shin H J, Takeda M, Enomoto A, et al. Interactions of urate transporter URAT1 in human kidney with uricosuric drugs[J]. Nephrology, 2011, 16(2): 156-162.
[38] Durante P, Romero F, Pérez M, et al. Effect of uric acid on nephrotoxicity induced by mercuric chloride in rats[J]. Toxicol Ind Health, 2010,26(3):163-174.
[39] Liu Y H, Di Y M, Zhou Z W, et al. Multidrug resistance-associated proteins and implications in drug development[J]. Clin Exp Pharmacol P, 2010, 37(1): 115-120.
[40] Boyer J L, Trauner M, Mennone A, et al. Upregulation of a basolateral FXR-dependent bile acid efflux transporter OSTα-OSTβ in cholestasis in humans and rodents[J]. Am J Physiol-Gastr L, 2006, 290(6): 1124-1130.
[41] Nandi S, Ma L, Denis M, et al. ABCA1-mediated cholesterol efflux generates microparticles in addition to HDL through processes governed by membrane rigidity[J]. J Lipid Res, 2009, 50(3): 456-466.
[42] Mangravite L M, Badagnani I, Giacomini K M. Nucleoside transporters in the disposition and targeting of nucleoside analogs in the kidney[J]. Eur J Pharmacol, 2003, 479(1): 269-281.
[收稿2016-02-21;修回2016-04-01]
(编辑:王静)
Repeated exposure to mercurial alters the expression of renal transporters in mice
WangYang,LuYuanfu,ZhouShaoyu,ShiJingshan,LiuJie
(Department of Pharmacology,Key Laboratory of Basic Pharmacology of Ministry of Education, Zunyi Medical University, Zunyi Guizhou 563099, China)
[Abstract]Objective To explore the effects of repeated exposure to mercurials on the expressions of renal transporters in mice.Methods Mice were administrated orally mercuric chloride (HgCl2, 32 mg/kg), methylmercury (MeHg, 2.6 mg/kg), cinnabar (HgS, 300 mg/kg) and An-Gong-Niu-Huang Wan (AGNH, 3.0 g/kg, containing 300 mg/kg HgS), daily for 44 days. Accumulation of Hg in kidneys was detected by Atom Fluorescence Photometer and the expressions of 29 renal transporters were quantified by RT-PCR.Results Renal accumulation of Hg after administration of HgCl2 (121 μg/g) and MeHg (35 μg/g) was much higher than that after HgS (3.7 μg/g) and AGNH (0.9 μg/g) administration. The expressions of the basolateral uptake transporters, namely the organic anion transporter Oat1(6-fold), Oat3 (17 to 60-fold) and organic anion transporting polypeptide Oatp4c1(3 to 8-fold) were increased by HgCl2, MeHg and HgS; the expressions of apical efflux transporters including the multidrug resistance-associated protein Mrp4 (3-fold), multidrug resistance protein Mdr1b (3 to 6-fold) and multidrug and toxin extrusion Mate2-k (3 to 11-fold) were also increased. In contrast, the apical uptake transporters, Oat2 (50%-95%) and Oatp1a1 (50%-85%), were decreased by HgCl2 and MeHg, whereas the uric acid transporter Urat1 was increased (5 to 10-fold) by HgCl2 and MeHg.Conclusion Accumulation of Hg after HgCl2,MeHg, Cinnabar and AGNH administration is dependent on the different effects on renal transporters.
[Key words]HgCl2; MeHg; HgS; Cinnabar; An-Gong-Niu-Huang Wan; Hg accumulation; renal transporter
[中图法分类号]R996
[文献标志码]A
[文章编号]1000-2715(2016)02-0114-08
[通信作者]陆远富,男,博士,教授,硕士生导师,研究方向:药物代谢与毒理,E-mail:yflu@zmc.edu.cn。
[基金项目]国家自然科学基金资助项目(NO:81460632)。
