一系列基于杂多酸构筑的配合物的合成,晶体结构及光催化性能
2016-05-03顾晓敏叶丹丹张文莉
王 蕾 顾晓敏 叶丹丹 张文莉 倪 良
(江苏大学化学化工学院,镇江 212013)
一系列基于杂多酸构筑的配合物的合成,晶体结构及光催化性能
王蕾*顾晓敏叶丹丹张文莉倪良
(江苏大学化学化工学院,镇江212013)
摘要:通过引入菲咯啉衍生物配体并改变反应体系中的金属盐,得到了3个新的含有杂多酸的金属-有机配合物(MOCs),{[Cu2(Do)2(H2O)4(SiW(12)O(40))]·10H2O}n(1),[Ag3(Do)5][Ag(Do)2](SiW(12)O(40)) (2),[Cu2(Do)4(H2O)2Cl](PMo(12)O(44))·2CH3OH (3)(Do=1,10-菲咯啉-5,6-二酮)。在配合物1中,Keggin型杂多酸与Cu连接形成了二维网状结构。配合物2中的Ag显示了多种不同的配位模式,构建了一个新颖的线性三核簇状结构。在配合物3中,Cu通过氯原子连接形成了一个双核结构。结构多样性表明金属离子与第二配体Do在构建不同结构的POMs中起到很重要的作用。光催化研究表明,配合物3不仅能在UV光照射下有效地催化降解罗丹明B(RhB),而且很稳定,能够从反应体系中分离以循环利用。
关键词:杂多酸;水热合成;晶体结构;催化性能
国家自然科学基金(No.21507047)、中国博士后基金(No.2015M571695)、江苏省博士后科学基金(No.1401176C)和江苏大学高级人才启动基金项目(No.14JDG053)资助。
*通信联系人。E-mail:wanglei86@ujs.edu.cn
0 Introduction
The design and construction of polyoxometalate (POMs)-based inorganic-organic hybrid compounds constitute a current topic within the fields of crystal engineering and materials chemistry. This is because their versatile applications in catalysis, photochemistry, electrochemistry, magnetism, and biochemistry[1-4]. In the process of synthesis, POMs can play different roles due to their features of oxygen-rich surface, high charge density and controllable size[5-6]. In our previous work, we report a series of POMs-based materials with various POMs as inorganic building blocks or noncoordinated templates.
As is known to all, the selection of organic ligands plays an important role in the self-assembly process of prospective POMs-based metal-organic complexes (MOCs), because organic ligands may control and adjust the final structures of target compounds. 1,10-Phenanthroline and its derivatives are used in coordination polymers due to their strong chelating abilities and fine conjugated character[7-9]. Especially, the conjugate aromatic ring will contribute to form novel structures by hydrogen bonds and π-π stacking. Different metal ions are of primary importance in the self-assembly process due to the unclear formation mechanism and multiple coordination modes. The transition metal ions Cuand Agdisplay an ability to coordinate readily to both hard and soft donor centers, and many compounds based on the coordination flexibility of the Cuand Aghave been synthesized and studied[10].
In this work, 1,10-phenanthroline-5,6-dione (Do) was selected as the multidentate ligand to assemble with Cu/Agions and various POMs for constructing new MOCs, in order to investigate whether the combination of multinuclear clusters with diverse metal ions could be realized, and to explore the role of polyoxoanions in the formation of the target compounds. Three POM-based MOCs containing multinuclear Cu/Agclusters and two types of POM were obtained: {[Cu2(Do)2(H2O)4(SiW12O40)]· 10H2O}n(1), [Ag3(Do)5][Ag(Do)2](SiW12O40) (2), [Cu2(Do)4(H2O)2Cl](PMo12O44)·2CH3OH (3). Their crystal structures, photoluminescent and photocatalytic properties have been studied in detail. The effect of various metal ions on the structures of the title compounds and the role of polyoxoanions was discussed. Additionally, photocatalytic activity is an appealing property of POMs[11-13]. Many organic pollutant compounds can be broken down into non-polluting small molecules and removed by light-excited POMs with high oxidizing ability[14-15]. So, the photocatalytic activities of 1~3 were also investigated in detail.
1 Experimental
1.1 Materials and methods
The chelating ligands Do was synthesized according to the literature method[16]. Other reagents and solvents for synthesis were purchased from commercial sources and used as received. Transmission mode FT-IR spectra were obtained as KBr pellets between 400 and 4 000 cm-1using a Nicolet Nexus 470 infrared spectrometer. Elemental analysis was carried out with a Perkin-Elmer 240C analyzer. Thermogravimetric analysis (TG) was performed on a Germany Netzsch STA449C at a heating rate of 10℃·min-1in nitrogen. Fluorescence measurement was carried out at room temperature with a Cary Eclipse spectrometer.
1.2 Syntheses of the compounds 1~3
{[Cu2(Do)2(H2O)4(SiW12O40)]·10H2O}n(1): A mixture of CuI (0.036 g, 0.19 mmol), H4SiW12O40(0.288 g, 0.10 mmol), Do (0.025 g, 0.12 mmol) and H2O (18 mL) were placed in a 25 mL Teflon-lined stainless steel vessel under autogenous pressure at 160℃for five days. After cooling to room temperature, Green block crystals of compound 1 were collected by filtration and washed with distilled water in 79% yield (based on [H4SiW12O40]). Anal. Calcd. for C24H40Cu2N4O58SiW12(%): C, 7.84; H, 1.09; N, 1.52. Found(%): C, 7.85; H, 1.06; N, 1.54. IR (KBr, cm-1): 3 507(m), 3 093(m), 1 704 (s), 1 619(s), 1 579(w), 1 487(m), 1 433(m), 1 305(m), 1 131(m), 1 015(m), 970(s), 921(s), 884(m), 794(m), 726(m) , 534(w).
[Ag3(Do)5][Ag(Do)2] (SiW12O40)] (2): Compound 2was also synthesized by using a method similar to that described for the synthesis of 1 except that AgNO3(0.033 g, 0.19 mmol) was used instead of CuI. Dark red block crystals of compound 2 were collected by filtration and washed with distilled water in 31% yield (based on Do). Anal. Calcd. for C84H42Ag4N14O54SiW12(%): C, 21.12; H, 0.87; N, 4.12. Found (%): C, 21.11; H, 0.88; N, 4.11. IR (KBr, cm-1): 3 075(m), 1 689(s), 1 570(w), 1 463(m), 1 297(m), 1 210(m), 1 120(m), 1 011(m), 966(s), 919(s), 882(m ), 793(m), 732(m), 533(w), 414(w).
[Cu2(Do)4(H2O)2Cl](PMo12O44)·2CH3OH (3): Compound 3 was also synthesized by using a method similar to that described for the synthesis of 1 except that CuCl2·2H2O (0.032 g, 0.19 mmol) and H3PMo12O40(1.183 g, 0.10 mmol) were used instead of CuI and H4SiW12O40. Green block crystals of compound 3 were collected by filtration and washed with distilled water in 61% yield (based on [H3PMo12O40]). Anal. Calcd. for C50H36ClCu2Mo12N8O56P(%): C, 20.07; H, 1.20; N, 3.75. Found(%): C, 20.06; H, 1.22; N, 3.73. IR (KBr, cm-1): 3 564(m), 3 096(m), 1 735(s), 1 703(s), 1 577(w), 1 487 (m), 1 432(m), 1 413(m), 1 300(m), 1 261(m), 1 063(m), 957(s),877(m),798(m),755(m),727(m),503(w),404(w).
1.3 Catalysis experiments
The photocatalytic activity of 1~3 was tested by degradation of Rhodamine B (RhB) under UV light irradiation at room temperature. For the experiment with a typical process, a suspension containing 1 (100 mg) and a 100 mL RhB (2.0×10-5mol·L-1) solution was stirred in the dark for about 30 min. It was then stirred continuously under Hg lamp irradiation. Every 45 min (or 10 min), 4 mL of samples were taken out of the reactor and centrifuged to remove the remnant photocatalyst from the liquid. The concentrations of RhB were estimated by checking the absorbance at 553 nm on a UV-2450 (Shimadzu) spectrophotometer.
1.4 X-ray crystallography
X-ray diffraction data for compounds 1~3 were collected on a Bruker SMART Apex CCD diffractometer, equipped with a graphite monochromatic Mo Kα radiation (λ=0.071 073 nm) at 293(2) K. The structures were solved by direct methods implemented in SHELXS-97[17]and refined by a full-matrix least-squares procedure based on F2using SHELXL-97[18]. All non-hydrogen atoms were refined anisotropically, whereas the hydrogen atoms were placed at calculated positions and treated using appropriate riding models. The detailed crystallographic data and structure refinement parameters for the three complexes are summarized in Table 1. Selected bond lengths and angles are listed in Table S1~S6 (Supporting Information).
CCDC: 1039184, 1; 1038117, 2; 1033951, 3.
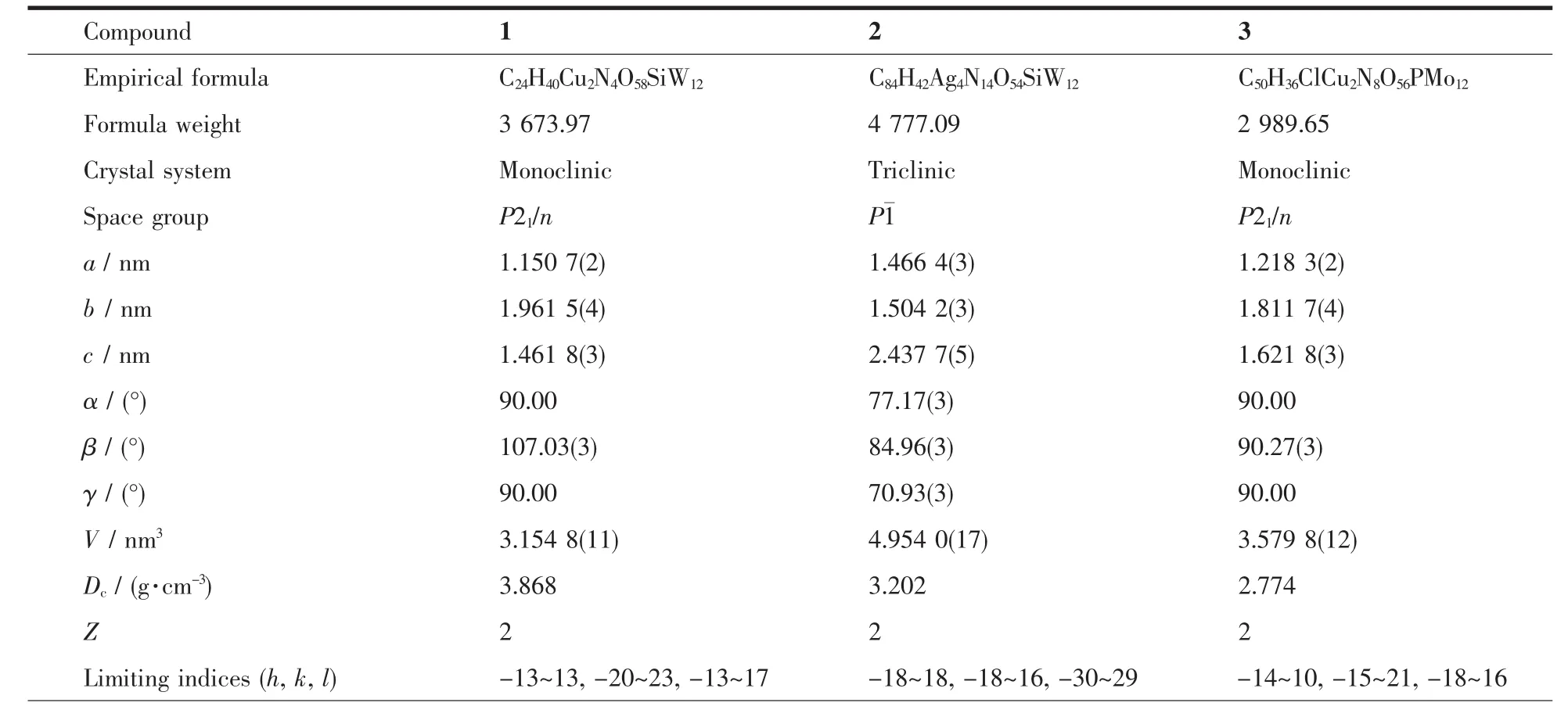
Table1 Crystal data and structure refinements for compounds 1~3
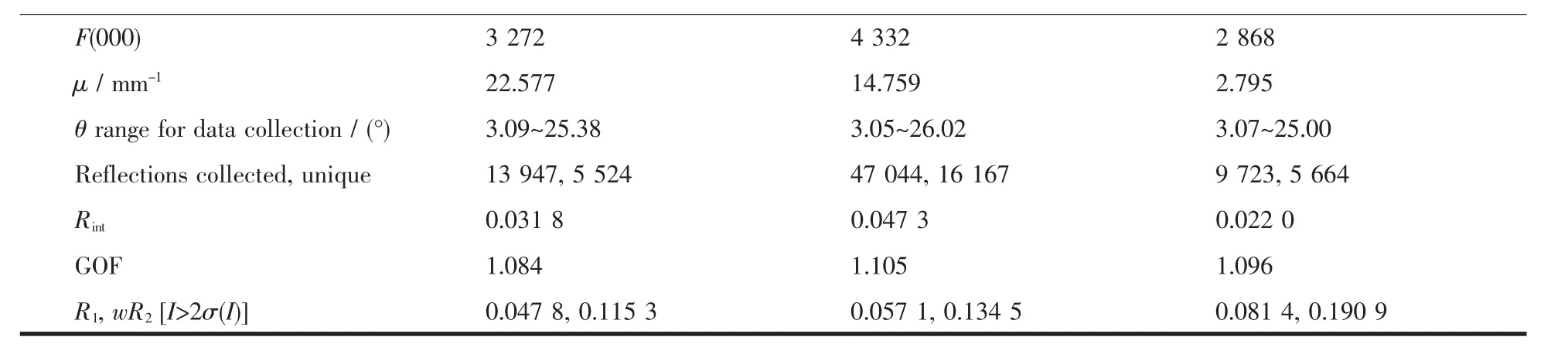
Continued Table 1
2 Results and discussion
2.1 Structural analysis of compounds 1~3
2.1.1 Crystal structure of {[Cu2(Do)2(H2O)4(SiW12O40)]· 10H2O}n(1)
Single-crystal X-ray diffraction reveals that compound 1 crystallizes in the monoclinic system with P21/n space group. Crystal structure analysis reveals that compound 1 consists of two Cuions, two Do ligands, a [SiW12O40]4-polyoxoanion and fourteen water molecules. As depicted in Fig.1a, Cu1 ions possess a distorted octahedral geometry[19], and are coordinated by two nitrogen atoms (N1, N2) from a Do ligand, two oxygen atoms (O5, O20A) from [SiW12O40]4-polyoxoanions and two oxygen atoms (O18, O19) from two coordinated water molecules, respectively. The bond distances and angles around the Cuions are in the normal ranges observed in other Cu-containing complexes[20].
The [SiW12O40]4 -anions link Cuions to generate a 2D inorganic layer, which results in the formation of channel with the space of area 1.257 3 nm×1.257 3 nm as presented in Fig.1b. The packing of the layers is dominated by hydrogen bonding interactions between water molecules and polyoxoanions (typical hydrogen bondings: O18 -H2W…O3 0.294 nm, O18-H2W…O5 0.291 nm) (Fig.1c). Such interactions led the formation of a 3D framework as shown in Fig.1d.
2.1.2 Crystal structure of [Ag3(Do)5][Ag(Do)2] (SiW12O40) (2)
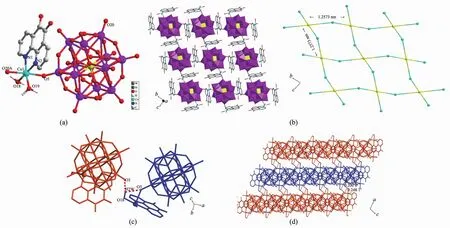
Fig.1 (a) Coordination environment of Cuion in 1; (b) Two-dimensional layer structure of 1; (c) Hydrogen bonds of 1; (d) Three dimensional supramolecular packing structures of 1
In order to investigate the effect of the metalcenters on the structure, Agion is selected to react with [SiW12O40]4-polyoxoanion and Do ligand under the same synthetic conditions. The asymmetric unit of compound 2 is shown in Fig.2a. It consists of one [SiW12O40]4-polyoxoanion, four Ag(I) ions and seven Do ligands. There are four crystallographically independent Agions exhibiting three kinds of coordination geometries as illustrated in Fig.2a. Ag1 possesses a distorted square pyramidal geometry[21a], and coordinated by three oxygen atom (O41, O42, O45) from two Do ligands and two nitrogen atoms (N3, N4) from a Do ligand. The interesting Ag-O bonds between oxygen atoms of organic ligands Do and metal ions are very rare in inorganic-organic hybrid compounds based on POMs. Ag2 and Ag3 are surrounded by four nitrogen atoms from two different Do ligands, and also respectively connected with O23 and O34 from [SiW12O40]4-polyoxoanions (Ag2-O23 0.281 1 nm, Ag3-O34 0.280 1 nm). Ag4 is in a slightly distorted quadrangular geometry[21b], and is surrounded by four nitrogen atoms from two Do ligands. The corresponding bond lengths and angles are in the normal ranges[22].
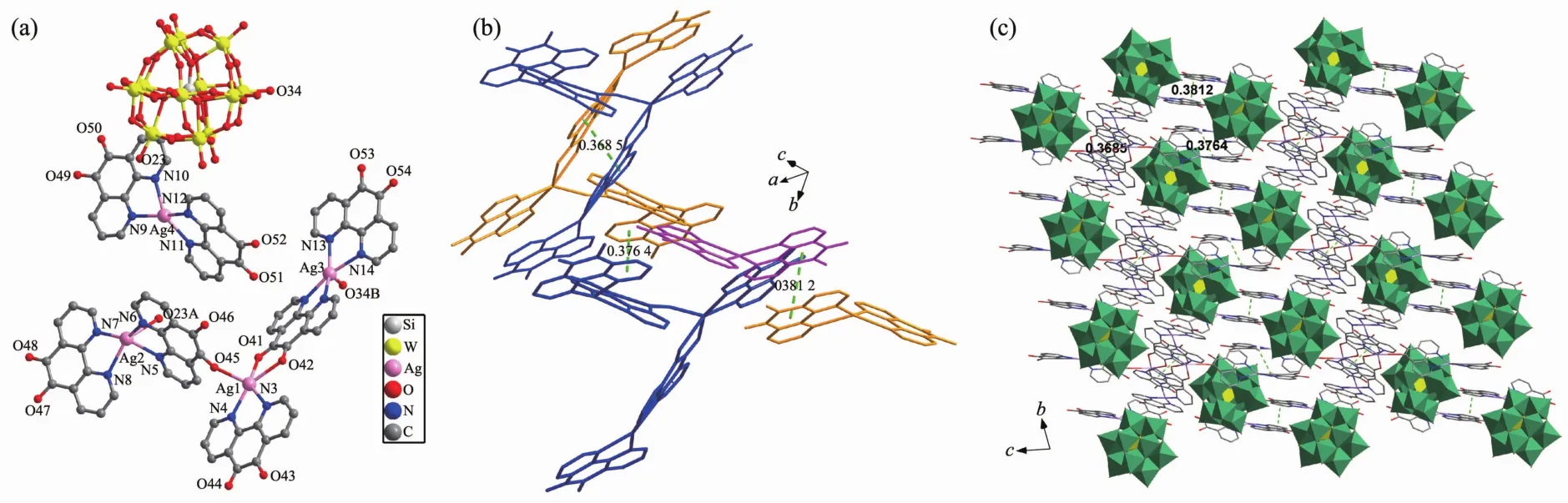
Fig.2 (a) Coordination environment of Agion in compound 2; (b)π-π stacking interactions in compound 2; (c) Three dimensional supramolecular packing structure of compound 2
2.1.3 Crystal structure of [Cu2(Do)4(H2O)2Cl] (PMo12O44)·2CH3OH (3)
To evaluate the effect of polyoxoanion on the framework formation of complex, we select [PMo12O44]3-polyoxoanion to react with copper salt in the presence of the same Do ligand. The X-ray structure analysis reveals that complex 3 contains two [Cu(Do)2(H2O)] units, a chlorine ion, a [PMo12O44]3-polyoxoanion and two methanol molecules. Cu1 is coordinated by four nitrogen atoms from two Do ligands, one oxygen atom from a water molecule and one chlorine ion (Fig.3a). The Cu-N distances vary from 0.200 5(11) to 0.229 7(15) nm, the Cu-O distance is 0.214 4(12) nm, and the Cu-Cl distance is 0.251 0(9) nm.
Two crystallographically independent Cuions in the complex 3 are bridged by one chlorine ion to form a binuclear structure with Cu-Cu distance of 0.528 99 nm (Fig.3b). The most interesting feature of the structure is that it contains strong intermolecular hydrogen bonding interactions between uncoordinated methanol molecules and oxygen atoms of Do ligands to form two dimensional layer (Fig.3d) (the main hydrogen bonding interactions included in the 2D layer: O1W-H1W…O27 0.240 nm,O27-H29B…O26 0.280 nm) (Fig.3c).
2.2 FT IR analysis
In the IR spectra (Fig.S1, Supporting Information), the band of 1 463~1 487 cm-1is attributed to the skeleton vibration of the benzene ring from the Do group. Characteristic vibration peaks of the Kegginpolyoxoanions are observed for ν(Si-Oa),ν(W-Ot), corner-sharing ν(W-Oc-W), and edge-sharing ν(W-Oe-W) at 1 015, 970, 921, and 794 cm-1for 1 and 1 011, 966, 919, and 793 cm-1for 2, respectively[23]. For compound 3, the characteristic peaks at 1 063, 957, 877, and 798 cm-1are attributed to ν(P-Oa),ν(Mo-Ot), ν(Mo-Oc-Mo), and ν(Mo-Oe-Mo)[24]. The signal at 534 cm-1for 1 (533 cm-1for 2, 503 cm-1for 3) also proves coordination of metal ions and nitrogen.

Fig.3 (a) Coordination environment of Cuion in compound 3; (b) View of dinuclear core structure in compound 3; (c) Hydrogen bonds of the compound 3; (d) Two dimensional supramolecular layer formed by hydrogen bonding interactions
2.3 Thermal properties
The thermal behavior was studied by a thermogravimetric (TG) analyzer to characterize the three compounds more fully in terms of thermal stability (Fig.S2). The TG curve of 1 shows a two-step weight loss: the first weight loss of 6.04% (Calcd. 6.86%) from 24 to 284℃may be assigned to the removal of H2O, and the second weight loss of 10.09% (Calcd. 11.43%) in the range of 414~774℃may be ascribed to decomposition of Do ligands. Compound 2 only has one-step weight loss of 29.16% (Calcd. 30.77%) from 460 to 770℃, which can be ascribed to the decomposition of Do ligands. In 3, a total weight loss of 32.90% is accordant with the calculated value of 31.44% in the range of 35~743℃, ascribed to the release of two methanol molecules, two water molecules and four Do ligands. These results, in good agreement with the calculated value, further confirm the formula of the title compounds.
2.4 Fluorescent properties
The solid-state fluorescence spectra of 1~3 were recorded at room temperature. Free Do ligand possesses intense emission at 594 nm upon excitation at 424 nm, assigned to π→π* transitions[25]. It can be observed that the three compounds all display a broad emission band at around 550 nm in the visible region upon excitation at 424 nm (Fig.S3), which can be attributed to the Do ligand. Blue shift of about 50 nm for 1~3 compared to the emission peak of free ligandDo is probably related to ligand-to-metal charge transfer[26].
2.5 Catalytic properties
The use of POMs, as one kind of green and cheap photocatalysts, to decompose waste organic molecules so as to purify the water resources has attracted great attention in recent years[27]. RhB is difficult to decompose in waste water. Herein we selected RhB as an organic pollutant to evaluate the photocatalytic efficiency of the three compounds as heterogeneous catalysts under UV light irradiation.
As shown in Fig.4, the absorption peaks of RhB aqueous solution decrease obviously with increasing reaction time for the compounds 1~3. For 1, the RhB solution has been taken out every 45 min, and 95.40% of RhB decompose after 315 min of irradiation. For 2, 92.13% of RhB decomposes after 450 min of irradiation. For 3, the RhB solution has been taken out every 10 min, and 98.84% of RhB decomposes after 80 min of irradiation. Changes in the concentration ratios (C/C0) of RhB solutions versus reaction time of the three compounds are plotted in Fig.5. It can be clearly seen that their photocatalytic efficiency is different. Clearly, the photocatalytic efficiency of compound 2 is lower than compound 1, and the photocatalytic efficiency of compound 3 based on [PMo12O40]3-is obviously higher than compounds 1 and 2 based on [SiW12O40]4-.
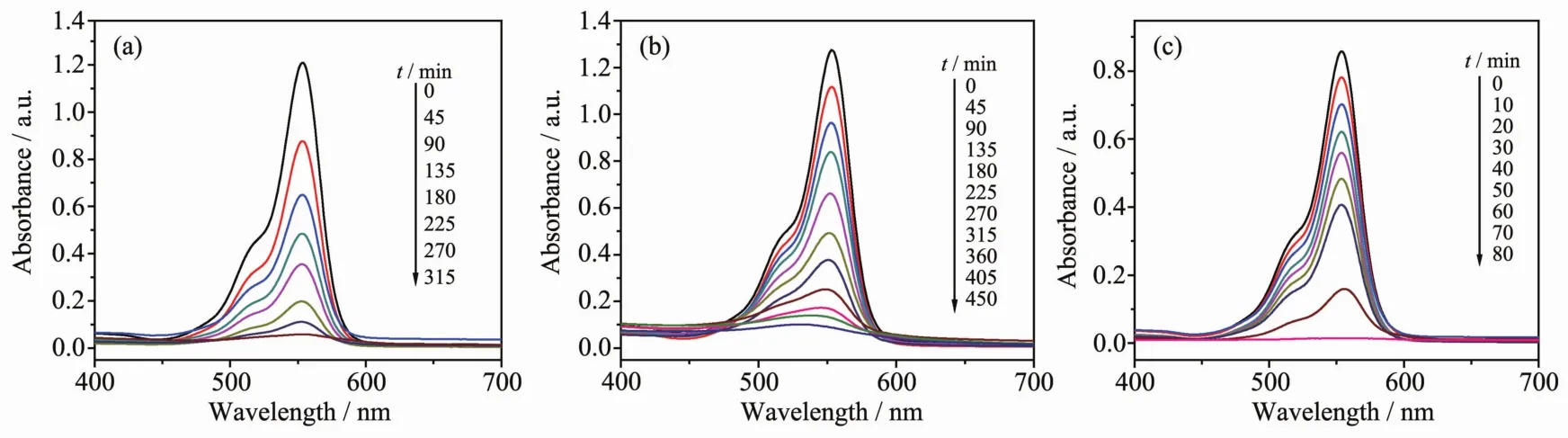
Fig.4 UV spectra of RhB solution after photocatalytic degradation by compound 1 (a), 2 (b) and 3 (c)
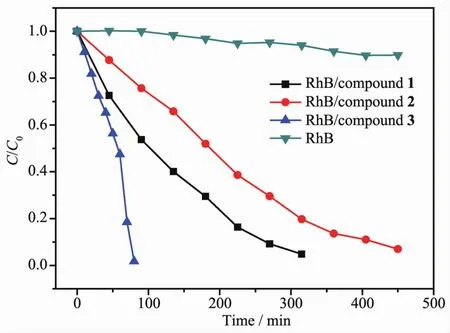
Fig.5 Plots of the concentration ratios of RhB (C/C0) vs irradiation time (min) in the absence and presence of 1~3 during the degradation reaction under UV irradiation
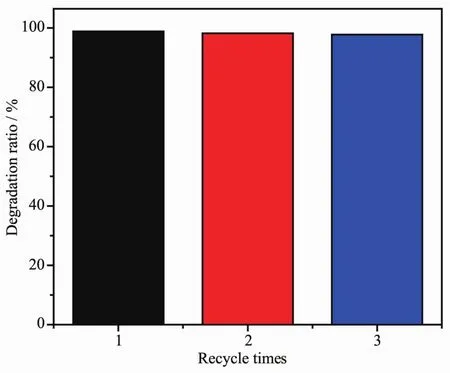
Fig.6 Effect of recycle times on the degradation ratio of RhB solution by compound 3
Additionally, the reproducibility of the photocatalytic degradation of RhB is an important factor to evaluate the applicability of photocatalyst, and further studies have shown that 3 are also very stable and easily separated from the reaction system for reuse. After each cycle of RhB degradation, 3 is collected by centrifugation, washed and dried at room temperature to repeat the test. As shown in Fig.6, no significant loss in the photocatalytic activity is observed for 3 after three cycles of the RhB degradation experiments (98.84% for the first cycle,98.18% for the second cycle and 97.73% for the third cycle). The results indicate that the photocatalytic activity of 3 has a good reproducibility. The different photocatalytic activity of 1~3 on the decomposition of RhB may be due to their different structures and different POMs in the title compounds.
3 Conclusions
In conclusion, by introducing two types of POMs into the Cu-Do and Ag-Do reaction system, three POM-based organic-inorganic hybrid compounds containing various multinuclear clusters have been successfullyprepared.Thecompounds 1~3 show diverse structures ranging from a 0D binuclear cluster, 0D trinuclear cluster, to a 2D layer. The structural diversities show that different polyoxoanions and metal ions play key roles in the construction of various architectures. In addition, photocatalytic activities indicate that the three complexes present good degradation activity and may be photocatalysts to decompose some organic dyes.
Acknowledgements: This work is supported from the National Natural Science Foundation of China (Grant No. 21507047), Postdoctoral Science Foundation of China (Grant No.2015M571695), Postdoctoral Science Foundation of Jiangsu Province (Grant No.1401176C) and the Programs of Senior Talent Foundation of Jiangsu University (Grant No.14JDG053).
Supporting information is available at http://www.wjhxxb.cn
References:
[1] Kamata K, Yamaguchi S, Kotani M, et al. Angew. Chem. Int. Ed., 2008,47:2407-2410
[2] Zhang J, Song Y F, Cronin L, et al. J. Am. Chem. Soc., 2008,130:14408-14409
[3] Miras H N, Wilsonw E F, Cronin L. Chem. Commun., 2009, 1297-1311
[4] Lisnard L, Mialane P, Dolbecq A, et al. Chem. Eur. J., 2007, 13:3525-3536
[5] (a)Long D L, Tsunashima R, Cronin L. Angew. Chem. Int. Ed., 2010,49:1736-1758
(b)Ma F J, Liu S X, Sun C Y, et al. J. Am. Chem. Soc., 2011,133:4178-4181
[6] Kan W Q, Yang J, Liu Y Y, et al. Inorg. Chem., 2012,51: 11266-11278
[7] Wang X L, Chen Y Q, Gao Q, et al. Cryst. Growth Des., 2010,10:2174-2184
[8] Wang L, Ni L. J. Coord. Chem., 2012,65:1475-1483
[9] Wang L, Ni L, Yao J. Polyhedron, 2013,59:115-123
[10](a)Han Z G, Wang Y N, Wu J J, et al. Solid State Sci., 2011,13:1560-1566
(b)Li S B, Ma H Y, Pang H J, et al. Cryst. Growth Des., 2014,14:4450-4460
[11]Hu Y, Luo F, Dong F. Chem. Commun., 2011,47:761-763
[12]Guo Y, Wang Y, Hu C, et al. Chem. Mater., 2000,12:3501-3508
[13]Wu Q, Chen W L, Liu D, et al. Dalton Trans., 2011,40:56-61
[14]Fontananova E, Donato L, Drioli E, et al. Chem. Mater., 2006,18:1561-1568
[15]Chen C C, Zhao W, Lei P X, et al. Chem. Eur. J., 2004,10: 1956-1965
[16]Hiort C, Lincoln P, Nordén B. J. Am. Chem. Soc., 1993, 115:3448-3454
[17]Sheldrick G M. SHELXS-97, Program for Automatic Solution of Crystal Structure, University of Göttingen, Germany, 1997.
[18]Sheldrick G M. SHELXL-97, Programs for Crystal Structure Refinement, University of Göttingen, Germany, 1997.
[19]Tian A X, Ying J, Peng J, et al. Inorg. Chem., 2009,48:100-110
[20]Sha J Q, Peng J, Zhang Y, et al. Cryst. Growth Des., 2009, 9:1708-1715
[21](a)Zhang Z, Yang J, Liu Y Y, et al. CrystEngComm, 2013, 15:3843-3853
(b)Wang X L, Hu H L, Liu G C, et al. Chem. Commun., 2010,46:6485-6487
[22]Yang G P, Wang Y Y, Liu P, et al. Cryst. Growth Des., 2010,10(3):1443-1450
[23]Thouvenot R, Fournier M, Franck R, et al. Inorg. Chem., 1984,23:598-605
[24]Rocchiccioli-Deltcheff C, Fournier M, Franck R, et al. Inorg. Chem., 1983,22:207-216
[25]LIU Chun-Bo(刘春波), WANG Qian-Qian(王倩倩), BAI Hong-Ye(白红叶), et al. Chinese J. Inorg. Chem.(无机化学学报), 2014,30(2):391-397
[26]Sun Y Q, Hu J, Zhang H H, et al. J. Solid State Chem., 2012,186:189-194
[27]Hu Y, Luo F, Dong F. Chem. Commun., 2011,47:761-763
Hydrothermal Syntheses, Crystal Structures and Catalytic Performance of a Series of Polyoxometalate-Based Hybrid Compounds
WANG Lei*GU Xiao-Min YE Dan-Dan ZHANG Wen-Li NI Liang
(School of Chemistry and Chemical Engineering, Jiangsu University, Zhenjiang, Jiangsu 212013, China)
Abstract:By using phenanthroline ligand and changing the metal salts in the reaction system, three new Kegginbased metal-organic complexes (MOCs), {[Cu2(Do)2(H2O)4(SiW(12)O(40))]·10H2O}n(1), [Ag3(Do)5][Ag(Do)2](SiW(12)O(40)) (2), [Cu2(Do)4(H2O)2Cl](PMo(12)O(44))·2CH3OH (3) (Do=1,10-phenanthroline-5,6-dione), have been successfully synthesized under hydrothermal conditions. In compound 1, the Keggin-type POMs act as highly connected inorganic ligands and connect Cuions to exhibit 2D network, which are modified by the Do polymeric motifs. For compound 2, the Agcenters exhibit various coordination types, and are linked by Do ligand to construct a linear trinuclear cluster structure. In compound 3, Cuions are connected by one chlorine ion to form a binuclear structure. The structural diversities indicate that the metal ions and the secondary ligand Do play an important role in the assembly and the structures of different POMs. Photocatalytic studies indicate that compound 3 is not only actively photocatalytic for degradation of Rhodamine B (RhB) under UV light irradiation, but also very stable and easily separated from the reaction system for reuse. CCDC: 1039184, 1; 1038117, 2; 1033951, 3.
Keywords:polyoxometalates; hydrothermal synthesis; crystal structure; catalytic properties
收稿日期:2015-11-03。收修改稿日期:2015-12-28。
DOI:10.11862/CJIC.2016.054
文献标识码:中国分类号:O614.121;O614.122A
文章编号:1001-4861(2016)04-0691-08
