4d-4f异金属配位聚合物的合成、结构和荧光性质
2016-05-03李彩红张瑞凤
李彩红 张瑞凤
(山西师范大学化学与材料科学学院,临汾 041000)
4d-4f异金属配位聚合物的合成、结构和荧光性质
李彩红张瑞凤*
(山西师范大学化学与材料科学学院,临汾041000)
摘要:在水热反应条件下成功地合成了3个4d-4f异金属化合物{[LnAg2(QA)4(H2O)5](ClO4)}n(Ln=Nd (1),Tb (2),Eu (3);HQA=3-喹啉羧酸)。用单晶衍射分析,元素分析和粉末衍射分析对晶体结构进行了表征。化合物1~3同构,在bc平面形成二维层结构,抗衡离子ClO4-与二维层通过氢键作用形成三维超分子结构。研究了配合物2和3的荧光性质。
关键词:异金属;配位聚合物;水热合成;原位脱羧;荧光性质
0 Introduction
In recent years, the design and synthesis of d-f heterometallic coordination polymers have attracted increasing interest due to their intriguing architectures and topologies as well as their potential applications as important functional materials in the fields of magnetism, sensors, adsorption, catalysis, and ionexchange[1-5]. Many heterometallic compounds have been successfully synthesized under hydrothermal conditions[6-10]. However, the assembly of extended Ln-TM heterometallic coordination frameworks is currently a formidable task because of competitive reaction between lanthanide and transition metal ions. According to the hard-soft acid base theory, lanthanide ions prefer O- to N-donors, while transition metal ions located in the d-block are borderline acids, having a strong tendency to coordinate to both N- and O-donors. Therefore, several types of ligands with N- and O-donors, such as CN-[11-12], carbonyl[13-14], amino acids[15-17],and pyridinecarboxylate[18-21], have been utilized to construct d-f heterometallic polymers.
Among them, 2,3-pyridinedicarboxylic acid (2,3-pydc), a ligand with pyridyl and carbonyl, has drawn extensive attention in the construction of heterometallic coordination polymers. For example, 3D Ln-Cuheterometallic coordination polymers with 3D (6,8) network[22]and 2D Ln-Znframeworks containing 1D lanthanide chain have been reported previously[23]. On the basis of previous studies, we think that 2,3-quinolinedicarboxylic acid (H2QDA), which contains 2,3-pydc moiety, is also an excellent ligand for building novel heterometallic polymers, and the π-π stacking interactions caused by benzene may further stabilize the supermolecular structure. Several coordination polymers based on H2QDA ligands have been reported[24-25], but most of them are homometallic. Recently the first Ln-Co heterometallic polymers with infinite lanthanide hydroxide chains have been reported by our group[26]. In this study, we report three Ln-Ag heterometallic coordination polymers, namely {[LnAg2(QA)4(H2O)5](ClO4)}n[Ln=Nd (1); Tb (2); Eu (3)]]. It is noteworthy that in situ decarboxylation of H2QDA occurs and H2QDA is transformed into 3-quinolinecarboxylate acid (HQA).
1 Experimental
1.1 Materials and instruments
All the materials and reagents were purchased commercially and used without further purification. Elemental analyses of C, H, and N were measured on a Perkin-Elmer 240Q elemental analyzer. IR spectra were recorded with KBr pellets in the range of 4 000~ 500 cm-1on a Varian 660-IR spectrophotometer. Thermogravimetric analysis (TGA) experiments were carried out on a Netzsch TG 209 apparatus with the heating rate of 10℃·min-1from 25 to 800℃under nitrogen. Powder X-ray diffraction (PXRD) patterns were collected on a Rigaku UltimaⅣdiffractometer using Cu Kα radiation (λ=0.154 18 nm) under ambient conditions. Solid-state photoluminescent spectra were measured at room temperature with an Edinburgh FLS920 fluorescence spectrophotometer. The emission spectra were recorded on an Edinburgh FLS920 fluorescence spectrophotometer.
1.2 Synthesis of complexes 1~3
A mixture of Ln2O3(0.297 mmol, 0.100 g for 1; 0.134 mmol, 0.100 g for 2; 0.284 mmol, 0.100 g for 3), AgNO3(0.588 mmol, 0.100 g), H2QDA (1.000 mmol, 0.217 g), one drop of HClO4and 10 mL water was sealed in a 23 mL Teflon-lined autoclave at 150℃for 4 days, and then cooled to room temperature at the rate of 5℃·h-1. Yellow lamellar crystals for 1~3 were obtained. Anal. Calcd. for Ag2NdC40H34ClN4O17(1)(%): C, 38.77; H, 2.75; N, 4.52; Found (%): C, 38.38; H, 3.04; N, 4.43. IR (KBr, cm-1): 3 434 (vs), 2 919(m), 2 852(m), 2 360(w), 2 341(w), 1 619(vs), 1 608(vs), 1 560(m), 1 463(w), 1 407(m), 1 311(w), 1 261(w), 1 209(w), 1 106(m), 931(w), 790(m), 744(w), 659(w), 622 (w), 555(w). Anal. Calcd. for Ag2TbC40H34ClN4O17(2)(%): C, 38.31; H, 2.71; N, 4.47; Found (%): C, 37.93; H, 3.11; N, 4.35. IR (KBr, cm-1): 3 440(vs), 2 921(m), 2 856(m), 2 364(w), 2 343(w), 1 606(vs), 1 590(vs), 1 558(s), 1 463(m), 1 409(s), 1 313(m), 1 261(w), 1 209(w), 1 106(s), 931(w), 792(s), 744(m), 663(w), 623(w), 555(w). Anal. Calcd. for Ag2EuC40H34ClN4O17(3)(%): C, 38.53; H, 2.73; N, 4.49; Found(%): C, 37.46; H, 2.53; N, 5.22. IR (KBr, cm-1): 3 440(vs), 2 920(m), 2 856(m), 2 361(w), 2 343(w), 1 606(vs), 1 590(vs), 1 558(s), 1 463(m), 14 09(s), 1 313(m), 1 261(w), 1 209(w), 1 106(s), 931(w), 792(s), 744(m), 663(w), 623(w), 555(w).
1.3 X-ray crystal structural determination
Single crystals suitable for the X-ray diffraction analysis were elaborately selected under microscope. Single-crystal determinations were performed on Bruker Smart APEX CCD diffractometer equipped with graphite monochromated Mo Kα radiation (λ=0.071 073 nm). Data were collected using φ-ω scans mode, and corrected for Lorentz and polarisation effects and absorption using SADABS software[27]. The structures were solved by direct methods and refined by fullmatrix least-squares methods on F2using the SHELXTL software package[28]. All non-hydrogen atoms were refined with anisotropic displacement parameters. The positions of hydrogen atoms were obtained byhydrogenation theoretically. Crystal data and structure refinement are listed in Table 1. Selected bond lengths and angles for complexes 1~3 are shown in Table 2.

Table1 Crystal data and structure refinement parameters for 1~3
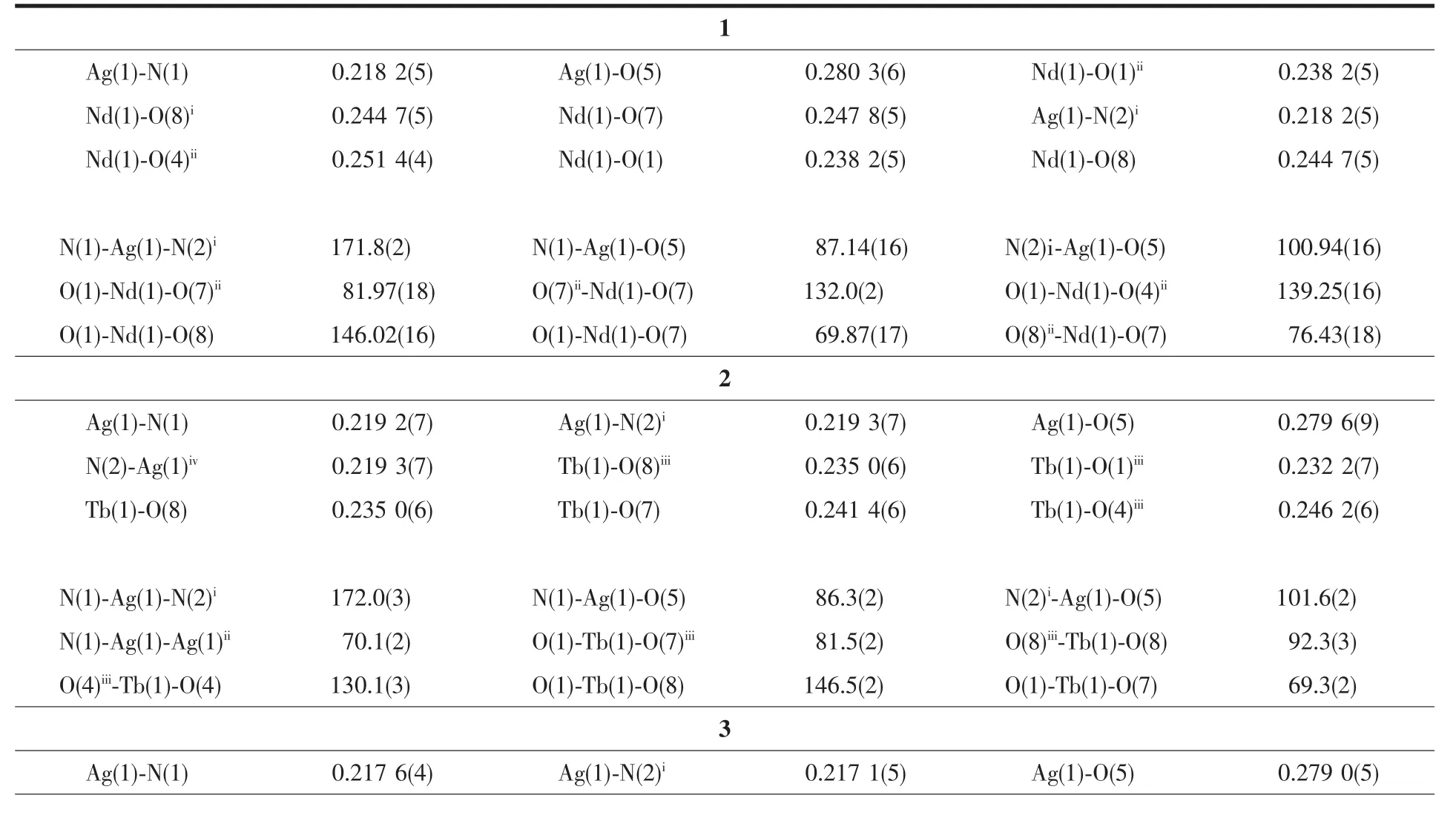
Table2 Selected bond lengths (nm) and angles (°) for 1~3
CCDC: 1038767, 1; 1039837, 2; 1038768, 3.

Continued Table 1
2 Results and discussion
2.1 Structural description of {[LnAg2(QA)4(H2O)5] (ClO4)}n
The structural analysis reveals that the compounds 1~3 are isostructural. Accordingly, the structure of 2 is described representatively here in detail. As shown in Fig.1a, each asymmetric unit comprises one Agion center, half of Tbion, two QA-ligands, two water molecules and half of perchlorate anion. The coordination geometry of Tbion center is a distorted bicapped trigonal prism formed by four oxygen atoms from water molecules and four carboxyl oxygen atoms from four QA-(Fig.1b). Tb-O bond lengths are ranging from 0.232 2(7) to 0.246 2(6) nm, comparable to those in eight-coordinated Tbion complexes with carboxylic acid ligands[29]. Since the radius of Ndion is larger than that of Tbion, most Nd-O bonds in 1 are slightly longer than the corresponding ones in 2, as shown in Table 1 and Table 2. As a result, the cell volume of 1 is larger than that of 2. Agion is surrounded by one oxygen atom and two nitrogen atoms from three QA-, and one oxygen atom from water molecule (Fig.1c). The average Ag-O and Ag-N bond lengths are 0.274 7(9) and 0.219 3(2) nm, respectively. The ligand in 2 presents two types of coordination modes:μ2-η1Nη1O (Scheme 1a) and μ3-η1Nη1Oη1O′(Scheme 1b).

30% ellipsoid probability; Symmetry codes:ix, -y+5/2, z+1/2;iii-x+1/2, y+0, -z+3/2;vx, 1/2+y, -z+3/2Fig.1 (a) ORTEP drawing of 2 with hydrogen atoms and perchlorate anion being omitted for clarity; (b) Coordination geometry of Tbion center; (c) Coordination environment of Agion

Scheme 1 Two types of coordination modes of QA-:μ2-η1Nη1O (a) and μ3-η1Nη1Oη1O′(b)
The most striking feature of 2 is the Tb (QA)4(H2O)4unit, which is surrounded by four QA-1and four coordinated water molecules. It should be noted that those four QA-ligands are arranged on two different planes (the dihedral angle is 69.707°), andeach plane contains one ligand of mode a (a-L) and one ligand of mode b (b-L) (Fig.2). Adjacent units are connected by Agions to produce a 1D infinite ribbon along the c axis (Fig.3a), which displays a zigzag chain on bc plane (Fig.3b). These chains are further extended into a 2D layer by O5 atoms from μ2-H2O molecules and O2 atoms from ligands of mode b (Fig.4). There exist two types of π-π interactions between the adjacent pyridyl rings of QA ligands (N1/ C9-C10 at (x, 2-y, 2-z) and N2vii/C19vii-C20viiat (x, 2-y, 2-z), N2viii/C19viii-C20viiiat (0.5-x, -0.5+y, z) and N2iii/C19iii-C20iiiat (0.5-x, 2.5-y, 2-z)). The centroidcentroid distances of two planes are 0.359 93(3) and 0.400 96(8) nm, and dihedral angles between two π planes are 0.678°and 1.526°, respectively. Moreover, π-π stacking interactions further enhance the stability of the 2D structure with average centroid-centroid distance of 0.380 45(2) nm (Fig.4)[30]. The counter ClO4-ions further connect these 2D layers into 3D supermolecular structure via hydrogen bonds.

Fig.2 Conformation and dihedral angle of structure unit Tb(QA)4(H2O)4
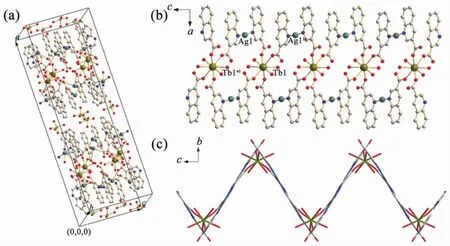
Symmetry codes:ivx, -y+5/2, z-1/2;vi-x+1/2, -y+5/2, -z+2Fig.3 (a) A cell frame of the compound 2; (b) 1D infiniteribbon along the c axis; (c) Ribbon displays a zigzag pattern on bc plane

Symmetry codes:iii-x+1/2, y, -z+3/2;viix, y-1/2, -z+3/2;viii-x+1/2, -y+2, z+1/2Fig.4 2D layer on bc plane built of 1D ribbons and the dotted lines represent π-π stacking interactions
2.2 PXRD
In order to check the phase purity of these compounds, powder X-ray patterns were recorded for all compounds at room temperature. As shown in Fig. 5, the experimental patterns are in good agreement with the simulated patterns, indicating the good phase purity of these compounds. The differences in intensity may be due to the preferred orientation of the crystalline powder samples.

Fig.5 PXRD patterns: (a) Simulated based on the X-ray single-crystal diffraction data of 1; (b) Assynthesized 1; (c) As-synthesized 2; (d) Assynthesized 3
2.3 Thermogravimetric analysis
To estimate thermal stability of compounds, thermogravimetric analyses (TGA) of compounds 1~3 were carried out from room temperature to 800℃at a heating rate of 10℃·min-1under N2atmosphere (Fig. 6). Because of the similarity of the thermaldecomposition behaviors in 1 ~3, a representative example of 1 is discussed here. For compound 1, the first weight loss of 7.24% (Calcd. 7.27% ) in the temperature range of 110~220℃corresponds to the successive release of five coordinated water molecules. On further heating, a plateau is observed from 220 to 245℃, indicating no further weight loss. The second weight loss between 245 and 800℃is due to the decomposition of the organic ligands and the collapse of the whole framework.

Fig.6 TGA curves of compound 1, 2 and 3
2.4 Fluorescent property
Because of the excellent luminescent properties of Tband Euions, the solid state photoluminescence of compounds 2 and 3 were measured at room temperature. When excited at 333 nm, compound 2 (Fig.7) emits an intense, characteristic transition spectrum of Tbions. The emission peaks at 490, 546, 586 and 622 nm are corresponding to transitions between the first excited state5D4and the ground multiplet7FJ(J=6~3), respectively[31]. As shown in the spectra of 3 (Fig.8), compound 3 exhibits characteristic transition of Euions upon excitation at 333 nm, and the emission peaks at 590, 615, 666 and 698 nm are corresponding to the transitions of5D0→7FJ(J=1~4), which implies an efficient ligand-tometal (europium) energy transfer (LMCT)[32-33]. The emission peak at 590 nm can be assigned to a magnetic-dipole5D0→7F1transition, and its intensity are associated with crystal field strength acting on Euions[34]. The strongest emission peak at 615 nm can be attributed to an electric-dipole5D0→7F2transition. The lower site symmetry Euions have, the stronger intensity5D0→7F2transition exhibits. The ratio of I5D0→7F2/I5D0→7F1is ca. 2.52 in 3, which indicates Euions have a low symmetric coordination environment[35-37], and it is consistent with the result of single-crystal X-ray analysis.
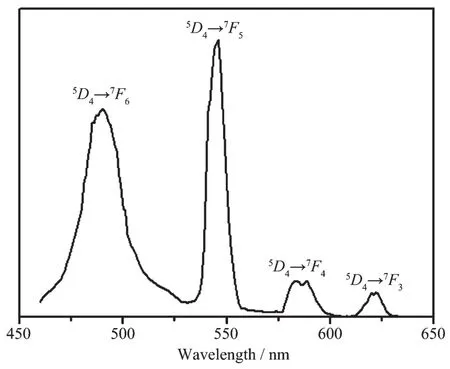
Fig.7 Solid-state emission spectra for compound 2 at room temperature
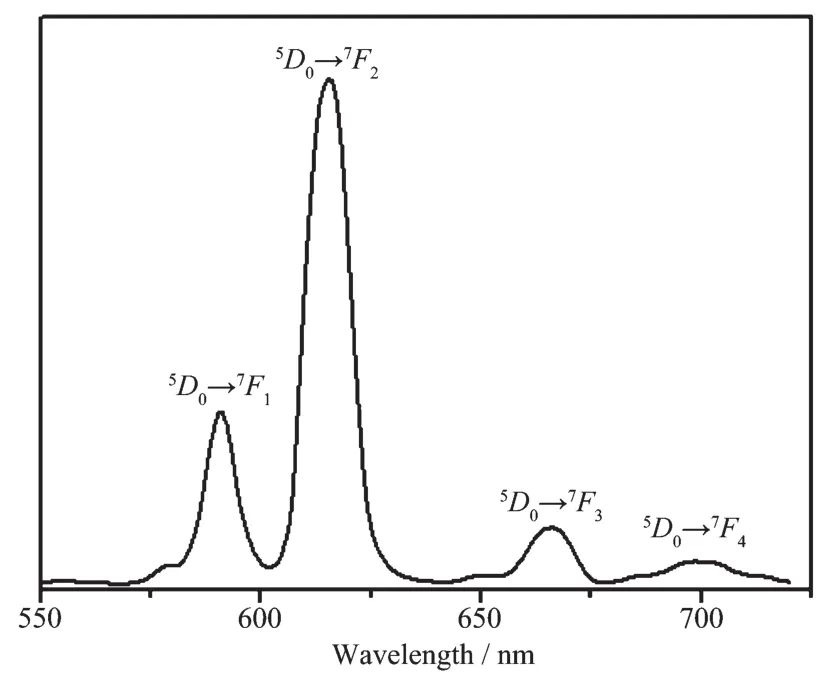
Fig.8 Solid-state emission spectra for compound 3 at room temperature
3 Conclusions
In summary, we have successfully obtained three novel d-f heterometallic coordination polymers based on 3-quinolinecarboxylic acid which was obtained from in situ decarboxylation of 2,3-quinolinedicarboxylic acid. In addition, we also study the fluorescent properties of them. The successful synthesis of the three complexes provides a method for the construction of other novel lanthanide-transition compounds.
Acknowledgments: This work was supported financially by the Initial Fund for Ph. Dr from Shanxi Normal University inShanxi Province.
References:
[1] Ibrahim M, Mereacre V, Leblanc N, et al. Angrew. Chem. Int. Ed., 2015,54(1):1-6
[2] Cheng P, Shi W, Xu N, et al. Cryst. Growth Des., 2013,13 (3):1218-1225
[3] Cheng P, Zhao X J, Wang Y. Inorg. Chem., 2015,54(9):4456-4465
[4] Srivastava S, Aggarwal H, Gupta R. Cryst. Growth Des., 2015,15(8):4110-4122
[5] Zhao B, Cui J Z, Wu Z L, et al. Inorg. Chem., 2015,54(11): 5266-5272
[6] Zhao B, Chen X Y, Cheng P, et al. J. Am. Chem. Soc., 2004,126(47):15394-15395
[7] Zhao B, Cheng P, Dai Y, et al. Angew. Chem. Int. Ed., 2003,42(8):934-936
[8] Zhao B, Cheng P, Chen X Y, et al. J. Am. Chem. Soc., 2004,126(10):3012-3013
[9] Liu K, Shi W, Cheng P. Coord. Chem. Rev., 2015,289-290: 74-122
[10]Shi W, Cheng P, Zhao B, et al. Chem. Eur. J., 2005,11(17): 5031-5039
[11]Prins F, Pasca E, Jongh L J D, et al. Angew. Chem. Int. Ed., 2007,46(32):6081-6084
[12]Estrader M, Ribas J, Tangoulis V, et al. Inorg. Chem., 2006, 45(20):8239-8250
[13]Deng H, Chun S, Florian P, et al. Inorg. Chem., 1996,35 (13):3891-3896
[14]Poplaukhin P V, Chen X N, Meyers E A, et al. Inorg. Chem., 2006,45(25):10115-10125
[15]Kong X J, Ren Y P, Long L S, et al. J. Am. Chem. Soc., 2007,129(22):7016-7017
[16]Kong X J, Ren Y P, Chen W X, et al. Angew. Chem. Int. Ed., 2008,47(13):2398-2401
[17]Shi W, Chen X Y, Zhao Y N, et al. Chem. Eur. J., 2005,11 (17):5031-5039
[18]Cheng P, Shi W, Yang A H, et al. Cryst. Growth Des., 2010,10(1):218-223
[19]Yang A H, Cui J Z, Zhao B, et al. CrystEngComm, 2011,13: 1870-1876
[20]Gao H L, Cheng P, Ding B, et al. Inorg. Chem., 2006,45(2): 481-483
[21]Zhao B, Cheng P, Shi W, et al. Chem. Eur. J., 2005,12(1): 149-158
[22]Cheng J W, Zheng S T, Liu W, et al. CrystEngComm, 2008, 10:1047-1051
[23]Chen L, Lin X M, Ying Y, et al. Inorg. Chem. Commun., 2009,12(8):761-765
[24]Wang M F, Hong X J, Zhan Q G, et al. Dalton Trans., 2012, 41:11898-11906
[25]Hong X J, Wang M F, Jia H Y, et al. New J. Chem., 2013, 37:933-940
[26]Cheng P, Wu W Y, Zhang R F, et al. Dalton Trans., 2015, 44:7144-7147
[27]Sheldrick G M. SADABS, Siemens Area Detector Absorption Correction Program, University of Göttingen, Germany, 1997.
[28]Sheldrick G M. Acta Crystallogr. Sect. A, 2008,64(1):112-122
[29]Yang A H, Zhao L H, Quan Y P, et al. Cryst. Growth Des., 2010,10(1):218-223
[30]Janiak C. J. Chem. Soc. Dalton Trans., 2000:3885-3896
[31]Cheng P, Zhao B, Shi W, et al. CrystEngComm, 2009,11: 1811-1814
[32]Deng H, Li Y H, Qiu Y C, et al. Inorg. Chem. Commun., 2008,11(10):1151-1154
[33]Cheng P, Zhao B, Shi W, et al. CrystEngComm, 2008,10: 1144-1146
[34]Liu Z H, Qiu Y C, Li Y H, et al. Polyhedron, 2008,27(17): 3493-3499
[35]Cheng P, Zhao B, Shi W, et al. CrystEngComm, 2009,11: 1261-1269
[36]Ma L, Qiu Y C, Peng G, et al. CrystEngComm, 2011,13: 3852-3861
[37]Bo Q B, Sun G X, Geng D L. Inorg. Chem., 2010,49(2):561-571
Syntheses, Structures and Photoluminescent Properties of Three 4d-4f Heterometallic Coordination Polymers
LI Cai-Hong ZHANG Rui-Feng*
(School of Chemistry & Material Science, Shanxi Normal University, Linfen, Shanxi 041000, China)
Abstract:Three 4d-4f heterometallic coordination compounds, namely, {[LnAg2(QA)4(H2O)5](ClO4)}n(Ln=Nd (1), Tb (2), Eu (3); HQA=3-quinolinecarboxylate acid), were successfully synthesized under hydrothermal conditions. Compounds 1~3 are isostructural and feature 2D layer structures on bc plane, which are further connected by the counter anions ClO4-by hydrogen bonds into 3D supermolecular structure. All these compounds are characterized by single-crystal X-ray diffraction, elemental analyses (EA) and powder X-ray diffraction (PXRD). Furthermore, the thermal stabilities and fluorescent properties of the selected compounds have been investigated. CCDC: 1038767, 1; 1039837, 2; 1038768, 3.
Keywords:heterometallic; coordination polymer; hydrothermal synthesis; in situ decarboxylation; luminescent properties
收稿日期:2015-11-16。收修改稿日期:2016-01-21。山西师范大学博士科研启动金(No.833114)资助项目。(*)通信联系人。E-mail:nkzrf@163.com
DOI:10.11862/CJIC.2016.081
中图分类号:O614.33+5;O614.341;O614.33+8
文献标识码:A
文章编号:1001-4861(2016)04-0713-07
