以4,4′-联苯二甲酸和咪唑基化合物为配体的钴配合物的合成和晶体结构
2016-05-03刘光祥
张 凤 刘光祥
(南京晓庄学院化学系,南京市新型功能材料重点实验室,南京 211171)
以4,4′-联苯二甲酸和咪唑基化合物为配体的钴配合物的合成和晶体结构
张凤刘光祥*
(南京晓庄学院化学系,南京市新型功能材料重点实验室,南京211171)
摘要:以4,4′-联苯二甲酸(H2BPDC)和4,4′-二咪唑基二苯醚(BIDPE)为原料,与硝酸钴在不同温度下反应,得到2个结构不同的配位聚合物{[Co2(BPDC)2(BIDPE)2(H2O)]·2H2O}n(1)和{[Co(BPDC)(BIDPE)]·H2O}n(2)。对它们进行了元素分析、红外光谱分析,并利用X射线衍射测定了它们的单晶结构。配合物1属于单斜晶系,C2/c空间群,a=1.456 02(15) nm, b=1.557 51(16) nm, c=2.522 6(3) nm,β=90.834 0(10)°, V=5.720 2(10) nm3, Z=4, Mr=1 256.98, Dc=1.460 g·cm(-3),μ=0.655, F(000)=2 592, R1=0.036 7, wR2=0.087 5(I>2σ(I))。配合物2属于三斜晶系,P1空间群,a=1.061 92(10) nm, b=1.098 51(11) nm, c=1.324 51(13) nm,α=112.725 0(10) nm,β= 92.112 0(10)°,γ=96.574 0(10)°, V=1.410 2(2) nm3, Z=2, Mr=619.48, Dc=1.459 g·cm(-3),μ=0.662, F(000)=638, R1=0.047 4, wR2=0.116 5 (I>2σ(I))。单晶结构分析显示,配合物1拥有一维链状结构,而配合物2具有二维两重贯穿结构,并表现出聚轮烷的结构特征。结果说明了反应温度在配合物组装过程中起着非常重要的作用。
关键词:钴配合物;双咪唑配体;芳香羧酸配体;晶体结构
国家自然科学基金(No.21271106)和教育部科学技术重点项目(No.210102)资助。*通信联系人。E-mail:njuliugx@126.com
0 Introduction
The design and synthesis of coordination polymers (CPs) are of great interest, not only because of their intriguing variety of architectures and topologies, but also because of their tremendous potential applications in nonlinear optics, catalysis, gas absorption, luminescence, and magnetism[1-4]. However, the rational design and synthesis of CPs with unique structure and function still remain a long-term challenge. The resultant structural framework is frequently influenced by various factors such as medium, pH value of solution, temperature, the nature of metal ions, coordination geometry, and a number of coordination donors provided by organic ligands[5-10]. From a synthetic point of view, the judicious selection of appropriate organic ligands and coordination geometries of the metals is proved to be one of the most effective ways to manipulate the versatile structures of CPs[11-12].
Among the various types of organic ligands, imidazole and its derivatives are often employed to fabricate CPs because of their strong coordination abilities and relatively versatile coordination geometries[13-15]. The V-shaped ligand 4,4′-bis(imidazole -l-yl)diphenyl ether (BIDPE) has previously been justified as an efficient and versatile organic building unit for construction of coordination architectures[16-18]. For BIDPE, which features three special characteristics: ⒤the free rotation of the imidazolyl ring can improve the flexibility of the polymeric frameworks to meet the requirement of coordination geometries of metal ions for tuning the fine structure.The long size makes it an excellent candidate to generate CPs of entangled topology.The V-shaped conformation can form coordinative loops or rings which are beneficial for the assembly of polyrotaxane- and polycatenane-like motifs. More importantly, recent studies indicate that utilizing mixed ligands is an effective route to construct intriguing CPs with attractive topological structures[19-22]. Such a dual-ligand strategy offers great promise for the construction of target frameworks with high complexities due to the presence of distinct donors which can coordinate with metal centers through different coordination modes. With a view to develop possible synthetic strategies, the employment of mixed N- and O-donor ligands would be a feasible method to build coordination architectures with interesting topologies and remarkable functionalities[23-27]. As is known, polycarboxylate ligands are excellent coligands for the construction of highly connected, different dimensional frameworks due to their versatile bridging modes. However, investigation of the BIDPE-carboxylate mixed-ligand system remains largely unexplored. Thus, the development of comprehensive research on this topic is necessary. Considering all of the above-mentioned, we prepared two new coordination polymers, namely, {[Co2(BPDC)2(BIDPE)2(H2O)]·2H2O}n(1) and {[Co(BPDC)(BIDPE)]·H2O}n(2). Herein, we report their syntheses, crystal structures and thermal properties.
1 Experimental
1.1 Materials and general methods
All chemicals and solvents were of reagent grade and used as received without further purification. The BIDPE ligand was synthesized according to the reported method[28]. Elemental analyses (C, H and N) were performed on a Vario ELⅢelemental analyzer. Infrared spectra were recorded on KBr discs using a Nicolet Avatar 360 spectrophotometer in the range of 4 000~400 cm-1. Thermogravimetric analyses (TGA) were performed on a Netzsch STA-409PC instrument in flowing N2with a heating rate of 10℃·min-1. Powder X-ray diffraction (PXRD) patterns wereobtained on Bruker D8 Advance X-ray diffractometer with Cu Kα radiation (λ=0.154 056 nm) at room temperature.
1.2 Synthesis of {[Co2(BPDC)2(BIDPE)2(H2O)]· 2H2O}n(1)
A mixture containing Co (NO3)2·6H2O (29.6 mg, 0.1 mmol), H2BPDC (24.2 mg, 0.1 mmol), BIDPE (30.2 mg, 0.1 mmol) and NaOH (8.0 mg, 0.2 mmol) in 15 mL of deionized water was sealed in a 25 mL Teflon lined stainless steel container and heated at 120℃for 3 days. Purple platy crystals of 1 were collected by filtration and washed with water and ethanol several times with a yield of 53% based on BIDPE ligand. Anal. Calcd. for C64H50N8O13Co2(%): C, 61.15; H, 4.01; N, 8.91. Found(%): C, 61.16; H, 4.03; N, 8.92%. IR (KBr, cm-1): 3 462 (br), 3 122 (w), 1 610 (s), 1 558 (s), 1 521 (s), 1 423 (m), 1 389 (s), 1 276 (w), 1 242 (s), 1 171 (w), 1 133 (w), 1 102 (w), 1 083 (s), 1 040 (m), 987 (w), 892 (s), 827 (w), 782 (w), 672 (w), 573 (w), 547 (w).
1.3 Synthesis of {[Co(BPDC)(BIDPE)]·H2O}n(2) Complex 2 was prepared by a process similar to that yielding complex 1 at 180℃by using Co(NO3)2·
6H2O (29.6 mg, 0.1 mmol), H2BPDC (24.2 mg, 0.1 mmol), BIDPE (30.2 mg, 0.1 mmol) and NaOH (8.0 mg, 0.2 mmol) in 15 mL of deionized water. Purple block crystals of 2 were collected by filtration and washed with water and ethanol several times with a yield of 44% based on BIDPE ligand. Anal. Calcd. for C32H24N4O6Co (%): C, 62.04; H, 3.90; N, 9.04. Found (%): C, 62.02; H, 3.89; N, 9.05. IR (KBr, cm-1): 3 459 (br), 3 061 (w), 1 608 (s), 1 523 (m), 1 358 (s), 1 275 (m), 1 200 (s), 1 124 (w), 1 034 (w), 1 009 (s), 835 (m), 802 (s), 638 (w), 594 (m), 519 (m), 478 (m).
1.4 X-ray crystallography
Two block single crystals with dimensions of 0.22 mm×0.20 mm×0.08 mm for 1 and 0.20 mm×0.18 mm× 0.16 mm for 2 were mounted on glass fibers for measurement, respectively. X-ray diffraction intensity data were collected on a Bruker APEXⅡCCD diffractometer equipped with a graphite-monochromatic Mo Kα radiation (λ=0.071 073 nm) using the φ-ω scan mode at 293(2) K. Data reduction and empirical absorption correction were performed using the SAINT and SADABS program[29], respectively. The structures were solved by the direct method using SHELXS-97[30]and refined by full-matrix least squares on F2using SHELXL-97[31]. All of the non-hydrogen atoms were refined anisotropically. The details of the crystal parameters, data collection and refinement for 1 and 2 are summarized in Table 1, and selected bond lengths and angles with their estimated standard deviations are listed in Table 2.
CCDC: 1429769 1; 1429770, 2.

Table1 Crystal data and structure refinement for 1 and 2
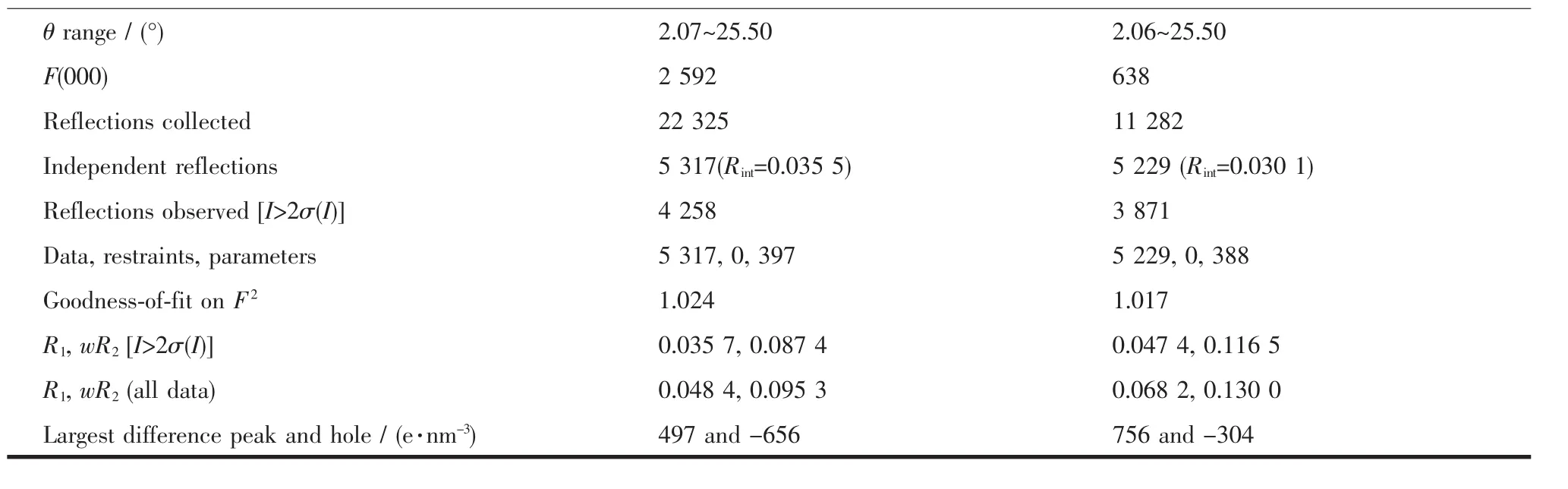
Continued Table 1
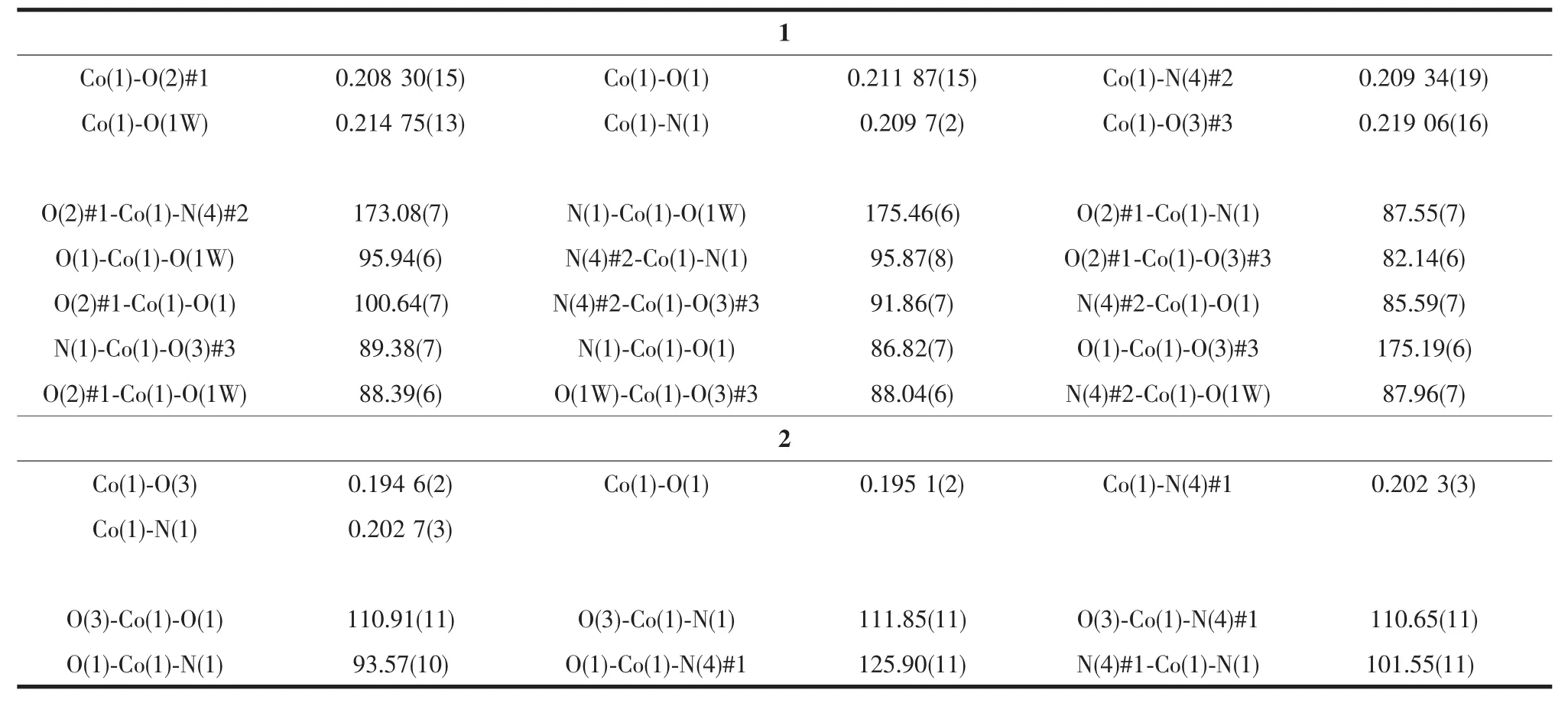
Table2 Selected bond lengths(nm) and angles(°) for 1 and 2
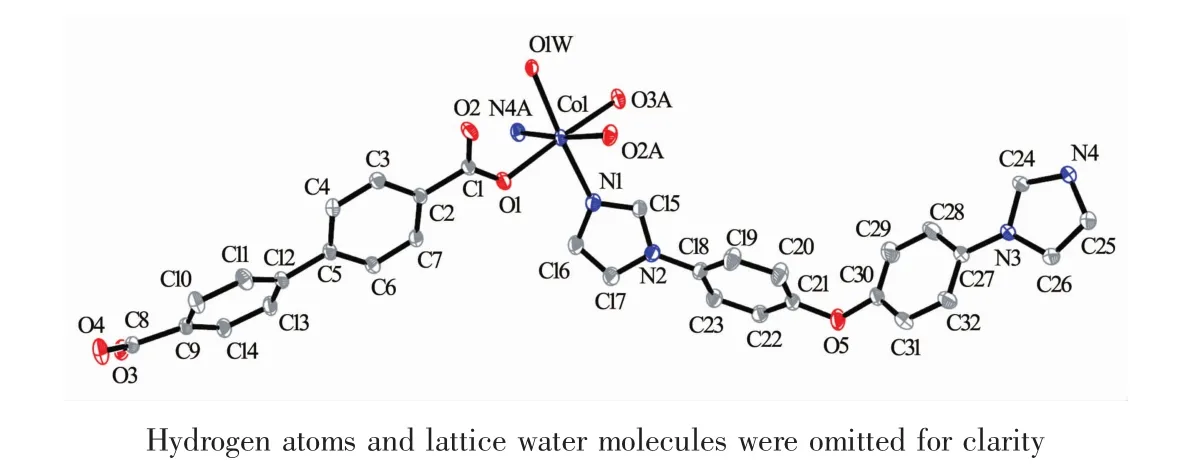
Fig.1 Coordination environments of the Coatoms in 1 with the ellipsoids drawn at the 30% probability level
2 Results and discussion
2.1 Crystal structure
Single crystal X-ray diffraction analysis revealed that complex 1 crystallizes in the monoclinic space group C2/c and features a one-dimensional chain structure based on triply bridged binuclear units. As illustrated in Fig.1, the asymmetric unit of complex 1 contains one crystallography independent cobalt ion, one unique BPDC dianions, one entire BIDPE molecule, a half ligated water molecule located on a crystallographic 2-fold axis and a lattice watermolecule. The cobalt ion possesses a distorted [CoO4N2] octahedral coordination environment with a cis orientation of its nitrogen donors, which belong to two different BIDPE ligands. Bond lengths and angles about the cobalt ion are standard for octahedral coordination (Table 2). Three of the oxygen donors belong to three different BPDC anions, while the remaining coordination site is occupied by an aqua ligand. Each BPDC anion serves as an exotridentate linker, connecting two cobalt ions at one carboxylate terminus in a bis-bridging binding mode with a third cobalt ion via the other carboxylate locus in a monodentate binding mode. The aqua ligand serves to bridge two Coions in a μ2-fashion; two Coions are in turn also bridged by two carboxylate termini from two different BPDC anions to form a triply bridged binuclear unit. These have a Co…Co distance of 0.357 7 nm, with the two bridging carboxylate groups situated 45.66°apart. Individual binuclear units are connected into a 1D [Co2(BPDC)2(BIDPE)2(μ2-H2O)]nchain motif, coursing along c-axis, by means of the monodentate carboxylate termini of the BPDC anions (Fig.2). The Co…Co contact distance through the full extent of the DBA dianionic tethers is 1.444 9 nm. The BIDPE ligands fosters a Co…Co distance of 1.468 4 nm. Adjacent [Co2(BPDC)2(BIDPE)2(μ2-H2O)]nchains are conjoined along the a and b axes by intermolecular weak interactions to construct the 3D supramolecular network (Fig.3).

Fig.2 View of 1D [Co2(BPDC)2(BIDPE)2(μ2-H2O)]nchain motif in 1
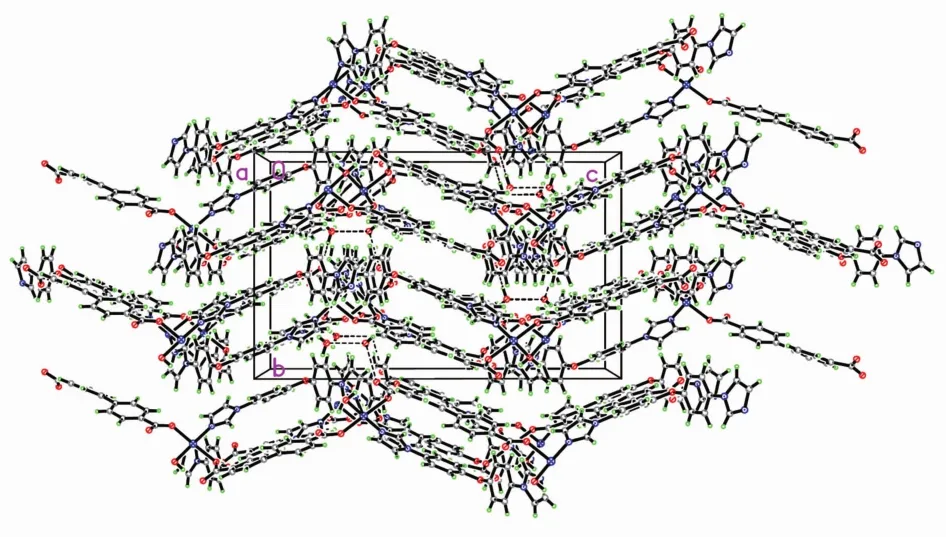
Fig.3 Molecular packing diagram projected along a axis in 1
Changing the temperature from 120 to 180℃for the same reaction mixture of 1 affords complex 2, which crystallizes in the triclinic P1 space group and shows a 2D→2D entanglement pattern with polycatenane features. The asymmetric unit of 2 contains one crystallographically independent Coion, one individual BIDPE ligand, two halves BPDC anions located on inversion centres and one lattice water molecule. As depicted in Fig.4, each Cois four-coordinated by two oxygen atoms from two BPDC anions and two nitrogen atoms from the imidazole groups of two BIDPE ligands, exhibiting a slightly distorted octahedral geometry. All chemical bonds fall in the normal ranges[32]. In 2, every two V-shaped BIDPE ligands are joined by two Coions to form a Co2(BIDPE)2ring with Co…Co separation of 1.504 8 nm and meanwhile all these rings are connected by BPDC anions to generate a 1D linear necklace-likechain along the b axis. These parallel chains are further bridged through the BPDC anions into a twodimensional layer (Fig.5) and neighboring layers are interpenetrated with each other in a parallel fashion to give an entangled 2D→2D double-layer (Fig.6). Undoubtedly, the structure of 2 can be described as a metal-organic polycatenane framework.
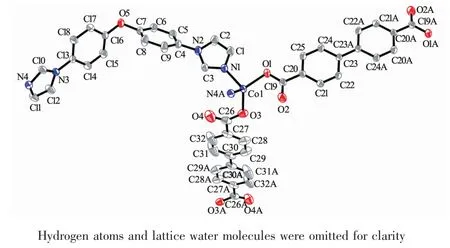
Fig.4 Coordination environments of the Coatoms in 2 with the ellipsoids drawn at the 30% probability level
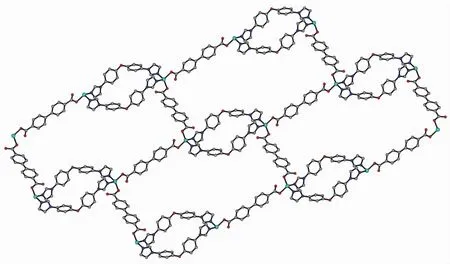
Fig.5 Perspective view of the 2D layer structure in 2

Fig.6 Perspective view of the 2D→2D polycatenane-like framework in 2
Complexes 1 and 2 were obtained under similar reactions at different reaction temperatures. At lower temperature, the carboxylate anions in 1 display monodentate and bis(bridging) modes, whereas in 2, there is only one bis(monodentate) coordination fashion. The different coordination modes of the Coion and different frameworks of 1 and 2 clearly showthat the formation of the framework is influenced by classical thermodynamic factors, such as condensation due to entropy driven dehydration reaction at higher temperature.Transformationreactionshavebeenstudied extensively by modifying reaction conditions[33-35], but to our knowledge this is the first attempt in CPs of aromatic carboxylates and V-shaped bis (imidazole) ligands where the temperature effects have been investigated and correlated with the structure.
2.2 FTIR spectra
The IR spectra of 1 and 2 show the absence of the characteristic bands at around 1 700 cm-1attributed to the protonated carboxylate group indicates that the complete deprotonation of H2BPDC ligand upon reaction with Co ion. The presence of vibrational bands of 1 610 ~1 558 cm-1are characteristic of the asymmetric stretching of the deprotonated carboxylic groups of BPDC2-anion. The difference between asymmetric and symmetric carbonyl stretching frequencies (Δν=νasym-νsym) was used to fetch information on the metal-carboxylate binding modes. Complex 1 shows two pairs of νasymand νsymfrequencies at 1 610, 1 423 (Δν=187) and 1 558, 1 389 (Δν=169) cm-1for the carbonyl functionality indicating two coordination modes as observed in the crystal structure. Complex 2 showed a pairs of νasymand νsymfrequencies at 1 608, 1 358 (Δν=250) cm-1corresponding to the carbonyl functionality of dicarboxylate ligand indicating a symmetric monodentate coordination mode. OH stretching broad bands at 3 462 cm-1for 1 and at 3 459 cm-1for 2 are attributable to the coordinated lattice water. The bands in the region of 640~1 250 cm-1are attributed to the -CH- in-plane or out-of-plane bend, ring breathing, and ring deformation absorptions of benzene ring. The IR spectra exhibit the characteristic peaks of imidazole groups at ca. 1 520 cm-1[36].

Fig.7 TGA curves of complexes 1 and 2

Fig.8 PXRD patterns of complexes 1 and 2
2.3 Thermal stability and powder X-ray diffraction (PXRD)
To examine the thermal stabilities of complexes 1 and 2, TG analyses were carried out (Fig.7). The TGA study of complex 1 shows a weight loss of 4.42% from 30 to 210℃, corresponding to the loss of two lattice water molecules and one coordinated water molecule (Calcd. 4.27% ). Then the TG curve presents a platform and frame starts to decompose at 310℃. In the case of complex 2, a little weight loss is observed from 30 to 90℃due to the release of one lattice water molecule, with a weight loss of 3.01% (Calcd. 2.90%). Furthermore, the decomposition of 2 occurs upon 315℃.
Powder X-ray diffraction analysis (PXRD)experiments were carried out for 1 and 2 at room temperature to characterize their purity. As shown in Fig.8, the measured peak positions closely match the simulated peak positions, indicative of pure products.
References:
[1] Long J R, Yaghi O M. Chem. Soc. Rev., 2009,38:1213-1214
[2] Liu K, Shi W, Cheng P. Coord. Chem. Rev., 2015,289:74-122
[3] Zhou H C, Kitagawa S. Chem. Soc. Rev., 2014,43:5415 -5418
[4] Ma L, Abney C, Lin W. Chem. Soc. Rev., 2009,38:1248-1256
[5] Guo X M, Guo H D, Zou H Y, et al. CrystEngComm, 2013, 15:9112-9120
[6] Zhao F H, Jing S, Che Y X, et al. CrystEngComm, 2012,14: 4478-4485
[7] Shen L J, Gray D, Masel R I, et al. CrystEngComm, 2012, 14:5145-5147
泰国、印度等国家医疗旅游发展经验告诉我们,一个地区之所以成为具有竞争力的医疗旅游目的地,是由许多因素共同决定的:医疗基础设施、休闲设施、高质量的医疗服务人才、合理的价格(交通、住宿、医疗等)等。因此,海南在加快提升医疗服务水平和能力建设的同时,还需要进一步提高旅游公共服务体系建设,包括安全便捷高效的交通基础设施建设,价格合理、服务优质、符合国际标准的酒店、餐饮以及景区建设;通晓国际规则和精通多国外语的国际旅游人才队伍建设;信息准确、及时的医疗旅游门户网站建设等。
[8] Stock N, Biswas S. Chem. Rev., 2012,112:933-969
[9] Guo H D, Guo X M, Zou H Y, et al. CrystEngComm, 2014, 16:7459-7468
[10]Liu G X, Zhu K, Chen H, et al. CrystEngComm, 2008,10: 1527-1530
[11]Pan M, Su C Y. CrystEngComm, 2014,16:7847-7859
[12]Ding J G, Yin C, Zheng L Y, et al. RSC Adv., 2014,4: 24594-24600
[13]Yao X Q, Pan Z R, Hu J S, et al. Chem. Commun., 2011, 47:10049-10051
[14]Li S B, Sun W L, Wang K, et al. Inorg. Chem., 2014,53: 4541-4547
[15]Phan A, Doonan C J, Uribe-Romo F J, et al. Acc. Chem. Res., 2010,43:58-67
[16]Hu J S, Shang Y J, Yao X Q, et al. Cryst. Growth Des., 2010,10:4135-4142
[18]Hu J S, Huang L F, Yao X Q, et al. Inorg. Chem., 2011,50: 2404-2414
[19]Hauptvogel I M, Bon V, Grünker R, et al. Dalton Trans., 2012,41:4172-4179
[20]Kim D, Lah M S. CrystEngComm, 2013,15:9491-9498
[21]Cao T T, Peng Y Q, Liu T, et al. CrystEngComm, 2014,16: 10658-10673
[22]Li Y W, Li D C, Xu J, et al. Dalton Trans., 2014,43:15708-15712
[23]Jiang H L, Tatsu Y, Lu Z H, et al. J. Am. Chem. Soc., 2010, 132:5586-5587
[24]Liu X M, Lin R B, Zhang J P, et al. Inorg. Chem., 2012,51: 5686-5692
[25]Han L W, Lu J, Lin Z J, et al. CrystEngComm, 2014,16: 1749-1754
[26]Kongpatpanich K, Horike S, Sugimoto M, et al. Chem. Commun., 2014,50:2292-2294
[27]Das M C, Guo Q S, He Y B, et al. J. Am. Chem. Soc., 2012, 134:8703-8710
[28]Liu G X, Zha X C, Wang Y, et al. J. Inorg. Organomet. Polym., 2012,22:258-263
[29]Sheldrick G M. SADABS. Program for Empirical Absorption Correction of Area Detector Data, University of Göttingen, Germany, 1996.
[30]Sheldrick G M. SHELXS-97, Program for Crystal Structure Solution, University of Göttingen, Germany, 1997.
[31]Sheldrick G M. SHELXL-97, Program for the Refinement of Crystal Structure, University of Göttingen, Germany, 1997.
[32]Liu Y, Qi Y, Su Y H, et al. CrystEngComm, 2010,12:3283 -3290
[33]Dan M, Rao C N R. Angew. Chem., Int. Ed., 2006,45:281-285
[34]Li B, Dai X M, Meng X G, et al. Dalton Trans., 2013,42: 2588-2593
[35]Ma J, Jiang F L, Chen L, et al. CrystEngComm, 2012,14: 4181-4187
[36]Nakamoto K. Infrared and Raman Spectra of Inorganic and Coordinated Compounds. 5th Ed. New York: Wiley & Sons, 1997.
Syntheses and Crystal Structures of Two CobaltCoordination Polymers Constructedfrom 4, 4′-Biphenyldicarboxylate and Bis(imidazole) Ligands
ZHANG Feng LIU Guang-Xiang*
(Key Laboratory of Advanced Functional Materials of Nanjing, Department of Chemistry, Nanjing Xiaozhuang University, Nanjing 211171,China)
Abstract:Two cobaltcoordination polymers, {[Co2(BPDC)2(BIDPE)2(H2O)]·2H2O}n(1) and {[Co(BPDC)(BIDPE)]· H2O}n(2) (H2BPDC=4, 4′-biphenyldicarboxylic acid and BIDPE=4, 4′-bis(imidazole-l-yl)diphenyl ether), have been synthesized and characterized by IR spectroscopy, elemental analyses and single-crystal X-ray diffraction. Complex 1 crystallizes in monoclinic, space group C2/c with a=1.456 02(15) nm, b=1.557 51(16) nm, c=2.522 6(3) nm,β= 90.834 0(10)°, V=5.720 2(10) nm3, Mr=1 256.98, Dc=1.460 g·cm(-3), F(000)=2 592,μ=0.655 mm(-1)and Z=4. The final R1is 0.036 7 and wR2is 0.087 5 for 4 258 observed reflections (I>2σ(I)). Complex 2 belongs to triclinic, space group P1 with a=1.061 92(10) nm, b=1.098 51(11) nm, c=1.324 51(13) nm,α=112.725 0(10)°,β=92.112 0(10)°,γ= 96.574 0(10)°, V=1.410 2(2) nm3, Mr=619.48, Dc=1.459 g·cm(-3), F(000)=638,μ=0.662 mm(-1)and Z=2. The final R1is 0.047 4 and wR2is 0.116 5 for 3 871 observed reflections (I>2σ(I)). Structural analyses reveal that complex 1 features a one-dimensional chain structure based on triply bridged binuclear units, which is further interlinked into a higher-dimensional supramolecular framework by intermolecular weak interactions, whereas complex 2 possesses a 2-fold parallel interpenetrating network consist of two identical sets of 2D layer motifs and shows polycatenane char-book=684,ebook=133acters. The results show that reaction temperature plays a significant role in the structure of the final products. CCDC: 1429769 1; 1429770, 2.
Keywords:cobalt coordination polymer; bis(imidazole) ligands; polycarboxylate; crystal structure
收稿日期:2015-10-26。收修改稿日期:2016-01-18。
DOI:10.11862/CJIC.2016.083
中图分类号:O614.81+2
文献标识码:A
文章编号:1001-4861(2016)04-0683-08
