Production and characterization of exopolysaccharides in mycelial culture of Cordyceps sinensis fungus Cs-HK1 with different carbon sources☆
2016-05-29XiaChenJianyongWuXiaotingGui
Xia Chen,Jian-yong Wu,Xiaoting Gui
Department of Applied Biology&Chemical Technology,State Key Laboratory of Chinese Medicine Molecular Pharmacology in Shenzhen,The Hong Kong Polytechnic University,Hung Hom,Kowloon,Hong Kong,China
1.Introduction
Edible fungi or mushrooms are nutritious and healthy foods,and some also have medicinal properties and are classified as medicinal fungi.Polysaccharides(PS)represent a major class of bioactive molecules from edible and medicinal fungi which have notable antitumor,immunomodulatory and other medicinal properties[1,2].Cordyceps(Ophiocordyceps)sinensis,generally called the Chinese caterpillar fungus,is a precious medicinal fungus in Chinese herbal medicine with a wide range of health bene fits and bioactivities[3,4].Since natural C.sinensis in the form of a caterpillar-fruiting body complex is very rare in natureand difficult to cultivate artificially,mycelial fermentation has become the main source of C.sinensis fungal materials.Cs-HK1 is a fungus isolated from the fruiting body of a natural C.sinensis and has been identified as a Tolypocladium fungus and a relative of C.sinensis[5].The mycelial culture of Cs-HK1 has been established and optimized for mycelial grow th with a liquid medium containing glucose as the major carbon source and a few other components[5].In addition to mycelial biomass,a signi ficant amount of exopolysaccharides(EPS)has been produced by the Cs-HK1 mycelial culture in the liquid medium.Microbial EPS produced by liquid fermentation have found wide industrial applications especially in the food and biomedical fields such as xanthan and gellan as food additives,scleroglucan as laxative tablet coating,and dextran as plasma expanders[6].More recently there has been increasing interest in the pharmaceutical applications of microbial EPS[7].
The crude EPS isolated from Cs-HK1 mycelial culture medium by ethanol precipitation was composed of polysaccharides and polysaccharide-protein complexes in a wide molecular weight range.Some of the completely and partially purified EPS fractions have show n antioxidant and immunomodulatory activities[8-10].The main components of EPS of Cs-HK1 are(1 → 3)-β-D-glucan[11]and galactomannan-protein[10]while the yield of EPS was about 3.2 g·L-1.How ever,(1 → 3)-β-D-glucan in EPS of Cs-HK1 was hardly dissolved in water and even after being dissolved in the water,the solution viscosity was very high and difficult to handle[11].The galactomannan-protein complex fractionated from EPS of Cs-HK1 showed stronger antioxidant activity with high water solubility,though its yield in the culture was much low er than(1→3)-β-D-glucan.Carbon source is one of the most important nutrients,and glucose is a common and favorable carbon source for biomass grow th and EPS production in most microbial fermentations[12,13].The addition of other monosaccharide sugars may be utilized by the fungal cells and converted to uridine-diphosphate(UDP)-monosaccharides for EPS synthesis[14].Galactose and mannose may be transferred to UDP-galactose and UDP-mannose during the fermentation process and further synthesize EPS.In this study,galactoseand mannose were each added asa secondary carbon source for production of EPS in the Cs-HK1 mycelial culture.
2.Experimental
2.1.Fungal species and mycelial culture conditions
Cs-HK1 was previously isolated from the fruiting body of a natural C.sinensis and its stock culture was maintained on solid potatodextrose-agar medium at 4°C[5].Cs-HK1 mycelial culture was routinely maintained in a liquid medium consisting of 40 g·L-1glucose,10 g·L-1yeast extract,5 g·L-1peptone,1 g·L-1KH2PO4and 0.5 g·L-1MgSO4·7H2O in shake- flasks at 150 rpm and 20 °C.In the experiments on the effects of a secondary monosaccharide carbon source,5 g·L-1of galactose or mannose was added to the liquid medium containing 35 g·L-1of glucose.To ensure the yield of EPS,sufficient glucose was added together with galactose or mannose at a mass ratio of 7:1.The Cs-HK1 mycelial liquid fermentation was carried out in 250 ml Erlenmeyer flasks each containing 50 ml of the liquid medium for an overall period of 8 days.On each day of the culture period,three flasks were taken out from the shaking incubator for measurement of biomass,EPS and carbohydrate concentrations.All experiments were performed in triplicate and the results were represented by mean±SD(standard deviation).
2.2.Determination of biomass and EPS in fermentation liquid
The fermentation liquid in the culture flasks was centrifuged at 10000 r·min-1(~14980 g)for 20 min to separate the biomass from the liquid medium.The precipitated biomass was washed twice with distilled water and freeze-dried to give the biomass dry mass.For isolation of crude EPS,the supernatant liquid collected from the centrifuge was subject to ethanol precipitation by adding three volumes of 96%(v/v)ethanol to each volume of the liquid medium.The precipitated EPS was collected by centrifugation at 10000 r·min-1(~14980 g)for 20 min,and then freeze-dried on an ALPHA 1-4 LD2 freeze dryer(Martin Christ Gefriertrocknungsanlagen GmbH,Germany)at condenser-60°C and 1.2×104MPa for 48 h.The moisture content of EPS samples after the freezedrying was negligible and applied for further analysis.
2.3.Determination of sugar consumption in fermentation medium
Concentration of glucose in the liquid medium was determined with a Biochemistry Analyzer(YSI Inc.,Yellow Springs,OH,USA).Concentrations of galactose and mannose were determined by the 1-phenyl-3-methyl-5-pyrazolone-high performance liquid chromatography(PMPHPLC)method as reported by Honda and co-workers[15].In brief,450 μl of fermentation broth was derivatized with 450 μl PMP solution(0.5 mol·L-1in methanol)and 450 μl of 0.3 mol·L-1NaOH at 70 °C for 30 min.The reaction was stopped by neutralization with 450 μl of 0.3 mol·L-1HCl,followed by extraction with chloroform(1 ml,3 times).The extract solution was then applied to HPLC analysis.HPLC analysis was performed on an Agilent 1100 instrument consisting of a G1312A Binpump and a UV detector with a ZORBAX Eclipse XDB-C18 column(5 μm,4.6 mm × 150 mm)at 25 °C.
2.4.Analysis of EPS composition and MW distribution
The total carbohydrate content of the crude EPS was determined by the anthrone-sulfuric acid assay using glucose as a standard and total protein content determined by the Low ry method using bovine serum albumin(BSA)as a standard[11].The monosaccharide constituents of EPS were analyzed by the PMP-HPLC method after complete hydrolysis with 2 mol·L-1trifluoroacetic acid(TFA)(110°C for 4 h)as described in detail previously[8].NMR spectroscopy was performed on a Bruker AV400 instrument.For the NMR analysis,the EPS sample(30 mg)was co-evaporated with D2O(Sigma-Aldrich,USA)twice by lyophilization before final dissolution in a mixed solvent(Me2SO-d6/D2O in the ratio of 6:1).The MW distribution of EPS was analyzed by high performance gel permeation chromatography(HPGPC)with the same instruments(a Waters 1515 isocratic pump and a Waters 2414 refractive index detector)and conditions as in a previous study[10].
3.Results and Discussion
3.1.Consumption of carbon sources during mycelial fermentation
The consumption of the monos accharide nutrients is shown in Fig.1.Glucose was consumed daily with about 32 g·L-1glucose utilized after 8 days of culture(Fig.1A).The absorption of mannose and galactose was much different as man nose was taken since the first day while galactose was not consumed even after 8 days(Fig.1B).Carbon source is a major limiting nutrient factor for microbial grow th.Although glucose is the common carbon source to achieve high production of biomass and EPS in mycelium fermentation[16,17],other monosaccharides,such as fructose,galactose and xylose,have also been used in the mycelial fermentation[18-21].Glucose can be directly transferred to UDP-glucose and further to synthesize EPS in mycelial fermentation,while mannose or galactose may be converted to UDP-mannose or UDP-galactose directly or transferred to UDP-glucose firstly by enzyme before the uptake[14].How ever,galactose was not utilized due probably to the lack of the related enzymes in the fungus.

Fig.1.Time courses of residual carbon sources in Cs-HK1 mycelial cultures:(A)glucose;(B)galactose and mannose.Error bars represent standard deviation(SD)of triplicate lf asks.
3.2.Effect of carbon sources on biomass and EPS production
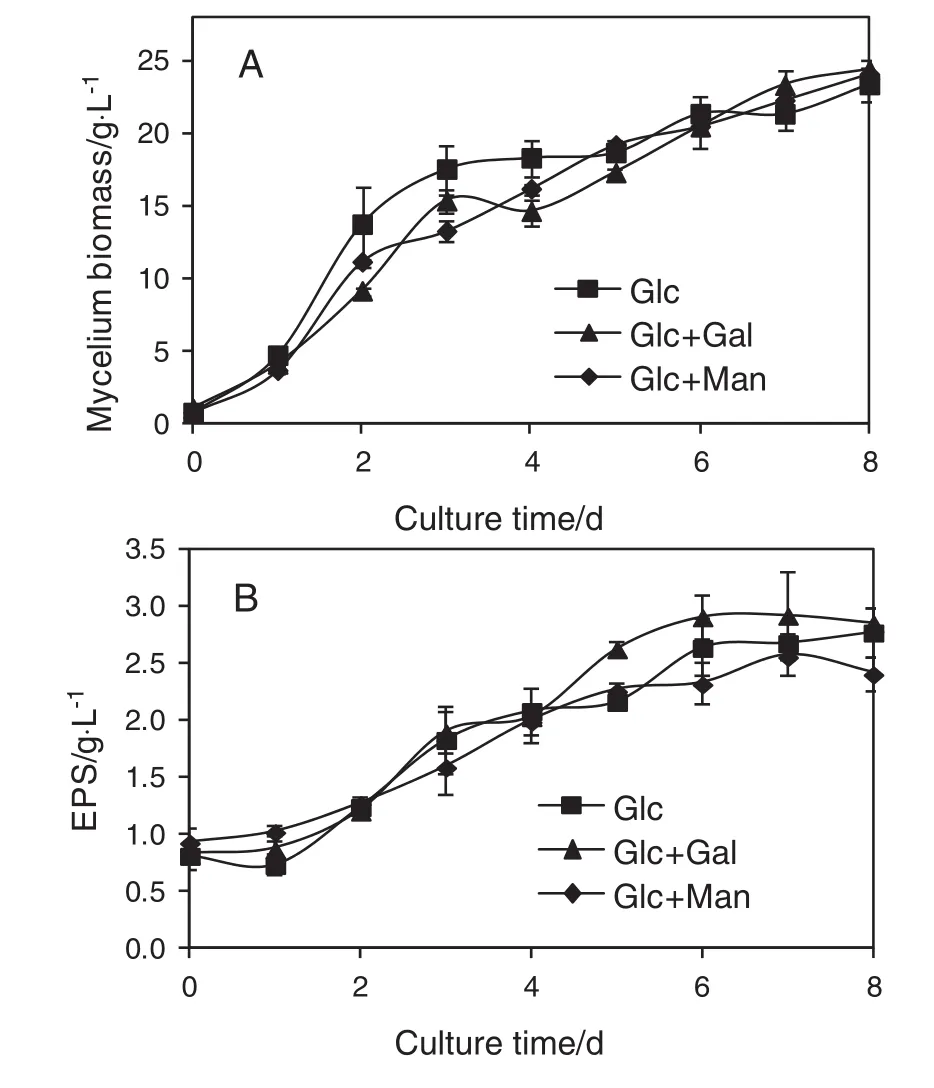
Fig.2.Time courses of biomass growth(A)and EPS production(B)in Cs-HK1 mycelial culture with Glc(40 g·L-1),Glc(35 g·L-1)+Gal(5 g·L-1)and Glc(35 g·L-1)+Man(5 g·L-1)as the carbon sources.Error bars represent standard deviation(SD)of triplicate flasks.
Fig.2 shows the biomass growth and EPS production by Cs-HK1 fungus on different carbon sources in liquid fermentation.Galactose could not be utilized in Cs-HK1 mycelial fermentation as discussed above.How ever,the production of mycelium and EPS in the presence of galactose was almost equal to the other tw o and the yield of EPS was also slightly higher than the other tw o from day 5 to day 7.A previous study has show n that galactose was consumed to promote the grow th of mycelia during the fermentation process[22].In this study,galactose also affected the biosynthesis of EPSal though it was not seen utilized.At day 8,the EPS production from the three types(Glc+Gal,Glc,Glc+Man)were 2.9 g·L-1,2.8 g·L-1and 2.4 g·L-1,respectively.As observed in our preliminary experiments(data not show n),galactose or mannose supplied as the sole carbon source in the Cs-HK1 mycelial culture could not support the biomass grow th and EPS production.
The Cs-HK1 mycelial culture parameters of biomass growth and EPS production derived from the above time courses(Figs.1 and 2)such as the biomass grow th rate,maximum concentrationsor yields of biomass and EPS were similar to those reported previously[23].
Table 1 show s the total carbohydrate and protein contents of EPS from Cs-HK1 mycelial culture with three different carbon sources on various days from days 3 to 8.Overall the total carbohydrate contents varied in the range of 14%-36%and the protein contents from 15%to 31%,which are higher in the later days with all carbon sources except for occasional fluctuations.
As show n in Table 2,the monosaccharide composition of EPS isolated from the Cs-HK mycelial cultures on different days varied with the carbon sources.Three monosaccharides,Glc,Gal and Man,were found in all EPS samples and Glc represents the major and most abundant monosaccharide constituent accounting for 50%-75%molar.The addition of galactose and mannose to the culture did not lead to a notable increase in their content in the EPS or even to a lower content.
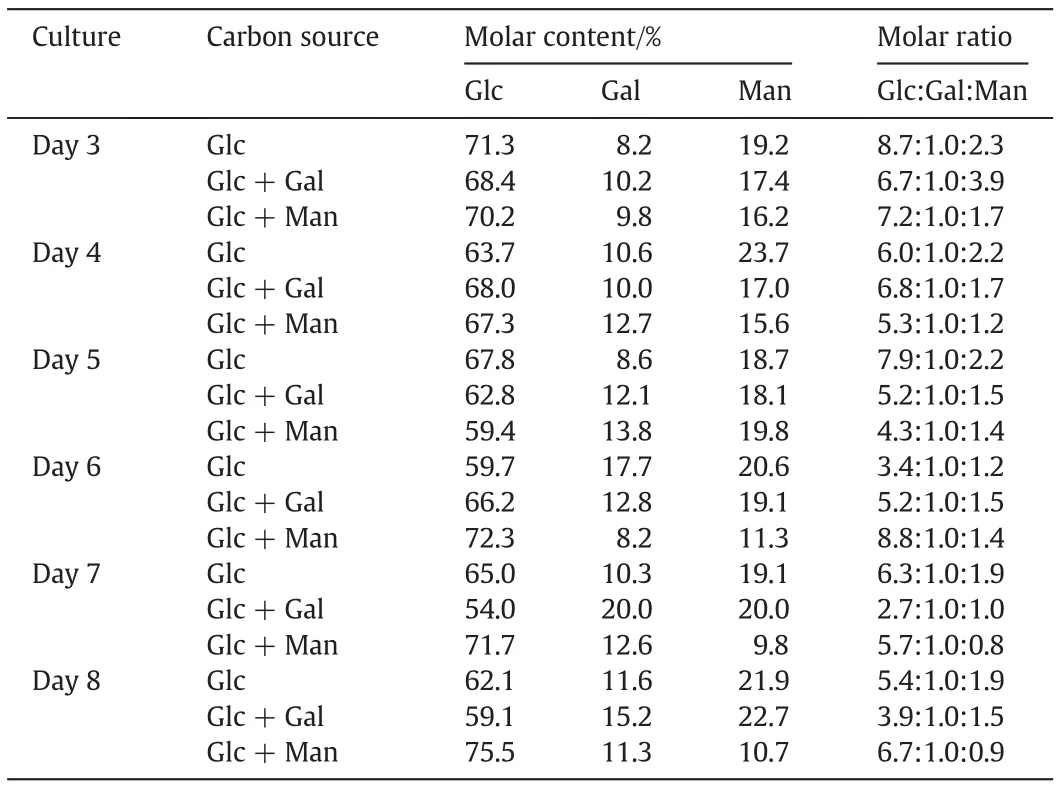
Table 2 Monosaccharide composition of EPS from Cs-HK1 mycelial culture with different carbon sources
3.3.Effect of carbon sources on EPS molecular properties
Fig.3 shows the HPGPC MW pro files of EPS collected on all 8 days of culture.In the first two days,the production of EPS was slow with three carbon sources and no significant differences were observed among the three EPS.After day 3,the MW pro files of EPS produced started to show notable differences.In the comparison of the peak area ratios(Table 3)which represent the relative amounts of different MW fractions of EPS,the amounts of the highest MW(with the shortest elution time of 10-15 min)and the low est MW EPS(eluted out at 22-32 min)were decreased,and the amount of middle MW EPS(eluted out at 12-22 min)increased with the culture period.Different monos accharides as carbon source have different pathways in the microbial fermentation process[14].The variation of EPS structural composition could be examined by NMR analysis.The1H NMR of EPS from the Cs-HK1 mycelial culture show s different proportions of β-glucan in the EPS produced with three different carbon sources(Fig.4).The proportion of β-glucanformed with galactose and glucose as the carbon source is higher(the shift around 4.8 and 4.2)than that in the EPS produced with glucose as the carbon source.Overall the results indicate that the EPS composition and MW distribution are affected significantly by the carbon sources and also vary with the culture period.

Table 1 Total carbohydrate and protein contents of EPS from Cs-HK1 mycelial culture with different carbon sources(mass%mean±SD,n=3)

Fig.3.HPGPC MW distributions of EPS from Cs-HK mycelial culture on days 1-8 with different carbon sources.The peak area ratios are summarized in Table 3.
Glucose and sucrose have been used as the favorable carbon sources for most of microbial fermentations[21-26].Although previous studies have also evaluated the use of galactose,mannose and some other mono-and oligo-saccharides for the production of biomass and EPS[27,28],few have assessed the composition and structure changes of EPS with these alternative carbon sources.In this study,the HPGPC and NMR analysis of EPS produced with alternative carbon sources show the changes in the molecular composition of EPS affected by galactose and mannose.When galactose and mannose were added as a secondary carbon source,man nose was consumed but galactose was not during the culture process.Galactose has been reported previously with low utilization in the fermentation of Listeria monocytogenes[29].Although galactose was not used by the mycelial culture,it affected the yield and composition of EPS.The mannose consumed did not increase the EPS yield and may be involved in the production of pyruvate[30].
4.Conclusions
The application of galactose or mannose as a secondary carbon source may influence the yield and composition of EPS in fungal mycelial culture.How ever,the influence appears to be irrelevant to their utilization or consumption during the mycelial fermentation process.The results suggest that galactose and mannose can be applied to vary the molecular properties of EPS composed of glucose,galactose and mannose.Variation of the carbon sources may be effective in the generation of new polysaccharide structures.Further studies should be conducted to investigate the physiological mechanisms such as the metabolism and biosynthetic pathways for the EPS with various carbon sources.
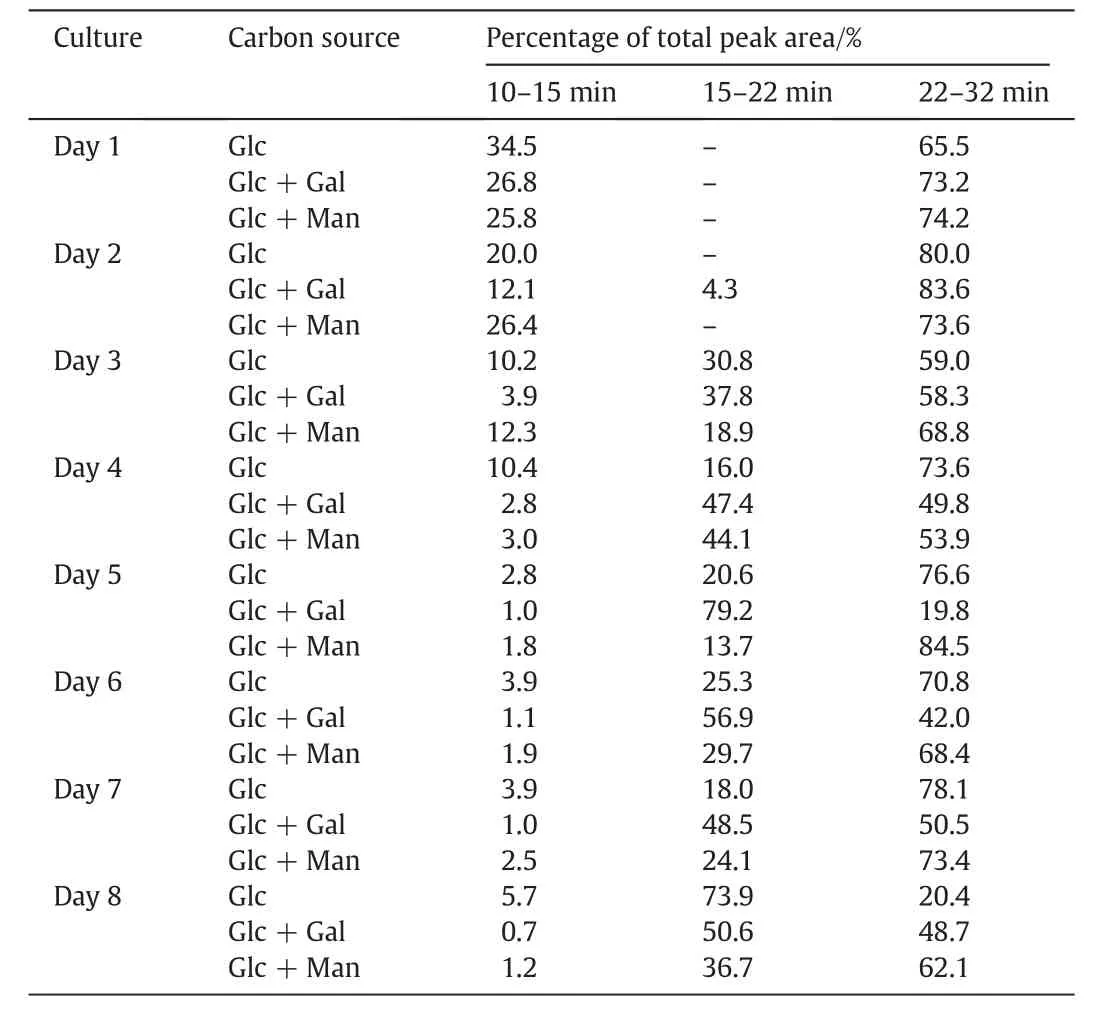
Table 3 The MW distributionsof EPSfrom Cs-HK1 mycelial culture with three carbon sources over 8 days of culture
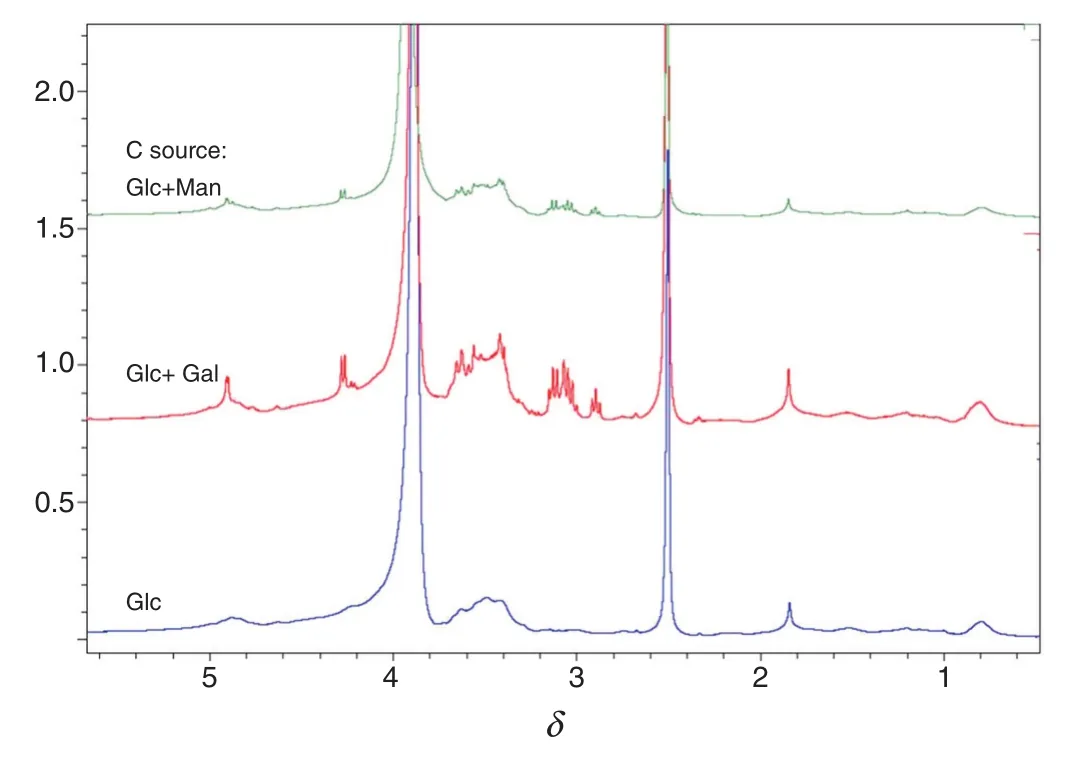
Fig.4.1H NMR of EPS collected on the day 3 from the Cs-HK1 fermentation broth on three different carbon sources.
[1]B.Stachow iak,J.Regula,Health-promoting potential of edible macromycetes under special consideration of polysaccharides:A review,Eur.Food Res.Technol.234(3)(2012)369-380.
[2]L.Ren,C.Perera,Y.Hemar,Antitumor activity of mushroom polysaccharides:A review,Food Funct.3(11)(2012)1118-1130.
[3]P.X.Chen,S.A.Wang,S.P.Nie,M.Marcone,Properties of Cordyceps sinensis:A review,J.Funct.Foods 5(2)(2013)550-569.
[4]M.G.Shashidhar,P.Girid har,K.U.Sankar,B.Manohar,Bioactive principles from Cordyceps sinensis:A potent food supplement—a review,J.Funct.Foods 5(3)(2013)1013-1030.
[5]P.H.Leung,Q.X.Zhang,J.Y.Wu,Mycelium cultivation,chemical composition and antitumour activity of a Tolypocladium sp.fungus isolated from wild Cordyceps sinensis,J.Appl.Microbiol.101(2)(2006)275-283.
[6]K.Kocharin,P.Rachathewee,J.J.Sanglier,W.Prathumpai,Exobiopolymer production of Ophiocordyceps dipterigena BCC 2073:optimization,production in bioreactor and characterization,BMC Biotechnol.10(1)(2010)51.
[7]A.Smelcerovic,Z.Knezevic-Jugovic,Z.Petronijevic,Microbial polysaccharides and their derivatives as current and prospective pharmaceuticals,Curr.Pharm.Des.14(29)(2008)3168-3195.
[8]S.Chen,K.C.Siu,W.Q.Wang,X.X.Liu,J.Y.Wu,Structure and antioxidant activity of a novel poly-N-acetylhexosamine produced by a medicinal fungus,Carbohydr.Polym.94(1)(2013)332-338.
[9]J.K.Yan,W.Q.Wang,J.Y.Wu,Recent advances in Cordyceps sinensis polysaccharides:mycelial fermentation,isolation,structure,and bioactivities:A review,J.Funct.Foods 6(2014)33-47.
[10]X.Chen,Z.Y.Ding,W.Q.Wang,K.C.Siu,J.Y.Wu,An antioxidative galactomannanprotein complex isolated from fermentation broth of a medicinal fungus Cs-HK1,Carbohydr.Polym.112(2014)469-474.
[11]X.Chen,K.C.Siu,Y.C.Cheung,J.Y.Wu,Structure and properties of a(1→3)-beta-D-glucan from ultrasound-d egraded exopolysaccharides of a med icinal fungus,Carbohydr.Polym.106(2014)270-275.
[12]S.W.Kim,H.J.Hwang,C.P.Xu,Y.S.Na,S.K.Song,J.W.Yun,In fluence of nutritional conditions on the mycelial growth and exopolysaccharide production in Paecilomyces sinclairii,Lett.Appl.Microbiol.34(6)(2002)389-393.
[13]J.Y.Wu,Z.Y.Ding,K.C.Zhang,Improvement of exopolysaccharide production by macro-fungus Auricularia auricula in submerged culture,Enzyme Microb.Technol.39(4)(2006)743-749.
[14]F.Mozzi,G.Rollan,G.S.de Giori,G.Font de Valdez,Effect of galactose and glucose on the exopolysaccharid e production and the activities of biosynthetic enzymes in Lactobacillus casei CRL 87,J.Appl.Microbiol.91(1)(2001)160-167.
[15]S.Honda,E.Akao,S.Suzuki,M.Okuda,K.Kakehi,J.Nakamura,High-performance liquid-chromatography of reducing carbohydrates as strongly ultravioletabsorbing and electrochemically sensitive 1-phenyl-3-methyl-5-pyrazolone derivatives,Anal.Biochem.180(2)(1989)351-357.
[16]B.C.Lee,J.T.Bae,H.B.Pyo,T.B.Choe,S.W.Kim,H.J.Hw ang,J.W.Yun,Submerged culture conditions for the production of mycelial biomass and exopolysaccharides by the edible Basidiomycete Grifola frondosa,Enzyme Microb.Technol.35(5)(2004)369-376.
[17]H.O.Kim,J.M.Lim,J.H.Joo,S.W.Kim,H.J.Hwang,J.W.Choi,J.W.Yun,Optimization of submerged culture condition for the production of mycelial biomass and exopolysaccharides by Agrocybe cylindracea,Bioresour.Technol.96(10)(2005)1175-1182.
[18]Y.J.Tang,L.L.Zhu,D.S.Li,Z.Y.Mi,H.M.Li,Significance of inoculation density and carbon source on the mycelial grow th and Tuber polysaccharides production by submerged fermentation of Chinese truffle Tuber sinense,Process Biochem.43(5)(2008)576-586.
[19]C.P.Pokhrel,S.Ohga,Submerged culture conditions for mycelial yield and polysaccharides production by Lyophyllum decastes,Food Chem.105(2)(2007)641-646.
[20]J.H.Xiao,D.M.Xiao,Z.H.Sun,Q.Xiong,Z.Q.Liang,J.J.Zhong,Nutritional requirements for the hyperproduction of bioactive exopoly saccharides by submerged fermentation of the edible medicinal fungus Cordyceps taii,Biochem.Eng.J.49(2010)241-249.
[21]X.B.Mao,T.Eksriwong,S.Chauvatcharin,J.J.Zhong,Optimization of carbon source and carbon/nitrogen ratio for cordycepin production by submerged cultivation of medicinal mushroom Cordyceps militaris,Process Biochem.40(5)(2005)1667-1672.
[22]N.Radchenkova,A.Tomova,M.Kambourova,Biosynthesis of an exopolysaccharide produced by Brevibacillus thermoruber 438,Biotechnol.Biotechnol.Equip.25(Suppl.1)(2011)77-79.
[23]J.Y.Wu,P.H.Leung,W.Q.Wang,C.P.Xu,Mycelial fermentation characteristics and anti-fatigue activities of a Chinese caterpillar fungus,Ophiocordyceps sinensis strain Cs-HK1(Ascomycetes),Int.J.Med.Mushrooms 16(2014)105-114.
[24]S.Rusinova-Videva,K.Pavlova,K.Georgieva,Effect of different carbon sources on biosynthesis of exopolysaccharide from Antarctic strain Cryptococcus laurentii AL62,Biotechnol.Biotechnol.Equip.25(Suppl.1)(2011)80-84.
[25]Z.Wu,J.Lu,X.Wang,B.Hu,H.Ye,J.Fan,M.Abid,X.Zeng,Optimization for production of exopolysaccharides with antitumor activity in vitro from Paecilomyces hepiali,Carbohydr.Polym.99(2014)226-234.
[26]S.Yoon,E.Hong,S.Kim,P.Lee,M.Kim,H.Yang,Y.Ryu,Optimization of culture medium for enhanced production of exopolysaccharide from Aureobasidium pullulans,Bioprocess Biosyst.Eng.35(1-2)(2012)167-172.
[27]M.Raza,K.Makeen,Y.Wang,Y.Xu,S.Qirong,Optimization,purification,characterization and antioxidant activity of an extracellular polysaccharide produced by Paenibacillus polymyxa SQR-21,Bioresour.Technol.102(10)(2011)6095-6103.
[28]P.Prasertsan,S.Wichienchot,H.Doelle,J.F.Kennedy,Optimization for biopolymer production by Enterobacter cloacae WD7,Carbohydr.Polym.71(3)(2008)468-475.
[29]A.Datta,M.H.Kothary,Effects of glucose,grow th temperature,and p H on listeriolysin O production in Listeria monocytogenes,Appl.Environ.Microbiol.59(10)(1993)3495-3497.
[30]M.Kleerebezem,J.Hugenholtz,Metabolic pathw ay engineering in lactic acid bacteria,Curr.Opin.Biotechnol.14(2)(2003)232-237.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Economic analysis in product design—A case study of a TCM dietary supplement
- Effect of ionic liquids on stability of O/W miniemulsion for application of low emission coating products☆
- Characterization and adsorption behaviors of a novel synthesized mesoporous silica coated carbon composite☆
- Experiment and simulation of foaming injection molding of polypropylene/nano-calcium carbonate composites by supercritical carbon dioxide☆
- Salt-free reactive dyeing of betaine-modified cationic cotton fabrics with enhanced dye fixation☆
- A computational analysis of the impact of mass transport and shear on three-dimensional stem cell cultures in perfused micro-bioreactors
