Single dose of intra-muscular platelet rich plasma reverses the increase in plasma iron levels in exercise-induced muscle damage: A pilot study
2016-04-25ZekinePunduk,OnurOral,NadirOzkayin等
Single dose of intra-muscular platelet rich plasma reverses the increase in plasma iron levels in exercise-induced muscle damage: A pilot study
Zekine Punduka,*,Onur Oralb,Nadir Ozkayinc,Khalid Rahmand,Rana VarolbaDepartment of Physical Education and Sports,University of Balikesir,Balikesir 10100,TurkeybDepartment of Physical Education and Sports,University of Ege,Izmir 35040,Turkey
cMedical Faculty,Department of Orthopaedics and Traumatology,University of Ege,Izmir 35100,Turkey
dFaculty of Science,School of Pharmacy and Biomolecular Sciences,Liverpool John Moores University,Liverpool,L3 3AF,UK Received 26 July 2014; revised 2 October 2014; accepted 17 November 2014
Available online 16 February 2015
Peer review under responsibility of Shanghai University of Sport.
* Corresponding author.
E-mail address: zkn1938@gmail.com (Z.Punduk).
http://dx.doi.org/10.1016/j.jshs.2014.11.005
2095-2546/©2016 Production and hosting by Elsevier B.V.on behalf of Shanghai University of Sport.
Abstract
Background: Platelet rich plasma (PRP) therapy is widely used in enhancing the recovery of skeletal muscle from injury.However,the impact of intramuscular delivery of PRP on hematologic and biochemical responses has not been fully elucidated in exercise-induced muscle damage.The purpose of this investigation the effects of intramuscular delivery of PRP on hematologic and biochemical responses and recovery strategy muscle damage induced by high intensity muscle exercise (exercise-induced muscle damage,EIMD).
Methods: Moderately active male volunteers participated in this study and were assigned to a control group (control,n = 6) and PRP administration group (PRP,n = 6).The subjects performed exercise with a load of 80% one repetition maximum (1RM) maximal voluntary contraction of the elbow flexors until point of exhaustion of the non-dominant arm was reached.The arms were treated with saline or autologous PRP post-24 h EIMD.Venous blood samples were obtained in the morning to establish a baseline value and 1-4 days post-exercise and were analyzed for serum ferritin,iron,iron binding capacity (IBC),creatinine kinase (CK),lactate dehydrogenase (LDH),aspartate aminotransferase (AST),and alanine aminotransferase (ALT).
Results: The baseline levels of plasma iron,ferritin,IBC,CK,LDH,AST,and ALT were similar in both the control and PRP groups.However,24-h following exercise a significant increase in these parameters was observed in both groups between 1 and 4 days during the recovery period.Interestingly,PRP administration decreased plasma iron levels compared to the control on the second day post-exercise.Plasma IBC increased in PRP group from Days 2 to 4 post-exercise compared to the control group whilst PRP administration had no effect on plasma ferritin,CK,AST,ALT,or LDH.
Conclusion: Acute exhaustive exercise increased muscle damage markers,including plasma iron,IBC,and ferritin levels,indicating muscle damage induced by exercise.PRP administration improves inflammation by reversing the increase in the iron levels post-exercise without displaying any myotoxicity and may have a role to play in the recovery of exercise-induced muscle damage.
©2016 Production and hosting by Elsevier B.V.on behalf of Shanghai University of Sport.
Keywords:Exercise-induced muscle damage; Ferritin; Plasma iron; Platelet rich plasma
1.Introduction
Recently platelet rich plasma (PRP),an autologous derivative of whole blood containing a supraphysiological concentration of platelets,has gained increasing popularity in both the scientific literature and the wider media for its potential application in the treatment of traumatic musculoskeletal and sports-related injuries,cancer biology,and dermatology.In addition,it has been reported that PRP administration may improve recovery from tendon and muscle injuries.1,2Biologic healing utilizes the normal mechanisms for tissue repair and incorporates these at the site of injury.Blood components such as platelets migrate to the injury site and play an important role in tissue repair.Platelets contain various growth factors and cytokines that initiate and promote healing by stimulating cell migration,cell proliferation,angiogenesis,and matrix.Other important bioactive factors released from platelets include histamine and serotonin,and these plateletgrowth factors enhance DNA synthesis,chemotaxis,and angiogenesis,increase collagen deposition,and stimulate synthesis of extracellular matrix.3
It is well established that an unaccustomed and strenuous exercise in the trained and untrained individual can induce skeletal muscle damage;4this phenomenon is commonly known as“exercise-induced muscle damage”(EIMD) and is determined by the type,intensity,and duration of exercise.5Moreover,in sports,the eccentric/concentric type of exercise has been used as a specific training model for muscle strength improvement during training sessions.However,symptoms of EIMD include reduced muscular force,increased stiffness,swelling delayed onset muscle soreness (DOMS),and an increased blood activity of muscle proteins such as creatine kinase (CK>1000 IU/L),4alanine transaminase (ALT),6aspartate transaminase (AST),6lactate dehydrogenase (LDH) activity,7and this may have a negative impact on performance.4Moreover,EIMD initiates an inflammatory response associated with secondary muscle damage and remodeling8sinceduring the acute phase,both neutrophils and phagocytic macrophages can release reactive oxygen and nitrogen species and remove debris by phagocytosis.9Moreover,recent studies have reported the levels of the ironregulatory hormone hepcidin are also increased after exercise.10-12Hepcidin is a liver-produced peptide hormone,up-regulated in response to elevated iron levels and the inflammatory cytokine interleukin-6 (IL-6),13,14and an increase in hepcidin levels usually occurs as a homeostatic response to inflammatory stimuli namely the IL-6 or elevated iron levels.13Peeling et al.11reported that inflammation,hemolysis,serum iron,ferritin,and urinary hepcidin were elevated in the high intensity interval post-running session.As such,the postexercise hepcidin response is likely to be homeostatic in nature,to help control and reduce the elevated levels of serum iron resulting from the exercise-induced hemolysis.15
Many studies have been published proposing various methods for treating DOMS,including cryotherapy,anti-inflammatory medication,stretching,hyperbaric oxygen,homeopathy,ultrasound,L-carnitine,rest,light exercise,and electromagnetic shields.16-21For example,non-steroidal anti-inflammatory drugs (NSAIDs) are routinely prescribed to alleviate EIMD-related symptoms and restore normal physical function of the muscle.22However,it has been reported that NSAIDs act by blocking Cyclooxygenase (COX) and thus they may have a detrimental effect on muscle regeneration and super-compensation.20Moreover,there is moderate to strong evidence that intramuscularly injected local anaesthetics and NSAIDs are myotoxic.The administration of PRP has also been reported to induce myotoxicity,however,the evidence is conflicting and further studies are required to confirm this as well as the possible myotoxic effects of corticosteroids.23Furthermore,clinical and histopathological studies have shown the potential myotoxicity of intramuscular injections in both animals and humans,24,25resulting in pain at the injection site and histopathological changes of inflammation,necrosis,and fibrosis.Besides histological changes,the local plasma CK concentration is the most commonly used valid marker for skeletal muscle myotoxicity.26-28There is conflicting evidence regarding the myotoxicity of intramuscular PRP injections.Two studies used an animal muscle injury model and reported increased signs of regeneration,less necrosis,and less granulomatous tissue in the muscles injected with PRP29,30andautologous conditioned serum (ACS),31than in control muscles on histological evaluation for up to 2 weeks.However,information regarding the myotoxicity of intramuscular PRP injection or the cross-talk between hematologic and biochemical response has not been reported in exercise-induced muscle damage.Therefore,we hypothesized that intramuscular PRP injection might improve inflammation and beneficial effect on DOMS and muscle damage induced by exercise without mytoxicity effects.The objective of the present study was to investigate whether the myotoxicity effects of the intramuscular PRP injection can provide an effective recovery strategy for attenuating DOMS and muscle damage induced by highintensity muscle exercise in humans.
2.Methods
2.1.Study design
Twelve moderately active male volunteers participated in this randomized double-blind placebo-controlled trial to verify the effects of the intramuscular PRP injection on hematologic,biochemical response,and mytoxicity on muscle recovery after an eccentric/concentric exercise.Subjects were randomly placed into two groups: PRP (n = 6) and control (n = 6),and they had not been involved in any regular weight-training program and had no history of injury to the arm,shoulder,and elbow region.The nature and the risks of the experimental procedures were explained to the subjects,and signed informed consent to participate in the study was obtained.Before the test session,participants were examined and checked by the use of routine blood analysis by a medically qualified practioner.Ethical approval was obtained from The Balikesir University Medical Faculty Ethics Committee (2013/14) and each participant gave written informed consent prior to the study.
2.2.Muscle damage exercise protocol
For the exercise-induced muscle damage test,subjects were seated on a bench with their arm positioned in front of their body and resting on a padded support,such that their shoulder was secured at a flexion angle of 0.79 rad (45°) and their forearm was maintained in the supinated position throughout the exercise.Subjects were repeatedly weight-loaded upon dumbbell lowering to achieve an 80% of maximum voluntary contraction (MVC),2-min rest between the sets of elbow extension from the flexed position at 90°to fully extended position slowly over 5 s,until exhaustion was experienced.The subjects were also given verbal encouragement by the investigator to maintain constant speed throughout the procedure.They were instructed to continue their normal activities and to abstain from any strenuous exercise at least 2 weeks before the experiment.Moreover,they were asked to continue their usual food intake,not to change the amount or frequencyof dietary meat and not to use any dietary supplements,antiinflammatory drugs,or anything else that could affect muscle soreness and damage until the end of the study.
2.3.Platelet-rich plasma and placebo
Each participant was assigned to either receive placebo (saline) injection or PRP injection in non-dominant arms with post-24 h DOMS exercise based on computerized randomization.PRP preparation was obtained from 8 mL of peripheral blood which was drawn from the dominant arm and the samples were centrifuged for 9 min at 3500 revolutions per minute (H-19F,RegenCentrigel,Regen ACR-C; Regen Lab,Switzerland) according to manufacturers recommendation.Subsequently,4 mL of PRP was injected using a 20-gauge needle into the pain full region of the non-dominant arm under sterile aseptic conditions.This kit produces 4 mL of PRP from 8 mL citrated blood.Therefore final platelet concentration is approximately≤2 fold over whole blood platelet concentration.Platelet recovery is reported to be>95% and a leukocyte recovery of 58%.
Venous blood samples were collected pre-,and 4 days postexercise,and analyzed for complete blood counts (WBC,RBC,Hb),serum ferritin,iron (Fe),iron binding capacity (IBC),CKLDH,AST,and ALT as markers of muscle damage and inflammation.
2.4.Hematological analysis
For analyses of serum Fe,IBC,and ferritin,blood samples were collected without any additive and after centrifugation sera were stored at-20°C until analyzed.Iron and IBC were measured spectrophotometricallyin an Advia 1800 analyzer (Siemens Healthcare,Erlangen,Germany) and ferritin was measured by immunoturbidimetric assay in an Olympus AU400 analyzer (Beckman Coulter,Brea,CA,USA).
2.5.Biochemical analysis
Following centrifugation at 825 g for 10 min,serum was analyzed for ALT,AST,CK,and LDH activities using commercially available kits in a chemistry autoanalyser (Cobas Integra 800; Roche Diagnostic GmbH; Mannheim,Germany).
2.6.Statistical analysis
All calculations were performed using SPSS software (SPSS Inc.,Chicago,IL,USA).The values of serum Fe,IBC,ferritin,ALT,AST,LDH,and CK were presented as raw values as the area under the curve (AUC) during the experimental period.The AUC was calculated as the sum of four or five trapezoid areas separated by each supplement time point.Two-way mixed model analyses of variance (2 groups×5 times) with repeated measures were used.Differences in continuous variables between groups were assessed using independent t test and between multiple points within the same group were analyzed using Student’s paired t test.Data were expressed as means±SE and the level of significance was set at p<0.05.
3.Results
There was no difference in body weight,height,age,and exercise performance had no significant differences between PRP and control (Table 1,p>0.05).The baseline values for plasma Fe,IBC,ferritin,AST,ALT,LDH,and CK values were similar between the control and the PRP administered group (p>0.05).However,at 24-h following exercise,the plasma Fe,IBC,AST,ALT,LDH,and CK values significantly increased in the control and the PRP administered group on Days 1-4 postexercise muscle damage (p<0.01,Fig.1).Acute exhaustive muscle exercise also increased the plasma ferritin levels in control and PRP group (p<0.01 and p<0.05,respectively,Fig.1C).Interestingly,PRP administration decreased plasma iron levels (p = 0.002) compared to the control group but this was only observed on the Days 2 and 3 post-exercise-induced muscle damage (Fig.1A).Moreover,plasma IBC levels were increased in PRP group from Days 1 to 4 post-exercise compared to control group (p<0.05,Fig.1B).In contrast,PRP administration had no effect on plasma ferritin,AST,ALT,LDH,and CK levels (Fig.1C-G).
4.Discussion
In this paper we report the effect of single dose of intramuscular PRP on Fe levels in exercise-induced muscle damage.This was a pilot study in which six subjects participated in the control group and test group,respectively.Acute exhaustive exercise increased muscle damage markers,including plasma Fe,IBC,and ferritin levels were increased confirming exerciseinduced muscle damage.PRP administration resulted in improved muscle recovery from injury without displaying any myotoxicity.Many methods have been utilised for the treatment of DOMS,including cryotherapy,anti-inflammatory medication,stretching,hyperbaric oxygen,homeopathy,ultrasound,L-carnitine,rest,light exercise,and electromagnetic shields.16-19Inflammatory conditions have been essentially treated by the use of non-steroidal anti-inflammatory drugs (NSAIDs) although they are ineffective in reducing muscle pain and do not increase muscle performance during DOMS.20-22,32-34As an alternative to conventional treatments,platelet-rich therapy has been applied due to its potential in accelerating muscle healing and reducing a player’s injury time.As far as we are aware,this study is the first to examine the effect of intramuscular PRP administration on DOMS and muscle damage markers also post-exerciseinduced muscle damage during the recovery period in healthy human volunteers.Importantly,our results show that the acuteexhaustive muscle exercise increased the plasma Fe,IBC,and ferritin level in both groups.Our findings on plasma Fe,IBC,and ferritin response to acute exhaustive exercise are in agreement with previous reports10-13which also reported an increase in serum Fe,IBC,and ferritin levels linked to the intensity of the exercise.It seems the increase in serum ferritin levels also led to an increase in plasma iron and hepcidin levels.A majority of publications have reported an elevated hepcidin levels 24 h post-exercise,preceded by acute increase in serum Fe and inflammation parameters.10-13,35However,we did not measure the post-exercise hepcidin levels in this study.The present study indicates that post-exercise serum Fe,IBC,and ferritin levels are induced as a result of inflammatory response due to exhaustive muscle exercise.Speculatively,acute exhaustive exercise increased the free Fe that enters the plasma and has a reduction-oxidation (redox) potential,which may promote free radical formation as a result of Fenton and Harber-Weiss reactions and can result in oxidative damage to tissues.36Interestingly,in this study it was observed that PRP administration reversed the observed increase in plasma Fe level due to muscle damage 2-3 days post-exercise.Furthermore,plasma IBC levels were up-regulated in PRP group from Days 1 to 4 post-exercise.On the other hand,PRP administration depressed the plasma ferritin levels during the recovery phase compared to control values,but did not reach statistical significance.These results are novel and to the best of our knowledge,no data exists concerning the acute effect of intramuscular PRP administration on plasma Fe,IBC,and ferritin levels parameters during recovery period in an acute exercise-induced muscle damage model.In general,related studies have reported that PRP treatment has anti-inflammatory properties through its effects on the canonical nuclear factor κB signalling pathway in multiple cell types including synoviocytes,macrophages,and chondrocytes.37In addition,PRP treatment has suppressed tendon cell inflammation in vitro and in vivo,marked by the upregulation of COX-1,COX-2,and mPGES-1 expression with highly PGE2 production.38Additionally,the present study demonstrated that intramuscular PRP injection plays a key role as an anti-inflammatory by suppressing effect of increased free iron in plasma during the muscle damage recovery.Evidently,we have previously shown that elbow flexors muscle strength peak torque values were improved after PRP administration when compared to the control arm,and this occurred on the same day (Day 2) when the serum Fe level declined post-exercise-induced muscle damage (Unpublished data).
Table 1The characteristics of the subjects (mean±SE).
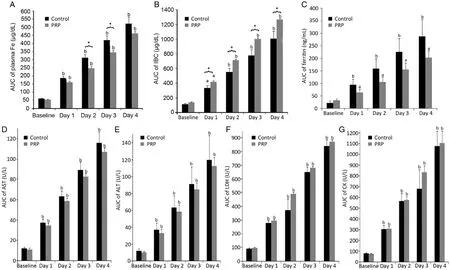
Fig.1.AUC of plasma (A) iron,(B) IBC,(C) ferritin,(D) AST,(E) ALT,(F) LDH,and (G) CK levels were calculated at baseline and on days 1,2,3,and 4 post-exercise induced muscle damage (mean±SE).ap<0.05,bp<0.01,compared with baseline analyzed by repeated measures by ANOVA.*p<0.05,significant differences between control and PRP,analyzed by independent-samples t test.AUC = area under the curve; IBC = iron binding capacity; AST = aspartate aminotransferase; ALT = alanine aminotransferase; LDH = lactate dehydrogenase; CK = creatinine kinase; PRP = platelet rich plasma.
Serum CK concentration is the most sensitive indicator of muscle damage and it begins to rise approximately 2-12 h after the exhaustive exercise.Exhaustive physical exercise increases serum enzyme activities such as CK,AST,LDH,and ALT,hence these are considered as markers for the muscular damage derived from intense exercise.39The increased activities of CK and LDH in serum after exhausted exercise could act as signals,attracting neutrophils to the damagedmuscle and initiating the inflammatory response.The maintenance of high CK activities after recovery could be and indicator of muscle repair.40-42Our result demonstrate that CK,AST,ALT,and LDH levels increased post-exercise during the DOMS period in both groups,indicating muscle damage.On the other hand,CK concentration is the most commonly used valid marker for skeletal muscle myotoxicity in the intramuscular injections.26-28Although intramuscular PRP injections are commonly used,there is only limited evidence base for myotoxicity in animals models29-31and these studies reported increased signs of regeneration of the muscle whilst necrosis and granulomatous tissue were decreased in the muscle injected with PRP when compared to the control,however,no response to CK levels was reported in these studies.29-31Limited human studies have been shown that intramuscular injection of lidocaine43and bupivacaine (20 mL)26lead to an increase in CK levels.Our findings showed that the plasma level of CK was increased in response to exhaustive exercise,however,PRP administration did not alter CK levels in the PRP group compared to control.Hence,intramuscular PRP injection did not show mytoxicity in exercise-induced model.
5.Conclusion
Our results indicate that acute exhaustive exercise increases muscle damage markers,including plasma Fe,IBC,and ferritin levels.PRP administration improved the inflammatory resoponse by reversing the observed increase in Fe levels and may have a role to play in the recovery of exercise-induced muscle damage.Evidently,intramuscular PRP injection had no effect on CK levels,indicating that it is not myotoxic.
Acknowledgment
The authors would like to thank the volunteers who took part in this study.
Authors’contributions
ZP designed the study,performed the study data and statistical analysis drafted the manuscript; OO conceived of the study,and participated in its design and coordination; NO applied the intra-muscular injection; KR edited the manuscript language and helped to draft the manuscript; RV participated to design study and coordination.All authors have read and approved the final version of the manuscript,and agree with the order of presentation of the authors.
Competing interests
None of the authors declare competing financial interests.No other organization or institution co-financed this research.
References
1.Gosens T,Peerbooms JC,van Laar W,den Oudsten BL.Ongoing positive effect of platelet-rich plasma vs.corticosteroid injection in lateral epicondylitis: a double-blind randomized controlled trial with 2-year follow-up.Am J Sports Med 2011;39:1200-8.
2.Peerbooms JC,Sluimer J,Bruijn DJ,Gosens T.Positive effect of an autologous platelet concentrate in lateral epicondylitis in a doubleblind randomized controlled trial: platelet-rich plasma versus corticosteroid injection with a 1-year follow-up.Am J Sports Med 2010;38: 255-62.
3.Grotendorst GR,Martin GR,Pencev D,Sodek J,Harvey AK.Stimulation of granulation-tissue formation by platelet-derived growth-factor in normal and diabetic rats.J Clin Invest 1985;76:2323-9.
4.Allen DG.Eccentric muscle damage: mechanisms of early reduction of force.Acta Physiol Scand 2001;171:311-9.
5.Malm C.Exercise-induced muscle damage and inflammation: fact or fiction? Acta Physiol Scand 2001;171:233-9.
6.Ahmetov II,Naumov VA,Donnikov AE,Maciejewska-Karłowska A,Kostryukova ES,Larin AK,et al.SOD2 gene polymorphism and muscle damage markers in elite athletes.Free Radic Res 2014;28:1-8.
7.Córdova MA,Martorell PM,Sureda GA,Tur Marí JA,Pons BA.Changes in circulating cytokines and markers of muscle damage in elite cyclists during a multi-stage competition.Clin Physiol Funct Imaging 2014;doi:10 .1111/cpf.12170,[Epub ahead of print].
8.Clarkson PM,Hubal MJ.Exercise-induced muscle damage in humans.Am J Phys Med Rehabil 2002;81(Suppl.11):S52-69.
9.Tidball JG,Villalta SA.Regulatory interactions between muscle and the immune system during muscle regeneration.Am J Physiol Regul Integr Comp Physiol 2010;298:1173-87.
10.Peeling P,Dawson B,Goodman C,Landers G,Wiegerinck ET.Training surface and intensity: inflammation,hemolysis,and hepcidin expression.Med Sci Sports Exerc 2009;41:1138-45.
11.Peeling P,Dawson B,Goodman C,Landers G,Wiegerinck ET,Swinkels DW,etal.Cummulative effects of consecutive running session on hemolysis,inflammation and hepcidin activity.Eur J Appl Physiol 2009; 106:51-9.
12.Peeling P,Dawson B,Goodman C,Landers G,Wiegerinck ET,Swinkels DW,et al.Effects of exercise on hepcidin response and iron metabolism during recovery.Int J Sport Nutr Exerc Metab 2009;19:583-97.
13.Nemeth E,Rivera S,Gabayan V,Keller C,Taudorf S,Pedersen BK,et al.IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin.J Clin Invest 2004;113: 1271-6.
14.Kemna E,Pickkers P,Nemeth E,van der Hoeven H,Swinkels D.Time-course analysis of hepcidin,serum iron,and plasma cytokine levels in humans injected with LPS.Blood 2005;106:1864-6.
15.Nemeth E,Tuttle MS,Powelson J,Vaughn MB,Ward DM,Ganz T,et al.Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalisation.Science 2004;306:2090-3.
16.Clarkson PM,Sayers SP.Etiology of exercise-induced muscle damage.Can J Appl Physiol 1999;24:234-48.
17.Cleak M,Eston R.Muscle soreness,swelling,stiffness and strength loss after intense eccentric exercise.Br J Sports Med 1992;26:267-72.
18.Cheung KH,Patria A,Maxwell L.Delayed onset muscle soreness: treatment strategies and performance factors.Sports Med 2003;33: 145-64.
19.Almekinders LC.Anti-inflammatory treatment of muscular injuries in sport.An update of recent studies.Sports Med 1999;28:383-8.
20.Paulsen G,Egner IM,Drange M,Langberg H,Benestad HB,Fjeld JG,et al.A COX-2 inhibitor reduces muscle soreness,but does not influence recoveryand adaptation after eccentric exercise.Scand J Med Sci Sports 2010;20:195-207.
21.Zhang J,Clement D,Taunton J.The efficacy of Farabloc,an electromagnetic shield,inattenuating delayed-onset muscle soreness.Clin J Sport Med 2000;10:15-21.
22.Schonfeld BJ.The use of nonsteroidal anti-inflammatory drugs for exercise-induced muscle damage: implications for skeletal muscle development.Sports Med 2012;42:1017-28.
23.Reuink G,Goudswaard GJ,Moen MH,Weir A,Verhaar JA,Tol JL.Myotoxicity of injections for acute muscle injuries: a systematic review.Sports Med 2014;44:943-56.
24.Zink W,Graf BM.Local anesthetic myotoxicity.Reg Anesth Pain Med 2004;29:333-40.
25.Blom L,Rasmussen F.Tissue damage at the infection site after intramuscular injection of drugs in hens.Br Poult Sci 1976;17: 1-4.
26.Nosaka K,Sakamoto K.Changes in plasma enzyme activity after intramuscular injection of bupivacaine into the human biceps brachii.Acta Physiol Scand 1999;167:259-65.
27.Diness V.Local tissue damage after intramuscular injections in rabbits and pigs: quantitation by determination of creatine kinase activity at injection sites.Acta Pharmacol Toxicol 1985;56:410-5.
28.Nyska A,Skolnick M,Ziv G,Gulkarov A.Correlation of injection site damage and serum creatine kinase activity in turkeys following intramuscular and subcutaneous administration of norfloxacin nicotinate.Avian Pathol 1994;23:671-82.
29.Hammond JW,Hinton RY,Curl LA,Muriel JM,Lovering RM.Use of autologous platelet-rich plasma to treat muscle strain injuries.Am J Sports Med 2009;37:1135-42.
30.Harris NL,Huffer WE,von Stade E,Larson AI,Phinney S,Purnell ML.The effect of platelet rich plasma on normal soft tissues in the rabbit.J Bone Jt Surg Am 2012;94:786-93.
31.Wright-Carpenter T,Opolon P,Appell HJ,Meijer H,Wehling P,Mir LM.Treatment of muscle injuries by local administration of autologous conditioned serum: animal experiments using a muscle contusion model.Int J Sports Med 2004;25:582-7.
32.Semark A,Noakes TD,St Clair Gibson A,Lambert MI.The effect of a prophylactic dose of flurbiprofen on muscle soreness and sprinting performance in trained subjects.J Sports Sci 1999;17: 197-203.
33.Kuipers H,Keizer HA,Verstappen FT,Costill DL.Influence of a prostaglandin-inhibiting drug on muscle soreness after eccentric work.Int J Sports Med 1985;6:336-9.
34.Bourgeois J,MacDougall D,MacDonald J,Tarnopolsky M.Naproxen does not alter indices of muscle damage in resistance-exercise trained men.Med Sci Sports Exerc 1999;31:4-9.
35.Peeling P,Sim M,Badenhorst CE,Dawson B,Govus AD,Abbiss CR,et al.Iron status and the acute post-exercise hepcidin response in athletes.Plos One 2014;9:e93002.doi:10.1371/journal.pone.0093002
36.Takami T,Sakaida I.Iron regulation by hepatocytes and free radicals.J Clin Biochem Nutr 2011;48:103-6.
37.Andia I,Maffulli N.Platelet-rich plasma for managing pain and inflammation in osteoarthritis.Nat Rev Rheumatol 2013;9:721-30.
38.Zhang J,Middleton KK,Fu FH,Im HJ,Wang JH.HGF mediates the anti inflammatory effects of PRP on injured tendons.PLoS One 2013;8:e67303.doi:10.1371/journal.pone.0067303
39.Kim H,Lee Y.Biomarkers of muscle and cartilage damage and inflammation during a 200 km run.Eur J Appl Physiol 2007;99:443-7.
40.Butterfield TA,Best TM,Merrick MA.The dual roles of neutrophils and macrophages in inflammation: a critical balance between tissue damage and repair.J Athl Train 2006;41:457-65.
41.Sureda A,Tauler P.Antioxidant supplementation influences the neutrophil tocopherol associated protein expression; but not the inflammatory response to exercise.Cent Eur J Biol 2007;2:56-70.
42.Evans WJ.Vitamin E,vitamin C,and exercise.Am J Clin Nutr 2000;72:647-52.
43.Sauerwein HP,Brouwer T,Dunning AJ.Creatine phosphokinase,myocardial infarction and intramuscular injection.Ned Tijdschr Geneeskd 1975;119:1399-402.
杂志排行
Journal of Sport and Health Science的其它文章
- Non-linearity in the dynamic world of human movement
- Comparing dynamical systems concepts and techniques for biomechanical analysis
- Multi-scale interactions in interpersonal coordination
- Can coordination variability identify performance factors and skill level in competitive sport? The case of race walking
- Multiscale entropy: A tool for understanding the complexity of postural control
- A history of low back pain affects pelvis and trunk coordination during a sustained manual materials handling task
