Continuous production of biodiesel from cottonseed oil and methanol using a column reactor packed with calcined sodium silicate base catalyst☆
2016-05-29XiaGuiSichenChenZhiYun
Xia Gui,Sichen Chen,Zhi Yun*
College of Chemistry and Chemical Engineering,Nanjing Tech University,Nanjing 210009,China
1.Introduction
In an age of worldwide fossil fuel depletion,global warming and resulting effects,a change from fossil feedstock to renewable resources could considerably contribute to a sustainable and greener future[1-3].As one of the most important renewable resources,vegetable oils have significant advantages in terms of environmental protection due to their unique chemical structures and physical properties.Biodiesel,derived from vegetable oils,bears a large potential for the substitution of fossil diesel.
Biodiesel is widely produced by chemical transesterification of vegetable oils with methanol or ethanol in the presence of a homogeneous catalyst,such as sulfuric acid or sodium hydroxide[4-9].However,existing biodiesel processes suffer from some serious problems with the use of homogeneous catalysts,such as equipment corrosion,waste effluent treatment,soap formation and catalyst removal,leading to severe economic and environmental penalties[10-14].Therefore,exploring heterogeneous catalysts is becoming more important in chemical and life science industry.
It is suggested that some supported solid base catalysts(alkali earth oxides,alkali metals or alkali earth salts loaded on metal oxide,etc.)are excellent catalysts for transesterification of triglyceride,but relatively higher temperatures and longer reaction time are required to achieve higher biodieselconversions[15-19].Sodium silicate is an effective heterogeneous base catalyst in biodiesel transesterification from soybean oil.In addition to high catalyst activity,sodium silicate retains the advantages of a supported solid base catalyst,and is often used as a starting materialto synthesize high catalytic activityγ-zeolite,NaY zeolite,NaX zeolite,etc.[20-25].
In this study,sodium silicate is exploited to catalyze the transesterification of cottonseed oil.The properties of calcined sodium silicate(CSS)are characterized by X-ray diffraction(XRD), field emission scanning electron microscope(FE-SEM)and FT-IR.Transesterification variables are systematically examined.And a new continuous process and apparatus are developed forthe biodieselproduction in the presence of CSS/θ ring solid base catalyst.
2.Materials and Methods
2.1.Materials
Cottonseeds were obtained from Lianyungang(Jiangsu,China).Methanol(>98%)and petroleum ether(60-90 °C)were obtained from Nanjing Huaqingnanfang ChemicalLtd.(Nanjing,China).Sodiumsilicate(AR)was obtained from Shanghai Lingfeng Chemical Ltd.(Shanghai,China).All other chemicalfor analyticalpurpose were obtained from ShanghaiAladdin Reagent Co.,Ltd.(Shanghai,China).All components used in this experiment were analytical reagent(AR)grade.
2.2.Method
2.2.1.Catalyst preparation and characterization
Sodium silicate was directly calcined in a muffle furnace at 100°C to 500°C for 1 h to 5 h.In order to eliminate coarse material,the resulting CSS was triturated and passed through a 120 mesh sieve.XRD was conducted using Cu Kα(λ=0.154 nm)as a radiation source in an automatic X-ray diffractometer(D8-Advance Bruker,GER).The samples were scanned in the range of 2θ =5°-60°at a scanning speed of 2(°)·min-1.Structural examination of the catalyst by FE-SEM was performed on a Hitachi S-4008(Hitachi,JPN)at an accelerated voltage of 20 kV.FT-IR spectra(Nicolet-8700,U.S.)were applied to characterize the structural change of catalysts.
The composition of reaction mixture was determined by gas chromatographic(GC-2014,Shimadzu,Japan)with a stainless PEG-20 column(2 m×4 mm).To detect the yield of biodiesel,methyl salicylate was used as internal standard and n-hexane as solvent.1 ml biodiesel sample was dissolved into 0.2 ml methyl salicylate and 8 ml n-hexane with vigorous stirring.1 μl of the mixture was injected into the GC.The column temperature was 180°C,the temperatures of injector and detector were 260°C.The flow rates of hydrogen,nitrogen,and air were 40,19,and 300 ml·min-1,respectively.
The yield of biodiesel Y is calculated as follows.

where msis the mass of internal standard added to the sample,miis the mass of biodiesel,m is the mass of total sample,Asis the peak area of internal standard,Aiis the peak area of biodiesel,fiis the correction factor of internal standard,and fsis the correction factor of biodiesel.
2.2.2.Base-catalyzed transesterification
After being dried at 105°C for 24 h,the industrial grade cottonseeds were milled into fine powder using an electric grinder and sifted through a 60-mesh screen sieve.According to the ISO 659-1988 and GB/T 14489.1-2008/ISO 665:2000 standards,the oil,moisture and free gossypol contents of the milled cottonseed were 35.4 wt%(wet basis),6.9 wt%and 0.94 wt%,respectively.
The polar solvent phase solution was prepared by mixing citric acid with methanol.Citric acid was used to increase the polarity of methanol solution and prevent mutual dissolution of methanol and petroleum ether in the two-phase solutions.An extracting solvent mixture consisting of polar solvent phase solution and petroleum ether was placed in a 500 mlthree-necked flask equipped with a reflux condenser,a mechanical stirrer and a thermometer.Fifty grams of milled cottonseed powder was added to the flask and extracted with 350 ml twophase extracting solvent at 30 °C and 600 r·min-1for 25 min.
The extraction mixture was filtered through a Buchner funnel to remove the cottonseed mealfrom the liquid phase.The filtercake was collected and dried at room temperature for 24 h before being extracted with a mixture of petroleum ether and methanol to obtain the residual oil and gossypol from the meals.The filtrate was transferred to a separating funnel and divided into two layers.The upper layer was the oil phase containing the petroleum ether and cottonseed oil,and the lower layer was the methanol phase containing methanol,free fatty acids,gossypol and citric acid.
A portion of the oil phase obtained,methanol and catalyst prepared were added to a 250-mlthree-necked flask fitted with a reflux condenser,a thermometer and a mechanical stirrer.After 2 h,the product was poured into a separating funnel and allowed to stand for 10 min to form a biphasic mixture.The lower layer was the crude glycerol phase containing glycerol,methanol and soap,while the upper layer was the cottonseed-based biodieselphase containing petroleumether,biodiesel and some unreacted triglyceride.
Transesterification was carried out under the conditions varied in the following ranges:temperature 30 °C to 70 °C,reaction time 0.5 to 4 h,methanol to oil mass ratio 6:1 to 18:1,and petroleum ether to oil mass ratio 0 to 5:1.The catalysts for transesterification were sodiumsilicate and CSS.The catalyst concentration was expressed as a mass ratio of total cottonseed oil,from 1 wt%to 3 wt%.
2.2.3.Continuous production of biodiesel using a column reactor packed with CSS base catalyst supported on θ ring
Continuous transesterification was performed in a packed column reactor at atmospheric pressure.The reactor was composed of a water-jacketed glass column randomly packed with CSS/θ ring solid base catalyst.The preparation of CSS base catalyst supported on θ ring and the scheme of the continuous production of biodiesel from cottonseed oil and methanol are shown in Figs.1 and 2.
Methanol and cottonseed oil were charged into the system using a plungerpump.The reactantswere mixed and preheated in a mixing column with random packing.The reaction temperature was controlled by a water bath.The sample was purified by reduced pressure distillation to remove excess methanol and water generated during the reaction.The effects of residence time(0.5 to 12 h),reaction temperature(40 °C to 60 °C),methanol/oil velocity ratio(1:6 to 1:3),and tower section ofcolumn reactor(1 to 5)on the conversion to fatty acid methylesters were investigated.Each tower section contained the same quantity of catalysts(4 g).All experiments were repeated 3 times and the standard deviation was never higher than 5%for any point.
3.Results and Discussion
3.1.Characterizations of catalyst
To obtain the optimal calcination conditions,especially calcination temperature and calcination time,CSS were prepared at 100°C,200 °C,300 °C,400 °C and 500 °C for 1-5 h.Their catalytic activity was investigated by transesterification of cottonseed oil with methanol.The effects of calcination temperature and particle size distribution on CSS activity for the transesterification of cottonseed oil are shown in Fig.3.The catalystcalcined at400°C for 2 h gave the bestcatalytic activity and highest biodiesel yield.Guo et al.also reported similar result[22].
The XRDdiffraction patterns ofsodiumsilicate(Fig.4a)show several characteristic peaks at 2θ≈ 17.6°,18.4°,24.1°,28.3°,30.7°,32.2°and 44.5°,indicating that the structure of sodium silicate is mainly assigned to Na2O·SiO2·9H2O.Calcined at 400 °C for 2 h,most of the combined water molecules will be removed and assigned to anhydrous Na2SiO3(Fig.4b).The calcination treatment enhances the intensity of some diffraction peaks(such as characteristic peaks at 2θ ≈ 17.9°,25.8°,30.4°,35.1°,37.8°,48.5°and 53.2°),and makes the catalyst more regular.The presence of H2O molecules and different structures of sodium silicate before and after calcination may result in different catalytic behaviors in the transesterification of cottonseed oil.
Images of sodium silicate calcined at 400°C for 2 h taken by FE-SEM show that a large number of agglomerates accumulate on the surface and they are loosely attached(Fig.5).Such structures of CSS are favorable to the entry of triglyceride and methanol due to considerable basic sites in the interior of the solid catalyst.
The effectofcalcination on the molecular structure ofsodium silicate was analyzed by FT-IR spectroscopy.The IR spectra of sodium silicate and CSS calcined at 400°C for 2 h are shown in Fig.6.The absorption band at 998 cm-1is attributed to the Si-O-Na stretching.After calcination the intensity of Si-O-Na stretching peak is reduced with a lowfrequency shift and a new Si-O-Si stretching peak at 896 cm-1.As the amount of Si-O-Si increases and the structure of catalyst becomes more regular,the absorption intensity for Si-O(at 486 cm-1)and Si-O-Na(at 998 cm-1)becomes weaker.Furthermore,the calcination process dehydrates most of the adsorbed water on the catalyst surface.Therefore,the intensity of water peaks(between 2500 and 3000 cm-1)is reduced with a low-frequency shift.
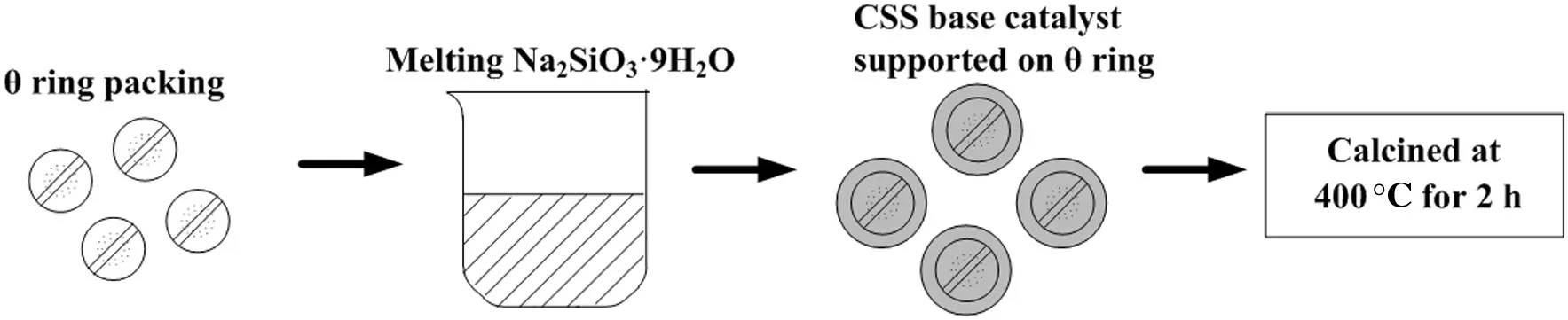
Fig.1.Preparation of CSS base catalyst supported on θ ring.
3.2.Effects of transesterification process variables
The mass ratio of petroleum ether to cottonseed oil was varied from 0-5:1 to investigate the effect of petroleum ether concentration on the conversion to fatty acid methyl esters.Fig.7 demonstrates the maximum conversion to biodiesel is at a mass ratio of 2:1.However,further dilution(3:1,4:1 and 5:1)results in a rapid decrease of biodiesel yield.These suggest that suitable petroleum ether content could improve the contact area of reactants and solid catalyst,while further dilution will decrease transesterification rate and biodiesel yield.The favorable petroleum ether/cottonseed oil mass ratio is 2:1.
To evaluate the effectofreaction time on the conversion to biodiesel,methanol petroleum ether and catalyst were added to cottonseed oil and the mixture was heated at 65°C for different reaction time in the range of 0.5 to 4 h.Fig.8 shows that the biodiesel yield increases with reaction time until it reaches a plateau.In this study,the optimum reaction time appears to be 3 h.
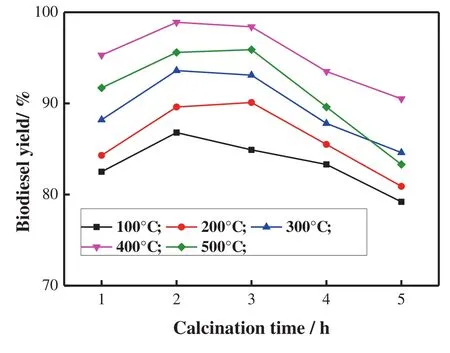
Fig.3.In fluence of calcination temperature and time on biodiesel yield.Temperature 65°C;time 3 h;mole ratio of methanol to oil=12:1;mass ratio of petroleum ether to oil=2:1;ratio of CSS to oil=2 wt%.

Fig.2.Scheme ofthe continuous production ofbiodiesel.1—column reactor;2—random packing;3—waterbath;4—globe valve;5—biodieseltank;6—check valve;7—plunger pump;8—methanol tank;9—cottonseed oil tank;10—return condenser.

Fig.4.XRD diffraction patterns of sodium silicate before and after calcination.

Fig.5.FE-SEM of CSS.
Sodiumsilicate and CCS in the range of1%-3%ofcrude cottonseed oil were used as catalyst to investigate the effect of catalyst content on transesterification of cottonseed oil.Fig.9 shows that the yield of biodiesel first increases with catalyst content,and then increases little as the content increases from 2 wt%to 3 wt%.The biodiesel yield is 98.9%at 2.0 wt%of CSS to cottonseed oil mass ratio,which is chosen for optimization of transesterification.

Fig.6.FT-IR spectra of catalyst.(a)Sodium silicate;(b)CSS,calcined at 400°C for 2 h.

Fig.7.In fluence of petroleum ether/cottonseed oil mass ratio on biodiesel yield.Temperature 65°C;time 3 h;mole ratio of methanol to oil=12:1;ratio of catalyst to oil=2.0 wt%.

Fig.8.'In fluence of reaction time on biodiesel yield.Temperature 65°C;mole ratio of methanol to oil=12:1;mass ratio of petroleum ether to oil=2:1;ratio of catalyst to oil=2.0 wt%.

Fig.9.'In fluence of catalyst/cottonseed oil mass ratio on biodiesel yield.Temperature 65°C;time 3 h;mole ratio of methanol to oil=12:1;mass ratio of petroleum ether to oil=2:1.
To examine the effect of temperature on transesterification,several runs were conducted for 3 h at different temperatures in the range of 30-70°C.The yield of biodiesel catalyzed by CSS increased with the temperature,reaching nearly 100%at 60 to 70°C(Fig.10).However,the yield of biodiesel catalyzed by sodium silicate decreased obviously at 70°C.This demonstrates that the catalytic activity of sodium silicate decreases due to some qualitative changes,such as melting of sodium silicate and loss of alkaline groups.Considering the biodiesel yield and energy economy,it is appropriate to choose 65°C as the reaction temperature for the transesterification of cottonseed oil.
The transesterification with methanol/oil molar ratio of 6:1 to 12:1(Fig.11)indicates that the conversion of cottonseed oil to biodiesel increases rapidly with the mole ratio,reaching 98.9 wt%at the mole ratio of 12:1 catalyzed by CSS.Further increasing mole ratio to 18:1,the conversion increases gradually to 99.3 wt%.Phan and Phan[26]and Shu et al.[27]also observed similar trend.In addition,stable emulsions may form at higher content of methanol(18:1),leading to complicated separation and purification of biodiesel.At the end of transesterification,the excess methanol was recovered by a vacuum distillatory and recycled in latter reactions.

Fig.10.In fluence of reaction time on biodiesel yield.Time 3 h;mole ratio of methanol to oil=12:1;mass ratio of petroleum ether to oil=2:1;ratio of catalyst to oil=2.0 wt%.

Fig.11.In fluence of methanol/cottonseed oil molar ratio on biodiesel yield.Temperature 65°C;time 3 h;mass ratio of petroleum ether to oil=2:1;ratio of catalyst to oil=2.0 wt%.
The reusability of sodium silicate and CSS was checked without any further purification and activation.Solid catalyst was collected after adding fresh reactants.The yield ofbiodiesel,determined after each catalystcollection,wasemployed to evaluate the reusability(Fig.12).With CSS,the cottonseed oil conversions maintained higher than 80%in 7 consecutive runs,while the yield with sodium silicate decreases apparently after 2 consecutive runs.
3.3.Effect of operating variables for continuous production
The number of tower section is associated with the residence time and the amount of catalyst in continuous transesterification.The yield of biodiesel is greatly dependent on the number of tower sections(Fig.13).For the reaction in one tower section,the biodiesel yield reached a plateau at 35.8%in about 1 h.The maximum yield increased rapidly from 35.8%to 98.9%with the increase of tower sections.It demonstrates that more catalystprovides longer reaction time and more active sites to promote the reaction between cottonseed oil and methanol at a given flow rate.
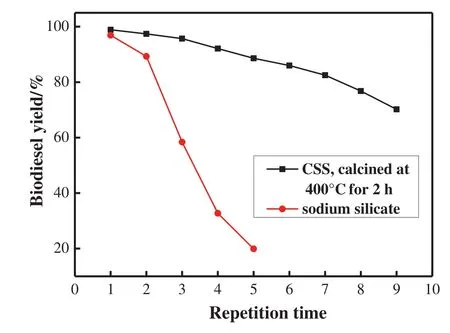
Fig.12.In fluence of repetition time on biodiesel yield.Temperature 65°C;time 3 h;mass ratio ofpetroleum ether to oil=2:1;mole ratio ofmethanolto oil=12:1;ratio ofcatalyst to oil=2.0 wt%.

Fig.13.In fluence of tower sections on biodieselyield.Temperature 55°C;methanolphase velocity=1 ml·min-1;oil phase velocity=3 ml·min-1;each tower section containing 4 g catalysts.

Fig.14.In fluence of reaction temperature on biodiesel yield.Five tower sections containing catalyst 20 g;methanol phase velocity=1 ml·min-1;oil phase velocity=3 ml·min-1.
Fig.14 shows the effect of reaction temperature on transesterification by the percentage of cottonseed oil converted to fatty acid methyl esters.The yield of biodiesel increased obviously from 83.9%to 98.8%as reaction temperature increased from 45 °C to 60 °C,because for an endothermic reaction,higher temperature will accelerate the rate of reaction.To preventa decline in the transesterification activity caused by methanolevaporation at higher temperature,the range of optimal reaction temperature may vary from 50 °C to 60 °C,depending upon the oils used[28-30].Moreover,unexpected and uncontrollable liquid back-up flooding was also observed at 60 °C after 2 h heating,so 55 °C was chosen as the favorable reaction temperature in this study.
For the effect of methanol/oil velocity ratio from 1/6 to 1/3,Fig.15 shows that the yield of biodiesel rapidly increases with the velocity ratio.As a reversible reaction,the biodiesel yield can be improved by introducing an excess amountofreactantmethanolto change the equilibrium.At the ratio less than 1/4,the velocity ratio of methanol/oil has a significant effect on the catalytic activity in the early stage of reaction.The increase in transesterification becomes slower as the velocity ratio increase to 1/6.Therefore,the ratio of methanol/waste oil of 1/6 with 99.1%yield is preferable.

Fig.15.In fluence of methanol/cottonseed oil velocity ratio on biodiesel yield.Five tower sections containing catalyst 20 g;temperature 55 °C;oil phase velocity 3 ml·min-1;methanol phase velocity=0.5,0.75 or 1 ml·min-1.
4.Conclusions
A novel solid base catalyst with good activity for transesterification was prepared by calcination.Sodium silicate calcined at 400°C for 2 h exhibited much higher catalytic activity and stability for biodiesel conversion.The maximum biodiesel yield was achieved with 2 wt%of calcined sodium silicate(CSS),mole ratio of methanol to cottonseed oil 12:1,and reaction temperature of 65°C for 3.0 h.However,a considerable loss in catalytic activity was observed when the catalyst was recycled for more than 7 times.
Considering technologicaland economic feasibility,CSS base catalyst supported on θ ring was prepared for continuous biodiesel production.The transesterification of cottonseed oil was performed in a column reactor packed CSS/θ ring solid base catalyst.A maximum biodiesel yield of99.1%was achieved at55°C.The results indicate thatthis new continuous biodiesel production process and apparatus have good potential for utilization and commercialization ofcottonseed with an inexpensive and easily available solid base catalyst.
References
[1]A.F.Faria-Machado,M.A.da Silva,M.G.A.Vieira,M.M.Beppu,Epoxidation of modified natural plasticizer obtained from rice fatty acids and application on polyvinylchloride films,J.Appl.Polym.Sci.127(5)(2013)3543-3549.
[2]H.C.Erythropel,M.Maric,D.G.Cooper,Designing green plasticizers:In fluence of molecular geometry on biodegradation and plasticization properties,Chemosphere 86(2012)759-766.
[3]U.Biermann,W.Friedt,S.Lang,W.Luhs,G.Machmuller,J.O.Metzger,M.R.Klaas,H.J.Schafer,M.P.Schneider,New syntheses with oils and fats as renewable raw materials for the chemical industry,Angew.Chem.Int.Ed.39(2000)2206-2224.
[4]F.R.Ma,M.A.Hanna,Biodiesel production:A review,Bioresour.Technol.70(1999)1-15.
[5]A.Kawashima,K.Matsubara,K.Honda,Development of heterogeneous base catalysts for biodiesel production,Bioresour.Technol.99(2008)3439-3443.
[6]M.J.Ramos,A.Casas,L.Rodriguez,R.Romero,A.Perez,Transesterification of sunlf ower oil over zeolites using different metal loading:A case ofleaching and agglomeration studies,Appl.Catal.A Gen.346(2008)79-85.
[7]C.V.McNeff,L.C.McNeff,B.Yan,D.T.Nowlan,M.Rasmussen,A.E.Gyberg,B.J.Krohn,R.L.Fedie,T.R.Hoye,A continuous catalytic system for biodiesel production,Appl.Catal.A Gen.343(2008)39-48.
[8]'E.Leclercq,A.Finiels,C.Moreau,Transesterification of rapeseed oil in the presence of basic zeolites and related solid catalysts,J.Am.Oil Chem.Soc.78(2001)1161-1165.
[9]P.P.Joaquín,D.Isabel,M.Federico,S.Enrique,Selective synthesis of fatty monoglycerides by using functionalised mesoporous catalysts,Appl.Catal.A Gen.254(2003)173-188.
[10]D.E.Lopez,J.G.Goodwin,D.A.Bruce,E.Lotero,Transesterification of triacetin with methanol on solid acid and base catalysts,Appl.Catal.A Gen.295(2005)97-105.
[11]M.D.Machado,P.J.Perez,E.Sastre,D.Cardoso,Selective synthesis of glycerol monolaurate with zeolitic molecular sieves,Appl.Catal.A Gen.203(2000)321-328.
[12]H.Kishida,F.M.Jin,Z.Y.Zhou,T.Moriya,H.Enomoto,Conversion of glycerin into lactic acid by alkaline hydrothermal reaction,Chem.Lett.34(2005)1560-1561.
[13]X.Deng,Z.Fang,Y.H.Liu,C.L.Yu,Production of biodiesel from Jatropha oil catalyzed by nanosized solid basic catalyst,Energy 36(2011)777-784.
[14]C.W.Wang,X.Gui,Z.Yun,Esterification of lauric and oleic acids with methanol over oxidized and sulfonated activated carbon catalyst,React.Kinet.Mech.Catal.113(2014)211-223.
[15]L.C.Meher,M.G.Kulkarni,A.K.Dalai,S.N.Naik,Transesterification of karanja(Pongamia pinnata)oil by solid basic catalysts,J.Lipid Sci.Technol.108(2006)389-397.
[16]H.P.Zhu,Z.B.Wu,Y.X.Chen,P.Zhang,S.J.Duan,X.H.Liu,Preparation of biodiesel catalyzed by solid super base of calciumoxide and its re fining process,Chin.J.Catal.27(2006)391-396.
[17]X.J.Liu,H.Y.He,Y.J.Wang,S.L.Zhu,X.Piao,Transesterification of soybean oil to biodiesel using CaO as a solid base catalyst,Fuel 87(2008)216-221.
[18]X.J.Liu,X.L.Piao,Y.J.Wang,S.L.Zhu,H.Y.He,Calcium methoxide as a solid base catalyst for the transesterification of soybean oil to biodiesel with methanol,Fuel 87(2008)1076-1082.
[19]W.L.Xie,H.Peng,L.G.Chen,Calcined Mg-Al hydrotalcites as solid base cata or methanolysis of soybean oil,J.Mol.Catal.A Chem.246(2006)24-32.
[20]D.L.Yun,F.Zhen,C.S.Tong,Y.Qing,Co-production of biodiesel and hydrogen from rapeseed and Jatropha oils with sodium silicate and Ni catalysts,Appl.Energy 113(2014)1819-1825.
[21]G.J.Suppes,K.Bockwinkel,S.Lucasa,J.B.Botts,M.H.Mason,J.A.Heppert,Calcium carbonate catalyzed alcoholysis of fats and oils,J.Am.Oil Chem.Soc.78(2001)139-149.
[22]F.Guo,Z.G.Peng,J.Y.Dai,Z.L.Xiu,Calcined sodium silicate as solid base catalyst for biodiesel production,Fuel Process.Technol.91(2010)322-328.
[23]'T.Selvam,B.Bandarapu,Hydrothermal transformation of a layered sodium silicate,kanemite,into zeolite Beta(BEA),Microporous Mesoporous Mater.64(2003)1387-1391.
[24]V.P.Sokolov,T.N.Shabalina,B.K.Nefedov,In fluence of silica-containing raw material on technology of NaY zeolite manufacture,Chem.Technol.Fuels Oils 23(1987)5-6.
[25]V.A.Patrikeev,M.L.Pavlov,B.I.Kutepov,R.A.Makhamatkhanov,O.S.Travkina,A.L.Shestopal,Crystallization of X-type zeolite from concentrated sodium silicate and aluminate solutions,Russ.J.Appl.Chem.80(2007)502-504.
[26]'A.N.Phan,T.M.Phan,Biodiesel production from waste cooking oils,Fuel 87(2008)17-18.
[27]Q.Shu,Q.Zhang,G.H.Xu,Z.S.Nawaz,D.Z.Wang,J.F.Wang,Synthesis of biodiesel from cottonseed oil and methanol using a carbon-based solid acid catalyst,Fuel Process.Technol.90(2009)1002-1008.
[28]'C.Samart,P.Sreetongkittikul,C.Sookman,Heterogeneous catalysis of transesterification of soybean oil using KI/mesoporous silica,Fuel Process.Technol.90(2009)922-925.
[29]J.M.Marchetti,V.U.Miguel,A.F.Errazu,Heterogeneous esterification of oil with high amount of free fatty acids,Fuel 86(2007)906-910.
[30]B.Achanai,C.Nattawut,L.Vorrada,R.Chao,C.Techit,K.Nanthakrit,Continuous process for biodiesel production in packed bed reactor from waste frying oil using potassium hydroxide supported on jatropha curcas fruitshell as solid catalyst,Appl.Sci.2(2012)641-653.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Synthesis of water-soluble acryl terpolymers and their anticorrosion properties on mild steel in 1 mol·L-1 HCl
- Prediction of pyrolysis kinetic parameters from biomass constituents based on simplex-lattice mixture design☆
- Evaluation of substrates for zinc negative electrode in acid PbO2-Zn single flow batteries☆
- Production of carbonaceous material from avocado peel for its application as alternative adsorbent for dyes removal
- Production of succinic acid in basket and mobile bed bioreactors—Comparative analysis of substrate mass transfer aspects☆
- Enhanced production of glycyrrhetic acid 3-O-mono-β-D-glucuronide by fed-batch fermentation using pH and dissolved oxygen as feedback parameters☆
