Synthesis of water-soluble acryl terpolymers and their anticorrosion properties on mild steel in 1 mol·L-1 HCl
2016-05-29GeethanjaliSubhashini
R.Geethanjali*,S.Subhashini
Department of Chemistry,Avinashilingam Institute for Home Science and Higher Education for Women,Coimbatore 641043,India
1.Introduction
Studies pertaining to corrosion inhibition of mild steel(MS)in acid are an attractive topic of research and have come out with a vast literature output.MS is a material of choice as constructional material owing to its excellent mechanical properties.MS also suffers from severe corrosion during certain processes that employ acid like pickling,cleaning,and descaling.The role of inhibitors comes in play to reduce the aggressiveness of acids and reduce the metallic loss due to corrosion.The important prerequisites for a compound to be an efficient inhibitor are:(1)Ability to form a defect free,compact barrier film;(2)capacity to chemisorb on to the metal surface;(3)polymerize in sites on the metal and(4)the barrier thus formed should increase the inner layer thickness.All these criteria are very well met by polymeric macromolecules and their adsorption at the solid/solution interface is governed by several factors listed below,
·Adsorption on the metal surface through segments but not directly in contact with the metal i.e.loops or tails rather than trains[1].A single polymeric molecule is capable ofreplacing severalwatermolecules on the metal surface by making the process entropically favourable and the presence of multiple bonding sites enables a slower desorption of the molecules[2].
·Polymer molecules generally lead to high affinity isotherms.
·When the polydispersity of the polymer is high the rate of adsorption becomes slower.
Solubility of the polymer is a major issue in corrosion inhibition studies,because of their increased chain length.But the presence of hydrophilic groups such as OH,NH2,COOH and SO3H increases the solubility of the polymer[3].Several water based acrylic terpolymers have been reported[4-8]as corrosion inhibitors as they have more stability,adhesive property and anchoring matrix for effective adsorption.The aim of the present work is to investigate the corrosion mitigating ability of water soluble acryl terpolymers viz.polyvinyl alcohol-g-poly(acrylamide-vinyl sulphonate)(PVA-AAm-VSA)and polyvinyl alcohol-g-poly(acrylic acid-vinyl sulphonate)(PVA-AAVSA)on corrosion of mild steel in 1 mol·L-1HCl.
2.Experimental
2.1.Synthesis of acryl terpolymers
The terpolymers PVA-AAm-VSA and PVA-AA-VSA were prepared by direct free radical polymerization of polyvinyl alcohol(PVA)and monomers acrylamide(AAm),acrylic acid(AA)and vinyl sulphonic acid sodium salt(VSA)in distilled water.PVA(2.5 g),AAm/AA(1 g),and VSA(1.5 g)were dissolved in 80 ml of water.The whole reaction mixture was purged with nitrogen gas for half-an-hour.10 mlofsodium dodecyl sulphonate solution(0.03 g)was mixed into the reaction solution.Then,0.273 g ofpotassiumpersulphate was dissolved in 10 ml was added as an oxidant initiator.The redox initiator pair Potassium persulphate-0.01 mol·L-1of tetra ethyl methyl ethylene diamine(TEMED)was added slowly to the reaction mixture in order to initiate the polymerization reaction.The reaction was continued for 3 h.The resulting white viscous solution was added to five-fold volume of acetone and the final product was precipitated.Few drops of ammonia were added to acetone to adjust the pH during the precipitation of the PVA-AA-VSA product.The precipitated product was a white rubbery mass that was washed several times with acetone-water mixture to get rid of the unreacted monomers,initiator and homopolymers.The product was dried under vacuum for 24 h and utilized for further studies.Total yield—PVA-AAm-VSA=94%,PVA-AA-VSA=83%.
2.2.Characterisation
2.2.1.FTIR
The functional groups of the grafted terpolymer was identified by IR spectroscopy(FTIR)using a Bruker Tensor-27 Fourier Transform spectrometer.Investigations have been performed in the transmission mode,using ATR assembly,at the resolution of 4 cm-1in the range of 400-4000 cm-1.
2.2.2.NMR
The grafted terpolymers were characterised by proton nuclear magnetic resonance(1H NMR)spectra with a DMX-500(Bruker,Germany)and the solvents were deuterated water and deuterated dimethyl sulphoxide.
2.2.3.TGA
The thermal stability of the polymers was determined using a thermogravimetric analyser with an Exstar SII TG/DTA6300 system at a heating rate of 20 °C·min-1from 30 to 650 °C under nitrogen purge.
2.2.4.DSC
Differential scanning calorimetry(DSC)analysis was carried out using an SDTQ 600 V8.0 Build 95 Instrument.The DSC thermograms were obtained at a heating rate of 10°C per minute in the temperature range of 30 to 500°C.
2.2.5.SEM
The surface examination of the metal samples was carried out using a scanning electron microscope(Bruker).An EDX system attached with the system was used for chemicalcharacterisation ofthe film formed on steel surface.
2.3.Gravimetric studies
Mass loss studies were conducted with the dried rectangular MS strips.The strips were immersed in triplicate in 1 mol·L-1HClin the absence and presence of various concentrations ofthe inhibitor for 1-12 h at room temperature at regular intervals.From the obtained mass loss results parameters such as corrosion rate(CR)and inhibition efficiency(IE)were calculated as follows:
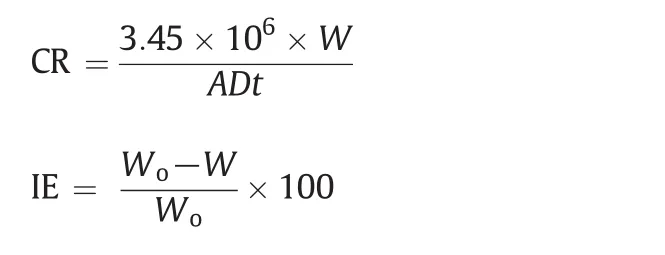
where Woand W are the mass loss(g)of the coupon in the absence and presence of inhibitor,A is the area of the coupon in cm2,D is the density of the material in g·cm-3,and t is the time of exposure in hours.
2.4.Electrochemical studies
Electrochemical studies were conducted using a Frequency Response analyser(Solartron 1280B)supported with corrware and zplot corrosion software.The conventional three-electrode system consisting of a saturated calomel electrode(SCE)as the reference electrode,platinum foil as the counter electrode,and MS strips having an exposed area of 1 cm2as the working electrode was used.The electrodes were immersed in 1 mol·L-1HCl solution for 30 min until a steady-state potential was reached.All the tests were performed at 30°C.
During the potentiodynamic polarisation measurements,specimens were polarised in cathodic and anodic directions from-0.1 V to-1 V at a scan rate of 2 mV·s-1.The corrosion current densities(Icorr)were determined from the intersection point of cathodic and anodic Tafel lines.The polarisation resistance RPwas determined from the slopes of the polarisation curves of linear polarisation region at the vicinity of the corrosion potential.Impedance spectra were recorded at corrosion potential in the frequency range 20000 Hz to 0.1 Hz with an AC amplitude of 10 mV.Inhibition efficiency was calculated from corrosion current Icorr(IEIcorr),polarisation resistance Rp(IERp),and charge transfer resistance Rct(IERct),using the following formulae:

andare parameters obtained for blank HCl,and Icorr,Rpand Rctare parameters obtained for inhibited solutions.
3.Results and Discussion
3.1.Characterisation
3.1.1.FTIR
The comparative FTIR spectra of PVA,PVA-AAm-VSA and PVA-AAVSA are depicted in Fig.1.In PVA-AAm-VSA,the broad peaks in the range of 3200-3400 cm-1can be attributed to the overlapped-OH and-NH groups of PVA and AAm respectively.But in PVA-AA-VSA,the large bands appearing in the region of 3000-3600 cm-1are due to the stretching vibrations of-OH arising from the intermolecular and intramolecular hydrogen bonds.The specific bands at 1653 cm-1and 1367 cm-1can be accounted to the CONHgroup ofpolyacrylamide,and 1661 cm-1to CO stretching of acrylic acid which is the evidence for grafting of polyacrylamide/polyacrylic acid onto the backbone of PVA[9].Orler etal.[10]observed peaks at1128,1175 and 1095 cm-1forstyrene sulphonic acid.For sodium styrene sulphonic acid Yang et al.[11]observed peaks at1184 and 1042 cm-1for antisymmetric and symmetric SO3-stretching.Butin ourcase the peaks for SO3-is observed at1097,1200 and 1265 cm-1for PVA-AAm-VSA and small peak at 1106-1044 cm-1for PVA-AA-VSA.
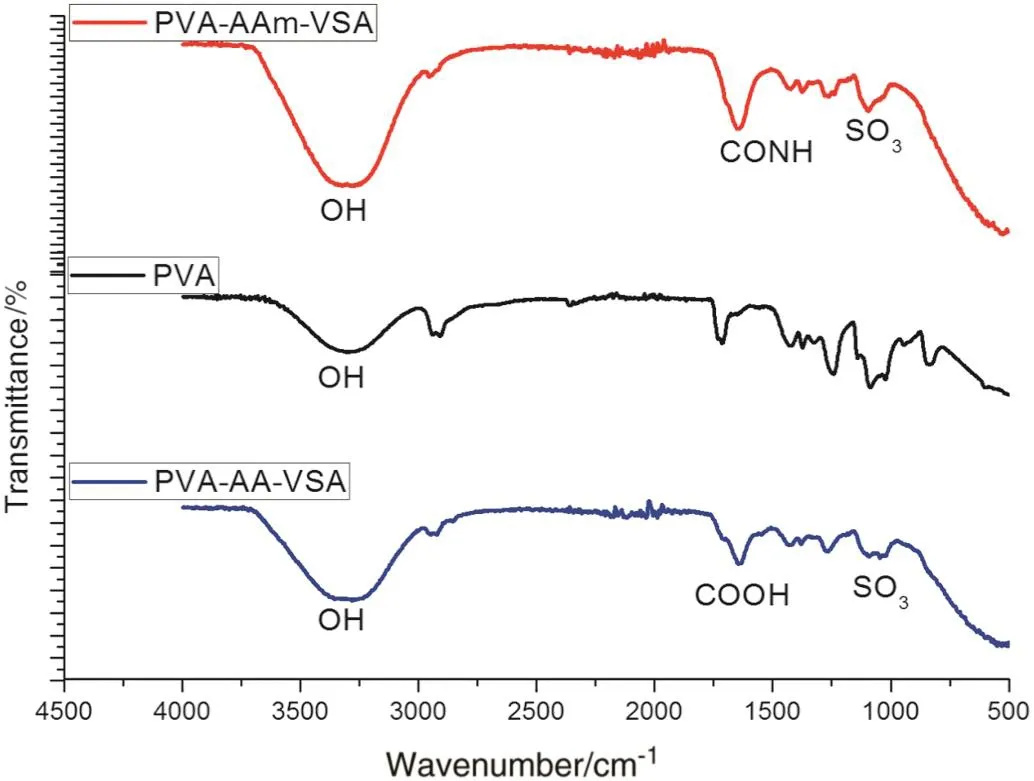
Fig.1.FTIR spectra of PVA-AAm-VSA and PVA-AA-VSA.
3.1.2.1H NMR
Fig.2(a)and(b)shows the1H NMR spectra of PVA-AAm-VSA and PVA-AA-VSA respectively.PVA-AAm-VSA:1H NMR(500 MHz,DMSO-d6,δ ppm):1.51-1.66(CH2),2-27(CH),3.9(CH-OH),5.5&5.9(d,NH2).PVA-AA-VSA:1.33-1.75(CH2),1.94-1.95(CH),3.6-3.8(CH-OH).
Chemical shifts in the range of 1.66-1.51 ppm can be assigned to the methylene-CH2-protons in the polymer chain.The OH and CH-OH protons are shifted and appear as a broad peak at 3.9,and 3.8-3.6 for PVA-AAm-VSA and PVA-AA-VSA respectively.In PVA-AA-VSA the OH of PVA and COOH of acrylic acid could have formed intermolecular hydrogen bonding and hence have shifted to an up field region[11,12].The less intense signals observed at 5.5 and 5.9 can be attributed to the NH2protons of polyacrylamide which are obviously absent in the case of PVA-AA-VSA[13].The proposed structure of the grafted terpolymers is shown in Fig.3.
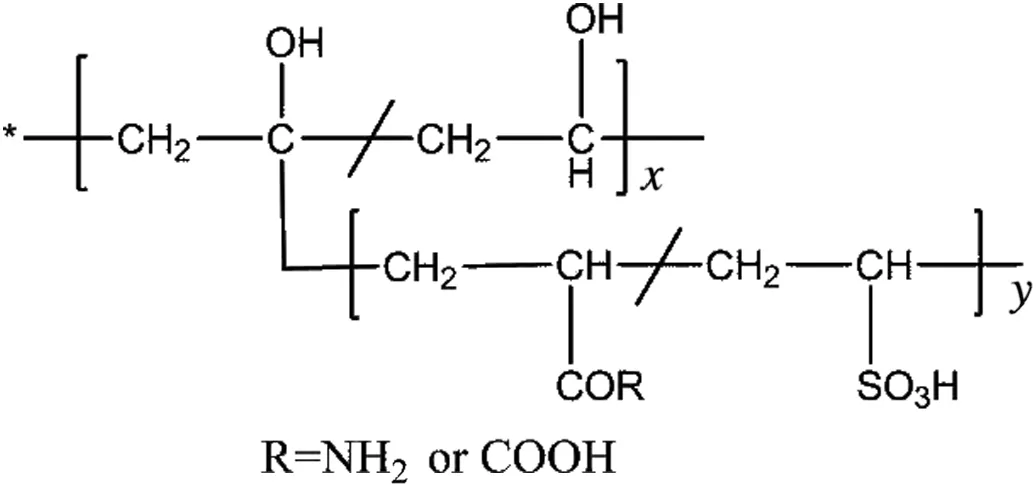
Fig.3.The proposed structure of the grafted terpolymers.
3.1.3.Thermogravimetric analysis
The TGand DTGcurves ofPVA-AAm-VSAand PVA-AA-VSAare presented in Fig.4(a)and(b)respectively.It is observed that the thermal degradation of all the samples has taken place within the programmed temperature range of 350 to 480°C.Decomposition of PVA proceeds in three stages.The first mass loss of 8%at 103°C is due to the moisture vaporization.The second stage,occurs in the range of 328°C corresponding to a mass loss of 5%may involve degradation of some volatile products.The third stage(431°C)with a maximum mass loss of 84%is correlated to the polyene residues which degrade on further heating to yield carbon and hydrocarbons[14].
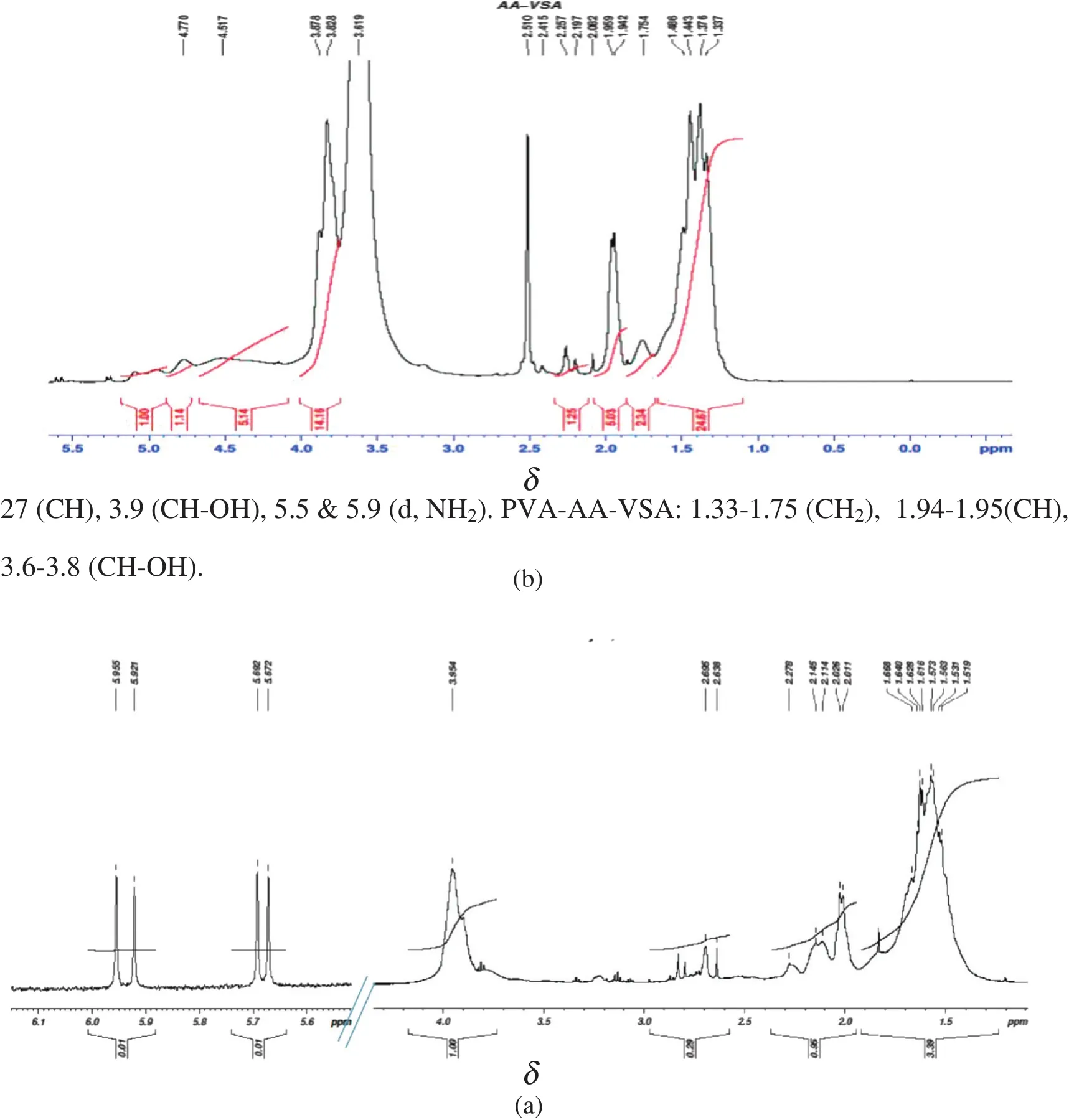
Fig.2.The 1H NMR spectra of(a)PVA-AAm-VSA and(b)PVA-AA-VSA.
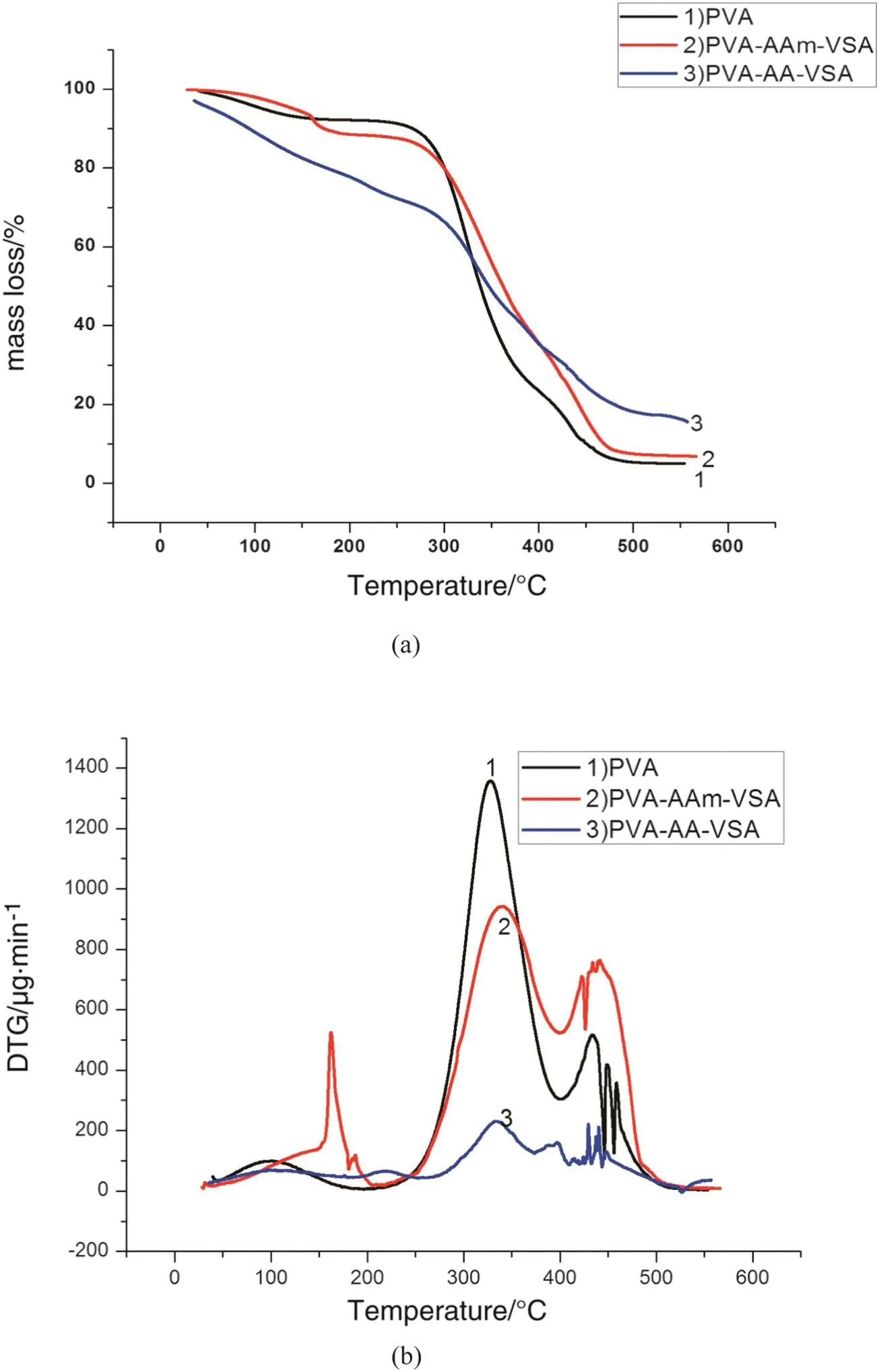
Fig.4.The TG and DTG curves of(a)PVA-AAm-VSA and(b)PVA-AA-VSA.
The TGA and DTG curves of PVA-AAm-VSA show 10%mass loss around 160°C due to the evaporation ofthe absorbed moisture.Further,the degradations are exhibited as two step degradation processes with two maxima at 337 °C and 440 °C in the DTG curve.These degradation steps can be possibly related to the imine reaction of amide group and desulphonation followed by thermal decomposition of polyacrylamide chain[15,16].The percentage of mass loss observed at these temperatures corresponds to 40%and 90%.The complete degradation starts from 470°C.The DTG curve of PVA-AA-VSA shows two small humps around 100 °C and 200 °C which can be attributed to the loss of water molecules and volatile groups.50%decomposition of PVA-AA-VSA started at 340°C exhibiting maxima in the DTG curve.At this stage decarboxylation and degradation of organosulphonic acid linkages could have taken place[17].Nearly 95%decomposition completed at 450°C after which a very less quantity of polymeric residue was left due to the thermal degradation of polymers into gaseous products at higher temperature.
The main degradation step of the terpolymers is slightly shifted to low temperature than that of the pure PVA.The thermal stability of PVA-AA-VSAis lowerthan thatofPVA-AAm-VSAdue to its hygroscopic nature and flexible chain linkages.In other words,polyacrylic acid in PVA-AA-VSA has a large number of strong hydrophilic groups that could impart the hygroscopic nature of the terpolymer.PVA-AAm-VSAhas a slightly improved thermalstability due to the strong intermolecular linkages resulting from increased hydrogen bonding formation and improved compact/stiff nature of the polymer[18].
3.1.4.DSC analysis
The DSC profile of the PVA-AAm-VSA(Fig.5(a)),shows three subsequent endothermic effects at 100,291 and 385°C which allows us to postulate stepwise transitions as follows.Tgis observed as a step down transition in the range of 40-162 °C(ΔH=48.1 mJ·mg-1)with a midpoint around 101°C.The Tgtransition is accompanied by a sharp peak at 185°C which could be accounted for glass transition.An endothermic peak in the temperature range of239-345°C,has been observed which is attributed to the melting of the polymer(Tm=291°C,ΔH=378.1 mJ·mg-1).The second melting point is recorded at the position of 385 °C with an enthalpy of 63.9 mJ·mg-1[19].
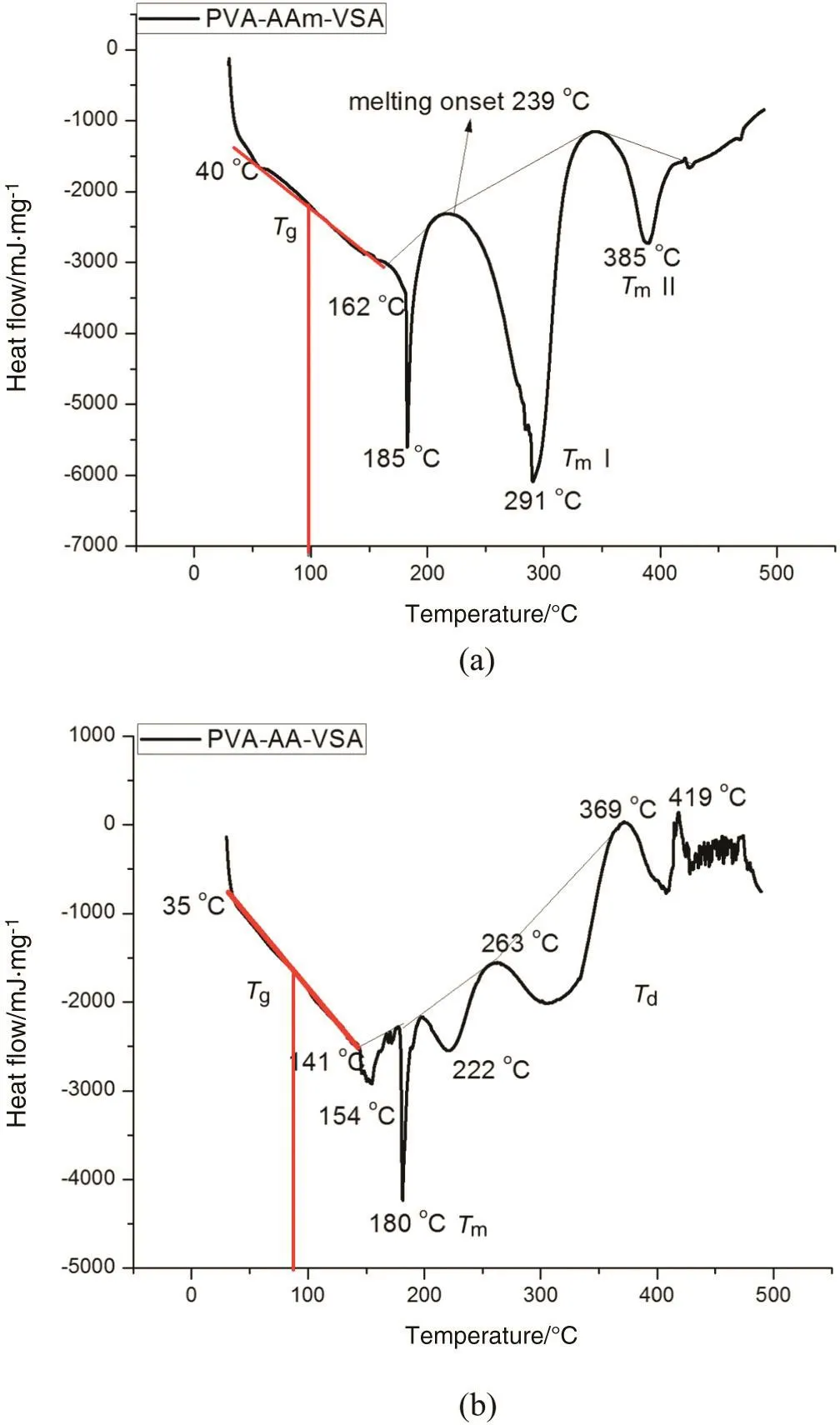
Fig.5.The DSC profile of(a)PVA-AAm-VSA and(b)PVA-AA-VSA.
The DSC thermogram of PVA-AA-VSA(Fig.5(b))reflected Tgin the temperature range of 35-141°C and Tgdetermined from the midpoint is 87 °C.The peak at 154 °C associated with the Tgrange is attributed to the enthalpy relaxation(ΔH=134 mJ·mg-1).The melting is observed as a sharp endothermic transition peak at 180°C associated with an enthalpy of194 mJ·mg-1.The firststep ofpolymerdegradation starts at 263 °C(ΔH=34 mJ·mg-1).Further polymer degradations are reflected in the form of strong exotherms at 369 and 419°C,having an enthalpy of 153 mJ·mg-1.
The occurrence of multiple melting endotherms could be due to the following reasons[20]:(1)melting-recrystallization-remelting cycle,(2)polymorphism,(3)variation in morphology of the polymer inherited in the form of lamellar thickness,perfection,stability or distribution,(4)physical ageing or/and relaxation of the rigid amorphous fraction,and(5)wide range of molecular weight distribution.
The complex melting behaviour exhibiting multiple endotherms is due to the domains with variable branching content[20].Hence in the above discussed polymers,the presence of two different melting peaks could be due to two different polymeric segments.The first melting point can be accounted for melting of the main polymeric chain and second melting point for broken polymeric chains that have actually segmented from the main polymer.Moreover,the melting isotherms are broadened,possibly due to the amorphous nature of the polymer[21-23].Random incorporation of the polyacrylamide and polyvinyl sulphonate in the polyvinyl alcohol backbone serves to further disrupt the crystalline regions of the polymer.
3.2.Gravimetric studies
3.2.1.Effect of concentration and time
The corrosion rate and IE data obtained on addition of PVA-AAm-VSA and PVA-AA-VSA in 1 mol·L-1HCl at 303 K is depicted in Table 1.It is very well established that inhibition efficiency increases with an increase in concentration whereas corrosion rate decreases.A maximum efficiency around 90%was observed for 0.45%at 12 h of immersion.The increase in inhibition efficiency with concentration is ascribed to the adsorption and effective surface coverage of the terpolymers.As a result the MS surface is efficiently separated from the corrosive medium[24].Both the polymers provide an IE in the range of 75%-80%even for the lowest mass concentration(0.03%)studied.This proves the effective surface coverage of the inhibitor even at low concentration.The corrosion rates in the absence of inhibitor increased till 3 h and then decreased due to the formation of oxide film.The presence of inhibitor however decreased the corrosion rates with increasing immersion time.At 6 h of immersion a maximum efficiency was achieved which got stabilized at the 9th hour followed by a slight increase in the 12th hour.The high IE after prolonged immersion time is due to the formation of stable adherent film of the inhibitor.The protective film is considered to be time-dependent on the MS surface[25].
PVA-AAm-VSA and PVA-AA-VSA are grouped as vinyl sulphonate polymers.The variation in their IE values can be correlated as follows:the terpolymers selected for the corrosion studies contain amide moiety in acrylamide/hydroxyl moiety in acrylic acid,in combination with sulphonic acid moiety in vinyl sulphonate and alcoholic OH in PVA.The mentioned groups could have contributed to the formation of barrier inhibitor film between metal and the corroding medium.In acid solution,the amide moiety of polyacrylamide can remain protonated to form polycations of polymeric material[26]thereby promoting electrostatic interactions.The lone pair of electrons of nitrogen,oxygen and sulphur present in the polymeric matrix can extend linkages with the empty d-orbitals of the iron surface thereby enabling the co-ordination[27,28].Further,the chains probably remain in the unfolded form due to the presence of COOH group hindrance along with the pi electron conjugation.The lack of pi electron conjugation is the reason for monomers or some polymers having least efficiency[29].
But when the IE values of PVA-AAm-VSA and PVA-AA-VSA are compared,maximum efficiency of 93.7%is provided by acrylamide containing polymer PVA-AAm-VSA and maximum efficiency of 90.7%is provided by acrylic acid polymer PVA-AA-VSA.The reason is simple and obvious i.e.due to the different hetero-atoms present in the polymers.Nitrogen atom is superior to oxygen atom incontrolling the corrosion.Moreover in acidic solutions,nitrogen becomes protonated which further aids the ease of adsorption on negatively charged metal surface as well.The protonated form of acrylamide gets adsorbed on the metal surface by electrostatic forces between ionic charges or dipoles with the potential zero charge of the metal.
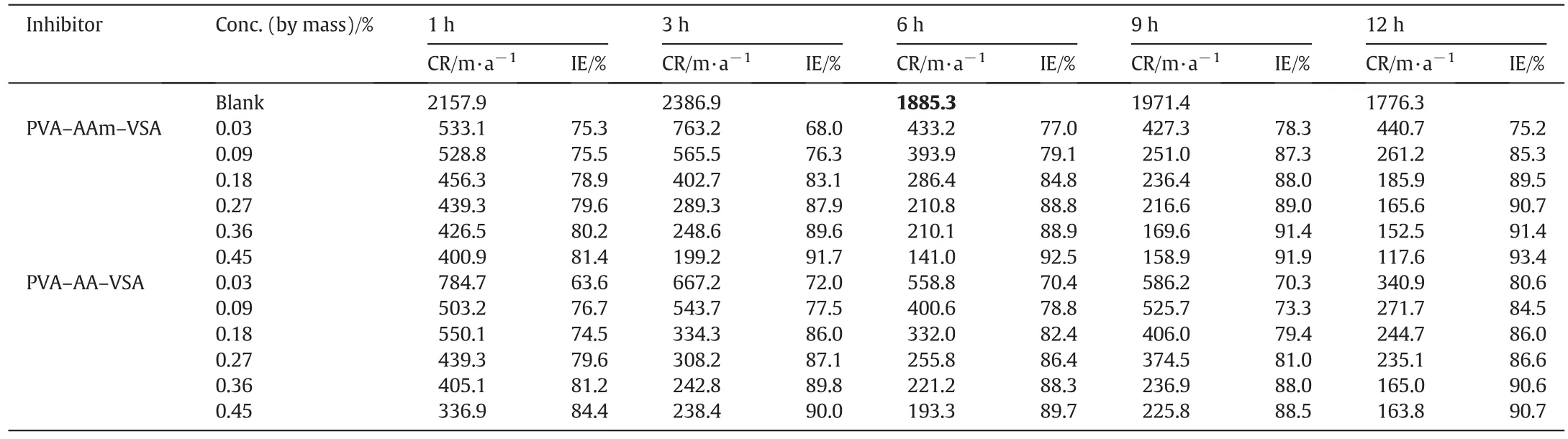
Table 1 Corrosion rates(CR)and corresponding IE values obtained for PVA-AAm-VSA and PVA-AA-VSA
3.3.Polarisation studies
Fig.6(a)and(b)shows the polarisation curves obtained for optimum concentration of PVA-AAm-VSA and PVA-AA-VSA respectively at 303 K and the corresponding polarisation data obtained are listed in Table 2.The polarisation studies were also carried out on MS forvarious immersion periods and trends in IE values obtained from Icorrvalues are depicted in Fig.7.
The current-potential curves show linear Tafel lines with different slope values.This indicates that the adsorption of the inhibitors in the electrical double layer does not affect the mechanism of the process.Baand Bcvalues are affected by the concentration and nature of the inhibitors[29].Both the Baand Bcvalues are decreased significantly in the presence of inhibitors when compared to that of blank[30].This behaviour suggests that all used inhibitors have an inhibitory effect on both cathodic and anodic reactions of the corrosion process and can be categorised as mixed type inhibitor.
The corrosion potential values remain almost the same with no definite shift in any particular direction[31].The relation between the inhibition efficiency and the shift/changes in corrosion potential can be explained as follows[32]:The anticorrosion ability of the inhibitors to some extent is due to the capability of the inhibitors to displace the electroactive interface from its original localization(metal/solution interface).This will lead to an electrical potential drop at the polymer/solution interface.But there will be almost no potential drop at the metal/polymer interface.If there is no potential drop,there will be no driving force for metal oxidation.Hence a better inhibition of corrosion can be expected for a polymer which is rich in availability of Pi-electrons.Moreover polymers being larger moieties impart the highest surface coverage of the metal surface with which various types of interactions can be established.
For all the inhibitor concentrations studied,the Icorrvalues significantly decrease corresponding to the blank HCl.The corrosion current density observed for blank HCl is 15 mA·cm-2which is reduced to 1.02 mA·cm-2and 1.21 mA·cm-2with 4.5%(by mass)of PVA-AAm-VSA and PVA-AA-VSA respectively.Rpvalues increase with increasing inhibitor concentration which leads to a decrease of Icorrvalues.An increase in Rpimplies higher protection of the interface against reactions associated with metal dissolution.

Fig.6.Polarisation curves of(a)PVA-AAm-VSA and(b)PVA-AA-VSA.
In order to study the stability of inhibitor film,the polarisation data was acquisitioned for every hour during the 12 h of immersion.The IEIcorrwas retrieved from each hour and the trend is plotted in Fig.7.It is well established that the IEIcorrvalues decrease till the 6th hour,and then tends to stabilize in the forthcoming hours.The initial decrease in IEIcorrvalues may be attributed to the instability of the film formed when the surface is polarised every next hour.
3.4.Impedance studies
The Nyquistplots in Fig.8(a)and(b)depicta setofdepressed capacitive loops generated for MS in blank HCl and PVA-AAm-VSA and PVAAA-VSA added solutions respectively.The depression at the high frequency region is a characteristic nature of the inhomogeneities and surface roughness of the electrode imputed during the electrochemical corrosion reaction[33].The solid electrodes are usually affected by these inhomogeneities and the resultanteffectis termed as the frequency dispersion effect[34,35].The addition of inhibitor poses no changes in the shape of the impedance indicating the activation-controlled nature ofthe reaction during the charge transferprocess[33].Butthe effectofthe addition ofthe inhibitor is reflected as an increase in diameter of the semicircle which takes place constantly at each level of inhibitor addition.
The increase in diameter of the impedance at increasing concentration of the inhibitor augments the charge transfer resistance as well as the inhibition effect of the polymer[35].The data acquisition from the Nyquist plot was carried out by fitting the data in the following equivalent circuit:R1+CPE/R2.Where R1is the solution resistance and R2is the charge transfer resistance.The use of CPE is generally required for the distribution of relaxation time arising as a result of inhomogeneity,roughness,adsorption or diffusion at the micro-and nano-levels of the metal surface[30].Double layer capacitance associated with the CPE element is calculated using the following formula:
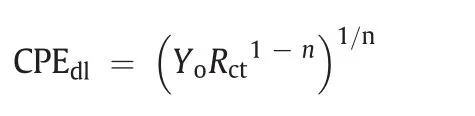
where Yois the magnitude of the CPE,and n value is a parameter that quantifies surface phenomena like surface inhomogeneities resulting from surface roughness,inhibitor adsorption,porous layer formation,etc.
Inspection of Table 3 reveals that the presence of both the inhibitors at all concentrations enhances the value of Rctin acidic solution when compared to that of blank.Double layer capacitance values are also brought down in the presence of inhibitor,and the decrease in the values of Cdlis due to the adsorption ofpolymeric inhibitoron metalsurface leading to the formation of barrier film that prevents the surface from further acid attack.The trend in the inhibition efficiency of the polymers is similar to that obtained for Icorrin the polarisation study[36].
In order to study the stability of inhibitor film,the impedance was measured for every hour during the 12 h of immersion.The IERctwas retrieved from each hour and is plotted in Fig.7.It is well established that the IERctvalues increase slightly and stabilize in the forthcoming hours.This trend is similar to the trend obtained in gravimetric studies.Since the impedance measurements employ AC current for polarisation,the film once formed is not much affected during consecutive analysis.Hence the IERctdoes not change distinctively in the course of the experiment.
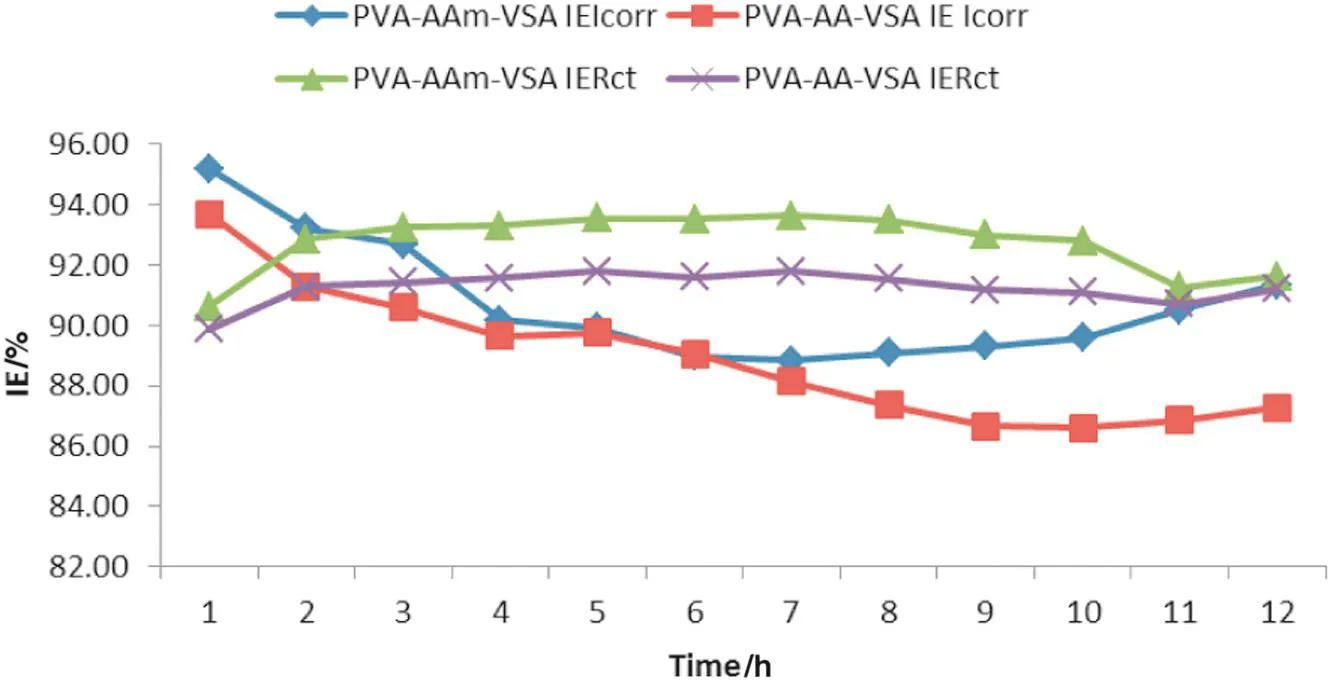
Fig.7.Variation of IE calculated from I corr and R ct of PVA-AAm-VSA and PVA-AA-VSA at various time intervals.
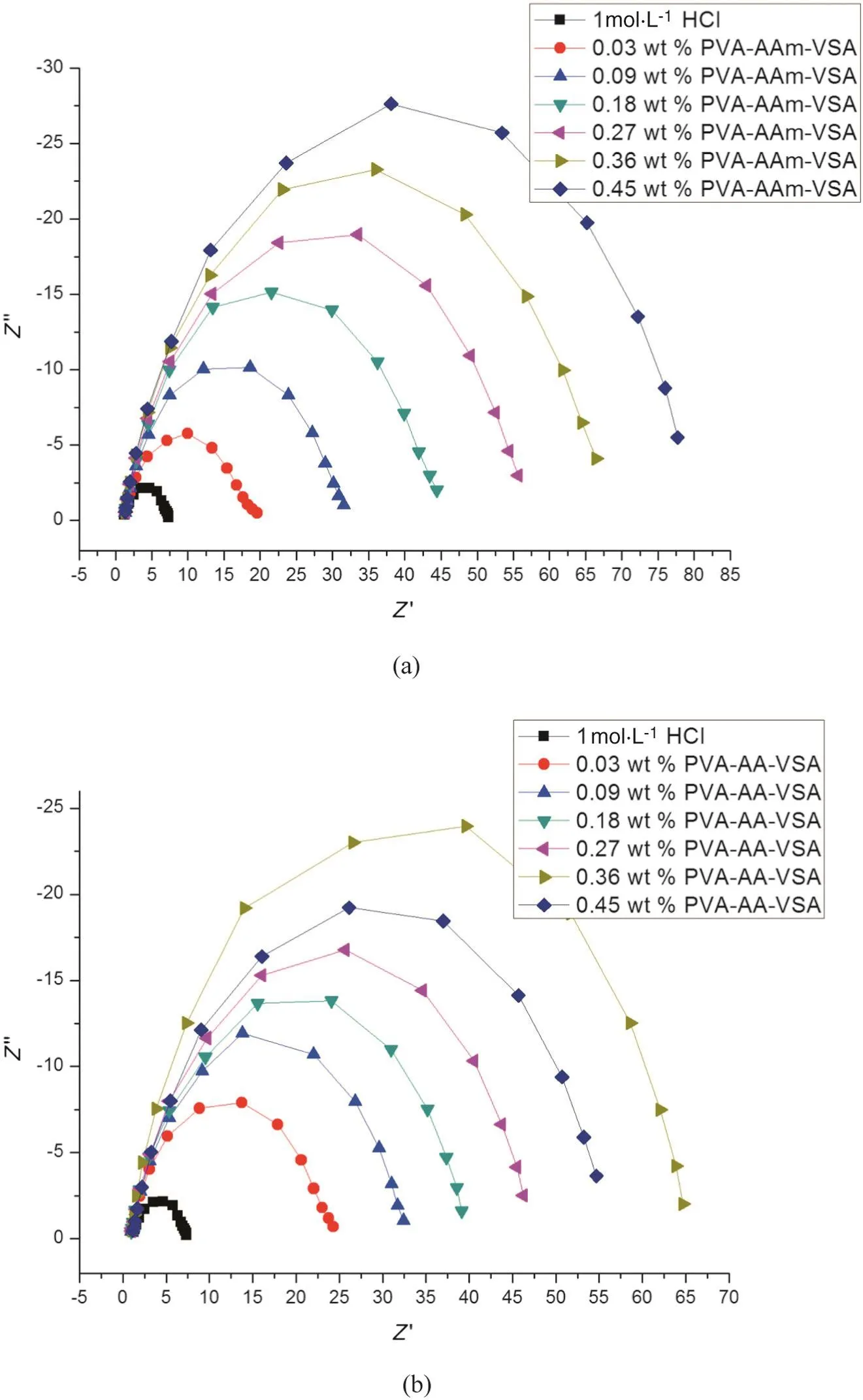
Fig.8.Nyquist plot of(a)PVA-AAm-VSA and(b)PVA-AA-VSA.
3.5.Surface analysis
In order to verify these results,the present research work has taken advantage of the bene fits offered by surface characterisation techniques:Scanning electron microscopy/energy dispersive X-ray analysis(SEM/EDX).Ex situ corrosion productanalysis was performed after a 6 h immersion in the respective inhibited solution.
The morphological dependence of the steel specimen on PVA-AAm-VSA and PVA-AA-VSA is illustrated through SEM micrographs in Fig.9(a)and(b)respectively.In the presence of inhibitors,we can observe a non-uniform distribution of particles as well as some clusters[37,38].Further magnification( fig inset)of distributed polymer particles to 2000×reveals clearly agglomerated particles of the polymer.The corrosion products are absent in the general view,and pits or crevices are not found when compared to the corroded metal specimen.The EDX analysis of the clustered polymeric particles confirms the presence of oxygen,nitrogen and/or sulphur in the particulates deposited on the surface.The mass of the elements present on the metal is provided in Table 4 which is an evidence for the surface coverage of the polymer on MS surface.
4.Conclusions
The terpolymers PVA-AAm-VSA and PVA-AA-VSA are synthesized by the free radical method and characterised byspectral and thermal techniques.PVA-AAm-VSA and PVA-AAVSA are found to be efficient in controlling the corrosion of mild steel in 1 mol·L-1HCl.The polymers are found to furnish an IE around 90% in gravimetric studies.Potentiodynamic polarisation studies show that the selected polymers inhibit both anodic and cathodic reactions,and thus act as mixed-type inhibitor.The impedance results also prove the inhibition ef ficiency of the polymers through increased Rctvalues.SEM and EDX analysis indicate that the surface morphology of the metal surface in the presence of the inhibitors clearly show the presence of adsorbed inhibitor particles.Inhibition efficiencies obtained from the methods viz.gravimetry and electrochemical are comparable and are in good agreement.At the same time,the inhibition ability of the two terpolymers follows the order:PVA-AAm-VSA>PVA-AA-VSA,which has been confirmed by the gravimetric and electrochemical measurements.
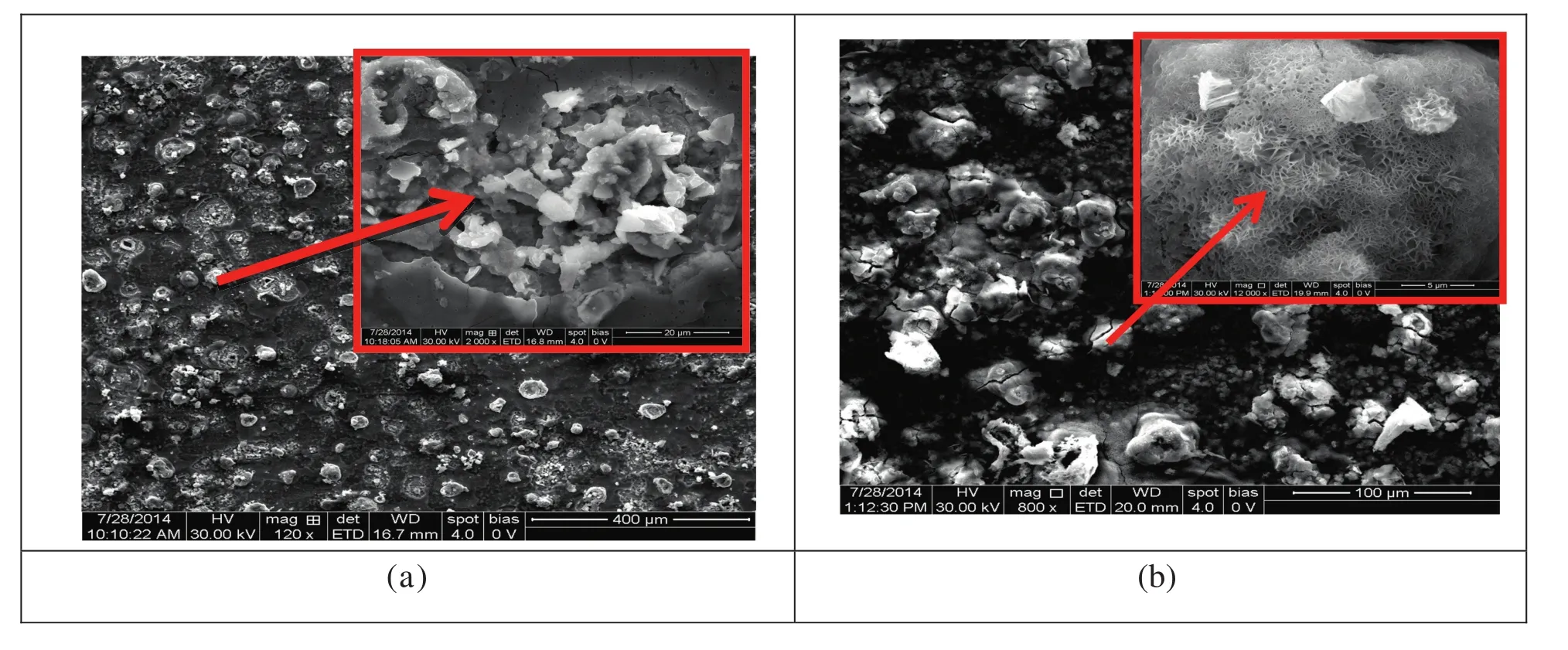
Fig.9.SEM image of(a)PVA-AAm-VSA and(b)PVA-AA-VSA(inset:magnified view).
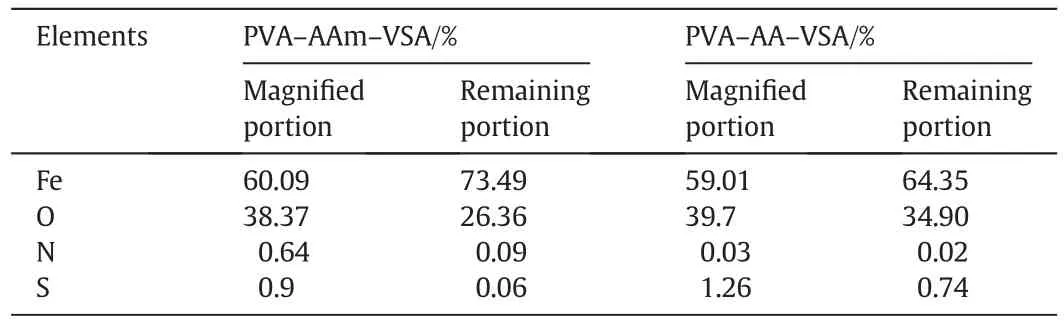
Table 4 Elemental composition determined using SEM-EDX analysis
Acknowledgements
One of the authors,R.Geethanjali thanks Tamil Nadu State Council for Science and Technology(Tnscst/rfrs/vr/2013-14)for catalysing and financially supporting the research work under the RFRS scheme.
References
[1]D.Chamovska,M.Cvetkovska,T.Grchev,Corrosion inhibition of iron in hydrochloric acid by polyacrylamide,J.Serb.Chem.Soc.72(2007)687-698.
[2]S.Umoren,Y.Li,F.Wang,Electrochemical study of corrosion inhibition and adsorption behaviour for pure iron by polyacrylamide in H2SO4:Synergistic effect of iodide ions,Corros.Sci.52(2010)1777-1786.
[3]M.Srimathi,R.Rajalakshmi,S.Subhashini,Polyvinyl alcohol-sulphanilic acid water soluble composite as corrosion inhibitor for mild steel in hydrochloric acid medium,Arab.J.Chem.5(2012)517-522.
[4]M.V.Azghandi,A.Davoodi,G.A.Farzi,A.Kosari,Corrosion inhibitive evaluation of an environmentally friendly water-base acrylic terpolymer on mild steel in hydrochloric acid media,Metall.Mater.Trans.A 44(2013)5493-5504.
[5]C.B.Verma,M.A.Quraishi,E.E.Ebenso,Electrochemical and thermodynamic investigation of some soluble terpolymers as effective corrosion inhibitors for mild steel in 1 M hydrochloric acid solution,Int.J.Electrochem.Sci.8(2013)12894-12906.
[6]R.Geethanjali,S.Subhashini,Synthesis of water soluble Polyvinyl alcohol-based Terpolymer and Evaluation of corrosion inhibition property on Mild steel in hydrochloric acid,Res.J.Rec.Sci.3(2014)170-176.
[7]Y.Ren,Y.Luo,K.Zang,G.Zhu,X.Tan,Lignin terpolymer for corrosion inhibition of mild steel in 10%hydrochloric acid medium,Corros.Sci.50(2008)3147-3153.
[8]M.V.Azghandi,A.Davoodi,G.A.Farzi,A.Kosari,Water-base acrylic terpolymer as a corrosion inhibitor for SAE1018 in simulated sour petroleum solution in stagnant and hydrodynamic conditions,Corros.Sci.64(2012)44-54.
[9]N.Tudorachi,R.Lipsa,Copolymers based on poly(vinyl alcohol)and acrylamide,J.Optoelectron.Adv.Mater.8(2006)659-662.
[10]E.B.Orler,D.J.Yontz,R.B.Moore,Sulfonation of syndiotactic polystyrene for model semicrystalline ionomer investigations,Macromolecules 26(1993)5157-5160.
[11]J.C.Yang,M.J.Jablonsky,J.W.Mays,NMR and FT-IR studies of sulfonated styrenebased homopolymers and copolymers,Polymer 43(19)(2002)5125-5132.
[12]B.L.Rivas,S.L.Nicolas,Poly(acrylic acid-co-vinylsulfonic acid):Synthesis,characterization,and properties as polychelatogen,J.Appl.Polym.Sci.88(2003)1698-1704.
[13]M.D.Fernández,M.J.Fernández,P.Hoces,Poly(vinyl acetal)s containing electrondonor groups:Synthesis in homogeneous phase and their thermal properties,React.Funct.Polym.68(2008)39-56.
[14]L.Calandrelli,G.De Rosa,M.E.Errico,Novel graft PLLA-based copolymers:Potential of their application to particle technology,J.Biomed.Mater.Res.62(2001)244-253.
[15]K.Lewandowska,Miscibility and thermal stability of poly(vinyl alcohol)/chitosan mixtures,Thermochim.Acta 493(2009)42-48.
[16]L.Y.Zhong,B.Y.Gao,C.X.Li,Q.Y.Yue,B.Liu,Synthesis and characterization of hydrophobically associating cationic polyacrylamide,Chem.Eng.J.161(2010)27-33.
[17]K.E.Lee,I.Khan,N.Morad,T.T.Teng,T.Poh,Thermal behavior and morphological properties of novel magnesium salt-polyacrylamide composite polymers,Polym.Compos.1515-1522(2011).
[18]Y.G.Devrim,Z.Rzaev,E.Pişkin,Physically and chemically cross-linked poly{[(maleic anhydride)-alt-styrene]-co-(2-acrylamido-2-methyl-1-propanesulfonic acid)}/poly(ethylene glycol)proton-exchange membranes,Macromol.Chem.Phys.208(2007)175-187.
[19]S.Cavus,Poly(methacrylamide-co-2-acrylamido-2-methyl-1-propanesulfonic acid)hydrogels:Investigation of pH-and temperature-dependent swelling characteristics and their characterization,J.Polym.Sci.B Polym.Phys.48(2010)2497-2508.
[20]USERCOM,1/2000 information for users of METTER TOLEDO thermal analysis systems(Google).
[21]G.Groeninckx,M.Vanneste,V.Everaert,Polymer Blends Handbook,Springer Science&Business Media,2003 233(chapter 3).
[22]M.F.Parveen,S.Umapathy,V.Dhanalakshmi,R.Anbarasan,Synthesis and characterization of nanosized Mg(OH)2and its nanocomposite with poly(vinyl alcohol),NANO 4(2009)147.
[23]M.F.Parveen,S.Umapathy,V.Dhanalakshmi,R.Anbarasan,Synthesis and characterizations of nano-sized Ni(OH)2and Ni(OH)2/poly(vinyl alcohol)nano composite,J.Mater.Sci.44(2009)5852.
[24]X.Li,G.Mu,Tween-40 as corrosion inhibitor for cold rolled steel in sulphuric acid:Weight loss study,electrochemical characterization and AFM,Appl.Surf.Sci.(2005)1254-1265.
[25]A.M.Al-Mayouf,A.K.Al-Ameery,A.A.Al-Suhybani,Inhibition of type 304 stainless steel corrosion in 2 M sulfuric acid by some benzoazoles—time and temperature effects,Corrosion 57(2001)614-620.
[26]S.A.Umoren,E.E.Ebenso,Blends of polyvinyl pyrrolidone and polyacrylamide as corrosion inhibitors for aluminium in acidic medium,Indian J.Chem.Technol.15(2008)355-363.
[27]A.Mansri,B.Bouras,B.Hammouti,I.Warad,A.Chetouani,Synergistic effect of AM-4VP-9 copolymer and iodide ion on corrosion inhibition of mild steel in 1 M H2SO4,Res.Chem.Intermed.39(2013)1753-1770.
[28]S.K.Shukla,M.A.Quraishi,R.Prakash,A self-doped conducting polymer“polyanthranilic acid”:an efficient corrosion inhibitor for mild steel in acidic solution,Corros.Sci.50(2008)2867-2872.
[29]A.Popova,M.Christov,A.Zwetanova,Effect of the molecular structure on the inhibitor properties of azoles on mild steel corrosion in 1 M hydrochloric acid,Corros.Sci.49(2007)2131-2143.
[30]M.Elayyachy,B.Hammouti,A.El Idrissi,New telechelic compounds as corrosion inhibitors for steel in 1 M HCl,Appl.Surf.Sci.249(2005)176-182.
[31]M.A.Amin,K.F.Khaled,S.A.Fadl-allah,Testing validity of the Tafel extrapolation method for monitoring corrosion of cold rolled steel in HCl solutions—Experimental and theoretical studies,Corros.Sci.52(2010)140-151.
[32]P.Herrasti,P.Oco,Polypyrrole layers for steel protection,Appl.Surf.Sci.172(2001)276-284.
[33]V.V.Torres,V.A.Rayol,M.Magalhães,G.M.Viana,L.C.S.Aguiar,S.P.Machado,E.D.Elia,Study of thioureas derivatives synthesized from a green route as corrosion inhibitors for mild steel in HCl solution,Corros.Sci.79(2014)108-118.
[34]T.Ghailane,R.A.Balkhmima,R.Ghailane,A.Souizi,R.Touir,M.EbnTouhami,K.Marakchi,N.Komiha,Experimental and theoretical studies for mild steel corrosion inhibition in 1 M HCl by two new benzothiazine derivatives,Corros.Sci.76(2013)317-324.
[35]W.Chen,H.Q.Luo,N.B.Li,Inhibition effects of2,5-dimercapto-1,3,4-thiadiazole on the corrosion of mild steel in sulphuric acid solution,Corros.Sci.53(2011)3356-3365.
[36]E.Navvaro-Flores,Z.Chong,S.Omanovic,Characterization of Ni,NiMo,NiW and NiFe electroactive coatings as electrocatalysts for hydrogen evolution in an acidic medium,J.Mol.Catal.A Chem.226(2005)179-197.
[37]K.Tebbji,H.Oudda,B.Hammouti,M.Benkaddour,M.El Kodadi,A.Ramdani,Inhibition effect of two organic compounds pyridine-pyrazole type in acidic corrosion of steel,Colloids Surf.A Physicochem.Eng.Asp.259(2005)143-149.
[38]M.A.Amin,K.F.Khaled,Q.Mohsen,H.A.Arida,A study of the inhibition of iron corrosion in HCl solutions by some amino acids,Corros.Sci.52(2010)1684-1695.(Please refer to the online version for the color figures)
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Prediction of pyrolysis kinetic parameters from biomass constituents based on simplex-lattice mixture design☆
- Evaluation of substrates for zinc negative electrode in acid PbO2-Zn single flow batteries☆
- Production of carbonaceous material from avocado peel for its application as alternative adsorbent for dyes removal
- Production of succinic acid in basket and mobile bed bioreactors—Comparative analysis of substrate mass transfer aspects☆
- Enhanced production of glycyrrhetic acid 3-O-mono-β-D-glucuronide by fed-batch fermentation using pH and dissolved oxygen as feedback parameters☆
- Continuous production of biodiesel from cottonseed oil and methanol using a column reactor packed with calcined sodium silicate base catalyst☆
