Multiscale entropy: A tool for understanding the complexity of postural control
2016-04-25MichaelBusaRichardvanEmmerikDepartmentofKinesiologyUniversityofMassachusettsAmherstAmherstMA01003USAReceivedMarch2015revised19June2015accepted20November2015Availableonline21January2016
Michael A.Busa *,Richard E.A.van EmmerikDepartment of Kinesiology,University of Massachusetts Amherst,Amherst,MA 01003,USA Received 3 March 2015; revised 19 June 2015; accepted 20 November 2015 Available online 21 January 2016
Multiscale entropy: A tool for understanding the complexity of postural control
Michael A.Busa *,Richard E.A.van Emmerik
Department of Kinesiology,University of Massachusetts Amherst,Amherst,MA 01003,USA Received 3 March 2015; revised 19 June 2015; accepted 20 November 2015 Available online 21 January 2016
Peer review under responsibility of Shanghai University of Sport.
* Corresponding author.
E-mail address: mbusa@kin.umass.edu (M.A.Busa)
http://dx.doi.org/10.1016/j.jshs.2016.01.018
2095-2546/©2016 Production and hosting by Elsevier B.V.on behalf of Shanghai University of Sport.
Abstract
Clinical disorders often are characterized by a breakdown in dynamical processes that contribute to the control of upright standing.Disruption to a large number of physiological processes operating at different time scales can lead to alterations in postural center of pressure (CoP) fluctuations.Multiscale entropy (MSE) has been used to identify differences in fluctuations of postural CoP time series between groups with and without known physiological impairments at multiple time scales.The purpose of this paper is to: 1) review basic elements and current developments in entropy techniques used to assess physiological complexity; and 2) identify how MSE can provide insights into the complexity of physiological systems operating at multiple time scales that underlie the control of posture.We review and synthesize evidence from the literature providing support for MSE as a valuable tool to evaluate the breakdown in the physiological processes that accompany changes due to aging and disease in postural control.This evidence emerges from observed lower MSE values in individuals with multiple sclerosis,idiopathic scoliosis,and in older individuals with sensory impairments.Finally,we suggest some future applications of MSE that will allow for further insight into how physiological deficits impact the complexity of postural fluctuations; this information may improve the development and evaluation of new therapeutic interventions.
©2016 Production and hosting by Elsevier B.V.on behalf of Shanghai University of Sport.
Keywords:Aging; Movement disabilities; Multiscale entropy; Postural control; Sensory loss
1.Introduction
The human body consists of a large network of interconnected structures,operating at a range of time scales.1,2In this paper we discuss how entropy techniques,multiscale entropy (MSE) in particular,have been used to quantify how disease and age related breakdowns in physiological processes impact postural fluctuations.Over the past 2 decades,entropy techniques based on the principles of information theory have been introduced to the study of postural control to quantify how individuals regulate their postural fluctuations.3The rationale behind the use of entropy techniques,which effectively quantify the probability that neighboring points in a time series will be within a predetermined range,is that healthy systems display dynamics indicative of a highly adaptable network of neuromuscular connections.Entropy measures have been used to estimate the amount of“complexity”3,4in a physiological system,whereby increases in entropy values are indicative of a system exhibiting a greater degree of complex dynamics.4,5
Analysis of entropy allows researchers to quantify how changes in physiological health impact the regulation of postural fluctuations.The multiscale entropy measure differs from previous entropy techniques (sample entropy (SE) and approximate entropy (ApEn)) by including multiple time scales of measurement.The inclusion of these multiple measurements allows for two distinct advantages: 1) the assessment of complexity (the SEvalue) at shorter and longer time scales,and 2) the quantification of the overall complexity of a system,calculated as the sum of the entropy values over all of the individual time scales.The combination of these features allows researchers to identify the time scales at which the breakdown in complexity occurs as well the overall complexity that takes all of the time scales into account.
The MSE technique enables researchers to assess how disease impacts postural fluctuations across a range of time scales that mirror the temporal dynamics of the complex network of structures (molecular,cellular,tissue,organ,organ system,and organism),each of which exhibits dynamics at adiverse range of time scales.6The dynamic interactions within and between physiological levels of the system afford the flexibility to tune outputs to the environment,through the perception-action cycle.7,8Additionally,it is this rich array of physiological interactions,operating at different time scales,that forms the basis of goal-directed movements that are stable and adaptable across a wide range of environmental conditions.Importantly,examination of physiological fluctuations at only one time scale limits the scope of interpretation to only that level and does not fully capture the dynamics of the entire system; for example,examining heart rate dynamics at only one time scale results in findings that are misleading with respect to the manner in which disease impacts the cardiac rhythm.9
Changes in the system resulting from aging and disease are not isolated to one time scale,as dysfunction at a lower level (e.g.,molecular,cellular,tissue) likely has a cascading effect on processes that occur at higher levels (e.g.,system and organism).Characteristically,lower-level processes occur at short time scales and represent interactions at the micro-scale,while higher-level (macro-scale) processes occur at longer time scales and represent the integration of these lower-level processes.While up to this point the multiscale approach has been focused on identifying how physiological changes impact the overall complexity of physiological processes,this approach also affords researchers the ability to investigate the individual time scales and may provide insight as to how particular physiological deficits impact postural control.The identification of specific time scales at which postural deficits occur may allow for the tailoring of interventions as well as the evaluation of these efforts.
Studies of human movement often make observations at the organismic level and assess the kinematic or kinetic mean and variability of these“task space”variables.These results often are interpreted with respect to the physiological processes that are thought to underlie these differences; however,these measures often focus on only one time scale and therefore lack the ability to assess the cascading impact of physiological dysfunction on the control and coordination of action patterns.Therefore,techniques that examine the multiscale temporal fluctuations of biological signals can provide additional insight into the way in which these levels of the system are related,and the organization of human movement from a complex/dynamical systems perspective.
Lipsitz and Goldberger3,4put forward the“loss of complexity hypothesis”as a framework to assess how the breakdown in physiological function,associated with disease and aging,is brought about by a reduction in the capacity of the complex network of interactions involved in the regulation of physiological outputs.This hypothesis suggests that deteriorations in health arise from a reduced capacity of the system to produce an adaptable set of solutions to execute tasks (Fig.1).Specifically,impairments are thought to manifest through either a breakdown in the dynamical processes between levels of the system,or by a reduction in the number of elements within a given level of the system; for example,multiple sclerosis (MS) results in the breakdown of the myelin sheath surrounding neurons of the central nervous system (CNS),resulting in a myriad of symptoms ranging from sensorimotor to cognitive.10Changes in physiological processes that accompany disease and aging can impact this system across multiple levels; identifying the relationship between the physiological deficit and the time scales at which they impact postural control will provide critical information for evaluating both disease severity and the effectiveness of treatments.
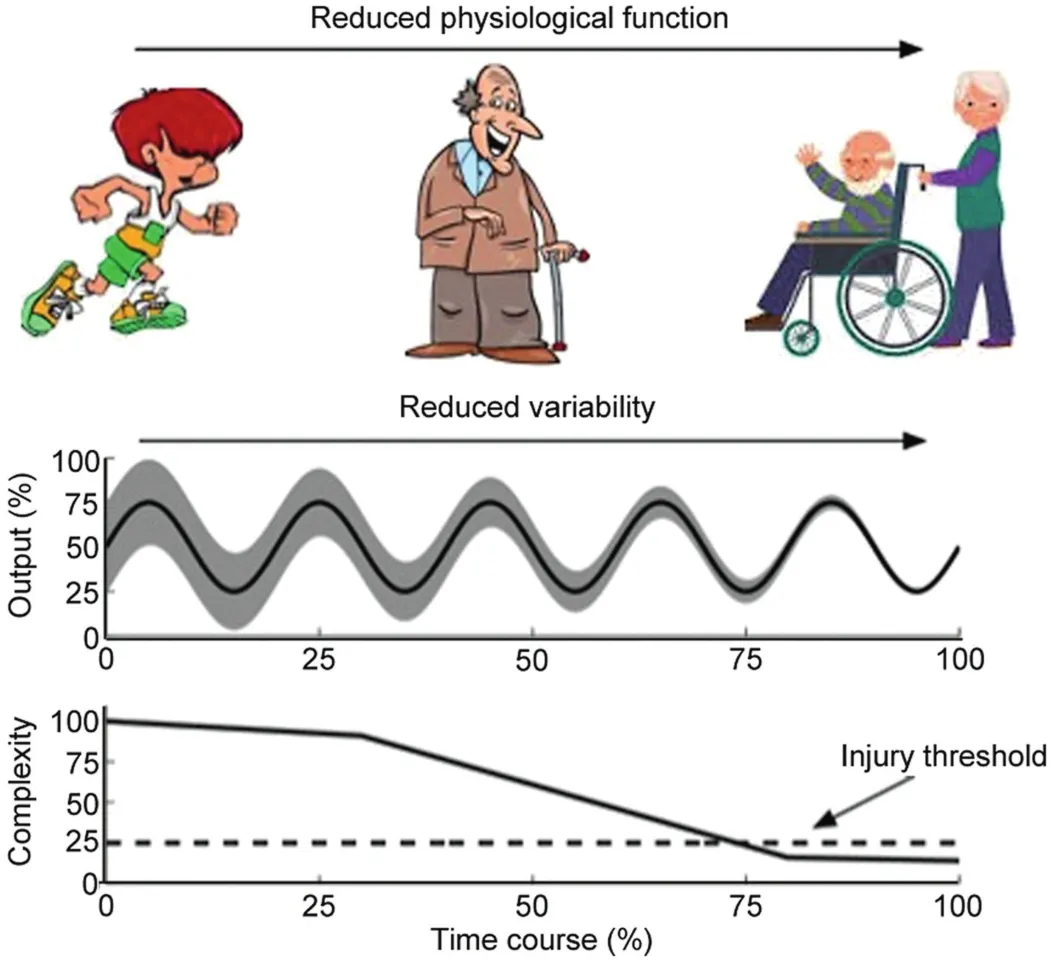
Fig.1.The relationship between physiological function,variability,and complexity.As physiological function deteriorates,interactions among elements in the system break down and variability is reduced,that manifest in lower overall complexity.
It is important to recognize,as noted by Duarte and Sternad,8that advanced age status in the absence of physiological deficit does not appear to impact the complexity of postural fluctuations.Furthermore,it should be noted that evidence supporting the loss of complexity hypothesis has come from measurements during baseline tasks,e.g.,quiet standing postures.Research into how the complexity of postural fluctuations is impacted during the performance of a greater array of ecologically relevant tasks,e.g.,postural-manual,is necessary to further elucidate the impact of health status on the complexity of postural fluctuations.It is entirely plausible that differences in the ability to perform tasks at the same level may confound the observed differences in complexity; future experiments should standardize task performance in a way that allows for the identification of how the complexity of postural fluctuations can be used for functional purposes,i.e.,afford a robust array of potential postural strategies that can be used to mitigate the effects of a perturbation.
The purpose of this paper is to: 1) review basic elements and current developments in using entropy to assess physiological complexity,and 2) demonstrate how the MSE technique has been used to gain insight into the changes in the complexity of postural sway due to disease and age related physiological changes.We conclude this paper with some suggestions for theexpanded use of MSE that may be useful for identifying how specific changes in physiological function manifest in changes in postural control.To fully understand why MSE can provide insights into systemic complexity that other entropy techniques do not,we must first identify the salient differences between these methods.Recently,there has been an increased interest in identifying how the complexity of physiological signals changes along with health and age status.In the pursuit of this goal several entropy measures have been developed,all of which have similar intentions: identify the nature of point-topoint fluctuations in a signal.The root of this approach can be found in Claude Shannon’s research on identifying information content in signals9,10and expanded into discrete signals in the form of Kolmogorov-Sinai Entropy.11,12
2.Overview of entropy techniques
2.1.ApEn
ApEn13was derived from the basic tenets of the Kolmogorov-Sinai Entropy12for signals that included both meaningful information as well as noise (Eq.1)
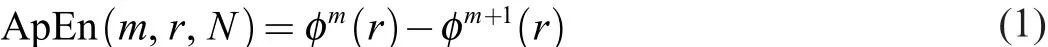
where m is the distance between time series points to be compared,r is the radius of similarity,N is the length of the time series,and ϕ is the probability that points m distance apart would be within the distance r.The purpose for the vector comparison term,r,is to identify a meaningful range in which fluctuations are to be considered similar.This technique has been used over the past 2 decades with the intent of identifying how disease states alter the regularity of physiological outputs.Pincus13noted that entropy values between populations should only be compared if they are calculated with fixed r and m values,as changing these parameters can affect the outcomes of the calculation; this is a practice that should be carried through any of the entropy calculations discussed here.While this technique has received a large amount of attention in the literature,it has been replaced by the SEtechnique,which has shown increased sensitivity and reliability brought about by eliminating the self-matching bias present in the ApEn algorithm.14-16
2.2.SE
SEprovides a technical improvement over that of the ApEn algorithm by eliminating the self-matching bias that is present in ApEn.This change is accomplished by altering the way in which the m and m + 1 windows are compared (Eq.2)

where m,r,N,and ϕ retain their meaning from Eq.(1).By eliminating a vector being compared to itself,SEhas demonstrated to be more reliable than the ApEn metric at identifying changes in the point-to-point fluctuations in naturally occurring physiological signals,e.g.,cardiac rhythms,and postural control.14,16The observed improvements in statistical reliability produced by the SEcalculation allow for consistent inferences to be made about the nature of point-to-point fluctuations over a single time scale.It should be noted that the SEmeasure requires stationary data,that is,time series where the local mean does not differ from the global mean; this requirement limits the type of signals that are appropriate for analysis by this technique.As statistical stationarity is required,it would be wise to select signals that occur under physiologically stationary conditions; that is,analyzing tasks that occur under a fixed set (e.g.,steady state) of demands at the level in which the observations are made.
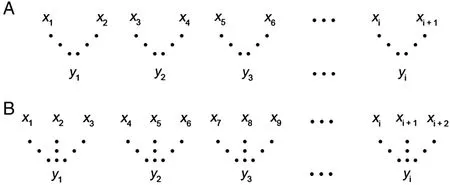
Fig.2.Coarse graining procedure.(A) scale 2,(B) scale 3,where the“x”series is the original time series and the“y”is the new time series constructed through an averaging of the data points.
2.3.Control entropy (CE)
To overcome the requirement of stationarity,Bollt and colleagues17-21introduced the CEmeasure to quantify changes in physiological complexity in non-stationary data.17-21This is accomplished by examining point-to-point fluctuations over short overlapping portions of the time series,with the assumption that the time series is stationary or near-stationary within these windows.This procedure yields a new time series,and CEhas been used to examine differences due to altered physiological requirements.For example,systematically increasing running speed leads to a reduction in complexity of the center of mass accelerations.19,20As with the other entropy measures,CEonly examines fluctuations at one time scale,thereby rendering it unable to examine changes in fluctuations at a level different than the specified window length m.
2.4.MSE
MSE builds on the SEtechnique by integrating a coarse graining procedure (Eq.3,Fig.2) which affords insight into the point-to-point fluctuations over a range of time scales
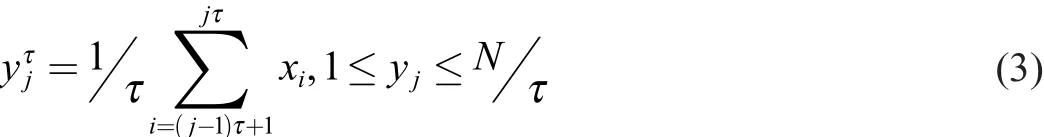
where τ is the timescale of interest,yjis a data point in the newly constructed time series,xiis a data point in the original time series and N is the length of the original time series.MSE uses the SEalgorithm to calculate an entropy value at each time scale.A major concern when applying the MSE algorithm(s) is to ensure that there are enough data points at the longest(largest) time scale,as too few values at a time scale yield unreliable probabilities from the SE.It has been suggested that 200 data points per window are needed to elicit consistent SEvalues,18though many studies have used 600 data points at the longest time scale.22-24We suggest performing a sensitivity analysis for each data set in order to identify the appropriate amount of data necessary to receive consistent SEvalues.
Once the time scales of interest (range) have been identified,the area under the SEvs.time scale curve,known as the complexity index (CI),can be calculated (Eq.4).

where SEis the SEvalue at time scale i and N is the total number of time scales used to calculate CI.CIprovides insight into the integrated complexity of the system,over the time scales of interest.Additionally,if it were thought that differences between the populations in question arise at specific time scales,MSE would allow for analysis at specific time scales by comparing the SEvalue at any specific level.This analysis allows identification how aging and disease impact the complexity of physiological outputs,which occur across multiple time scales.This information can then be used to compare health states and evaluate the impact of interventions.A recent review by Gow et al.,22focusing primarily on methodological choices of the experiments which have used MSE up to this point and makes recommendations for future study design,provides a valuable resource to consider when designing studies that will use MSE analysis.
2.5.Entropy and the loss of complexity hypothesis
Now that we have identified each of the entropy measures and their relationship to one another,it is important to identify how the information gained from these techniques can be used to identify meaningful changes in the fluctuations of postural dynamics.Furthermore,it is pertinent to contextualize the expected pattern of results within a conceptual understanding of how the multiscale variability in a signal can be useful in identifying the health of a system.The measures of entropy identified above all attempt to identify the probability of pointto-point fluctuations in a time series falling within a specific range r.Time-series that exhibit a high degree of regularity would yield a low entropy value; whereas a more freely fluctuating,complex signal would generate higher entropy values.Relating these values to the health status can be done within the loss of complexity hypothesis,3,4where a reduction in the dynamic interactions of a complex network of physiological pathways is predicted to lead to reduced point-to-point fluctuations and entropy,suggesting a reduced adaptive capacity.It should be noted that the signals examined within this framework should be free of special constraints,i.e.,functioning near their limits; this is why signals like resting heart beat interval and postural sway during quiet standing are nicely suited for these explorations.
3.Applications of MSE
We will now demonstrate the utility of the MSE technique to identify changes in postural fluctuations due to aging and disease.The focus of this section will be part tutorial and part review of the existing literature of how MSE can be used to identify dynamical changes in postural sway due to altered physiological function.Specifically,we will discuss evidence from the literature where the MSE technique has been used to identify changes in the complexity of postural fluctuations among cohorts of individuals with: multiple sclerosis,23adolescent idiopathic scoliosis,24and age related changes in sensory function.25,26Finally,we will discuss how MSE can be used as a method for evaluating the efficacy of postural aids.27
3.1.MSE analysis of postural changes in multiple sclerosis
In a recent study we examined the changes in postural dynamics in individuals with mild-to-moderate MS compared to age-matched healthy controls without neurological impairment.23Our aim was to identify if those with MS attempt to overcome impairments in cutaneous sensation by relying more on visual information for the control of upright standing.To pursue these questions we had individuals stand in a variety of postures,including maximum leans in both the anteriorposterior and medial-lateral direction,in addition to standing upright.For the purpose of this paper we will only focus on the upright quiet standing results,as this condition is frequently used in other reports that use MSE to investigate the control of posture.25-27In our previous investigation individuals stood quietly on a force platform for 25 s in two visual conditions,eyes-open and eyes-closed,while center of pressure (CoP) position was collected at 100 Hz.
In both conditions the time series of the CoP position was band pass filtered with cutoff frequencies of 2 Hz and 20 Hz,with a fourth-order,zero-lag Butterworth filter.This choice was made in order to remove any non-stationarities from the data.As 2500 data points were available for the MSE analysis,we calculated the SEvalue for 10 time scales to assess fluctuations occurring at a range of 33 to 330 ms,using an r of 15% of the standard deviation of the time series,and an m of 2.This range is computed by dividing the sampling rate (100 Hz) by the m + 1 term in Eq.(2) at each time scale,which in this case results in a denominator of 3.
The results of this experiment showed that the presence of MS had a strong effect of reducing CIin both the anteriorposterior and medio-lateral direction (Cohen effects sizes d = 0.9 and 0.8,respectively).Beyond the differences in overall complexity,it is apparent that even at very short time scales,individuals with MS display reduced complexity compared to those without neurological impairment (Fig.3).These differences in CIprovide insight into the nature of the changes that occur due to MS,that is,a physiological breakdown in neuronal function likely impacts processes that occur at very short time scales yet appear to have a widespread,systemic impact on the control of the postural CoP.The information gained from the MSE technique allows for additional insight into the nature of change due to MS,in a manner that is not apparent from singletime scale techniques.The MSE analysis shows that even in a cohort of individuals with MS who are not yet limited by significant balance impairment and show mild-to-moderate MS symptoms,postural dynamics are affected across a range of different time scales (Fig.3).
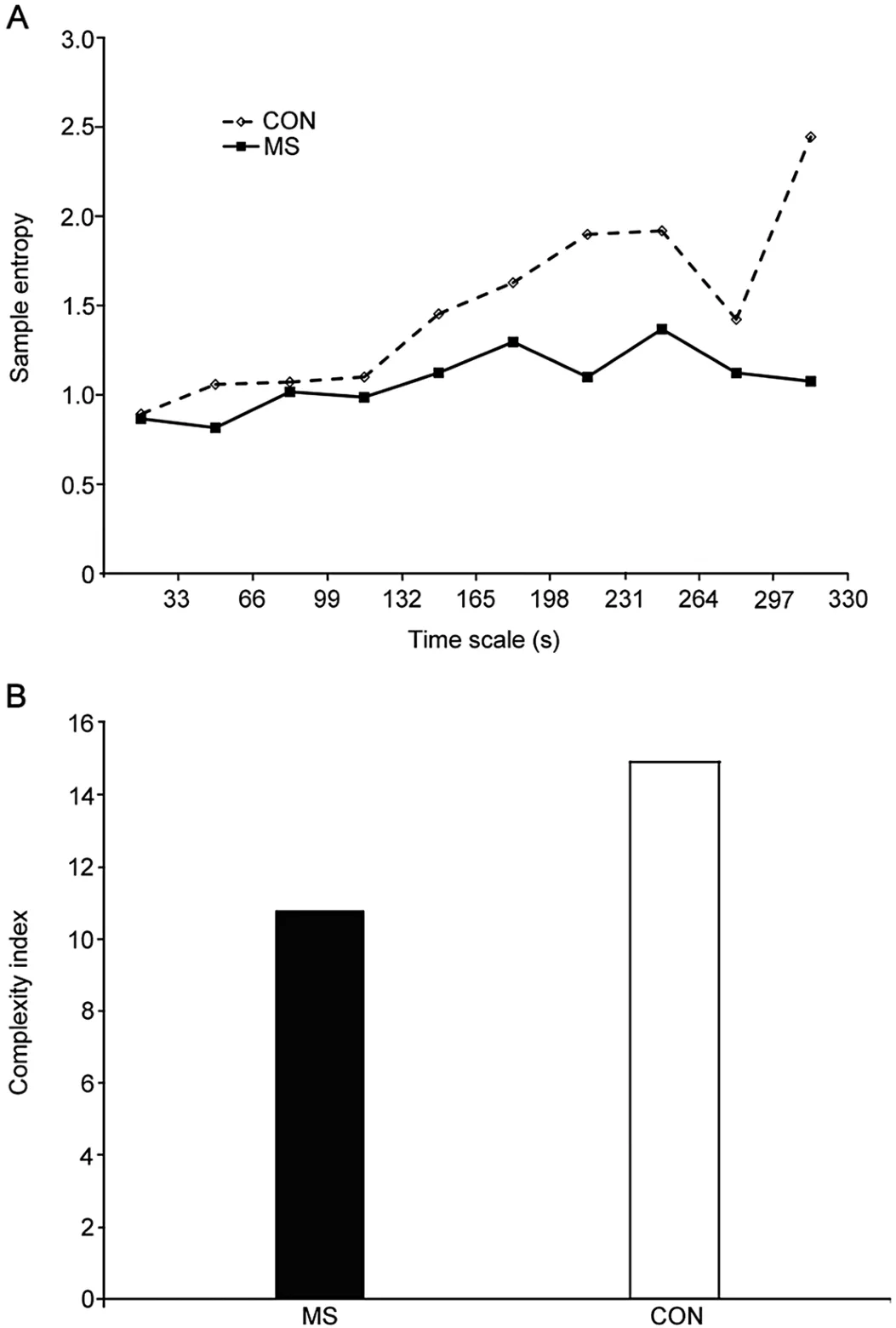
Fig.3.Plots from representative individuals with (MS) and without (CON) multiple sclerosis.(A) Sample entropy and (B) Complexity index from a 30-s trial where sample entropy was calculated for 10 time scales.
Furthermore,we used MSE to explore the role of vision in the control of upright standing in people with MS compared to controls.We observed that those with and without MS did not differ in their responses to the removal of vision (Cohen’s d = 0.1 and 0.3,for the anterior-posterior and medio-lateral fluctuations,respectively).23These results suggest that vision plays similar roles in the regulation of the point-to-point fluctuations and adaptability of the postural CoP in individuals with mild-to-moderate MS compared to healthy controls.Whether or not this is observed in more severely impacted people with MS needs further investigation.
3.2.Adolescent idiopathic scoliosis
We have also used MSE to identify how the presence and progression of adolescent iodiopathic scoliosis (AIS) influences the control of upright standing.24In this study we examined (1) if individuals with AIS display altered postural dynamics compared to those without AIS,and (2) whether two clinically distinct subgroups of individuals of AIS (prebracing and preoperative) differed in the complexity of their CoP fluctuations.
In this study the MSE parameters we used were similar to those in the previously discussed study,with the same m and r values; however,in this study we only applied a 20-Hz lowpass filter,as detrended fluctuation analysis provided evidence that the data sets were stationary.Analysis of both the anteriorposterior and medio-lateral CoP position time series revealed distinct differences between the groups.The CIof the mediallateral CoP fluctuations was reduced in those with AIS compared to those withoutAIS.The CIof the anterior-posterior CoP also revealed differences between the AIS subgroups,with the more severe preoperative group exhibiting lower complexity than the prebracing group.
This study showed that the MSE analysis was able to identify systematic differences between those with and without AIS as well as between groups with different AIS severity,while other techniques did not (e.g.,CoP range,velocity,acceleration,sway area,and mean time-to-boundary).These results suggest that both directions of postural sway provide evidence that the progression of AIS impacts the complexity of postural sway.24Medio-lateral CoP fluctuations were able to distinguish between those with and without AIS,while the anteriorposterior fluctuations were able to distinguish between clinically distinct AIS subgroups.These changes in CIalign with the loss of complexity hypothesis in that individuals with AIS exhibit reductions in medio-lateral complexity,while those with more severe AIS displayed reduced postural complexity in the anterior-posterior direction.
3.3.Examples from the aging literature
MSE has also been used to examine the effects of aging on the complexity of postural control.Several studies have used MSE to explore the effect of aging on the control of upright standing,8,25,26as well as the impact of subsensory stochastic resonance vibrations to the feet for the improvement of postural control in older individuals.27It appears that the complexity of postural fluctuations between groups of unimpaired young and older individuals is not different,as there were no differences inAdditionally,the results from Costa et al.27suggested that MSE is able to differentiate between older individuals with and without a history of falls.The evidence reported in these studies suggests that changes in physiological function that emerge as a part of the aging process contribute to the complexity of postural fluctuations along a continuum such that increased impairments leads to greater reductions in complexity (Fig.1).
Age related physiological changes in vision and somatosensation may also impact postural complexity,and allow for insight into how perceptual information impacts the regulation of postural fluctuations.25,26Manor et al.25provided evidence that age related decline(s) in single and multiple sensory modalities impact the complexity of postural fluctuations among older individuals.Specifically,the complexity of postural fluctuations is reduced among groups of olderindividuals with visual and/or somatosensory impairments,with deficits in somatosensation appearing to have a greater impact than vision on the complexity of postural control.Furthermore,the cohort of individuals with concurrent visual and somatosensory impairment demonstrated lower complexity in their postural fluctuations than the groups with deficits to one or none of these sensory modalities,suggesting that among older individuals,functional deficits in multiple sensory modalities likely contribute to the increased incidence of falls.23
Postural tasks rarely take place in isolation and are often integrated with manual or cognitive tasks during activities of daily living.28Dual-task paradigms combining cognitive and postural elements can serve as a model for eliciting stress in the system and allow for insight into how postural control processes are integrated in order to produce complex and adaptable responses.Among older individuals,those who display frailty and pre-frailty symptoms29exhibit reduced postural complexity compared to non-frail older individuals; this pattern is observed in both baseline (postural task only) and stressed (postural and cognitive tasks) conditions.26Further evidence suggests that those with greater baseline reductions in the complexity of postural control fluctuations,due to impairments in somatosensation,vision,or both,also display greater reductions in complexity in stressed conditions.25This work nicely demonstrated that loss of complexity in postural control occurs under an ecologically relevant stressor,cognitive load; it also provides insight into how groups who display larger reductions in complexity during baseline postural control conditions may be at even greater risk for adverse events under these dual task conditions.These inferences still need to be experimentally confirmed,through postural perturbation tasks.
3.4.Evaluating assistive devices
The MSE analysis technique has also been used to evaluate the effects of subsensory tactile stimulation,stochastic resonance,to the feet in improving postural control.Stochastic resonance consists of the application of white noise signals at a level that enhances the likelihood that naturally occurring phenomena will elicit a response that would not have otherwise occurred.30The application of stochastic resonance has been shown to improve cutaneous sensory function.15,31As has been previously discussed,age related impairments in cutaneous sensation are linked to reductions in the complexity of postural fluctuations; therefore,methods that directly improve cutaneous sensation could serve to increase postural complexity.This is in fact what has been observed,as Costa et al.27have shown that the application of a stochastic resonance stimulus,known to improve cutaneous sensation,improves the complexity of postural fluctuations.Their results showed increased postural complexity to levels similar to those observed in younger individuals when stochastic resonance signals are applied to the plantar surface of the feet.27This example demonstrates that MSE can be used to evaluate the efficacy of a potential treatment modality that aims to improve postural stability/adaptability,by addressing the underlying multiscale cause of dysfunction.
3.5.Summary of findings
The MSE analysis has expanded our understanding of how the degeneration of physiological function that accompanies aging and disease manifests in the reduction of complexity in the postural CoP; these changes appear to be brought about in a manner that is indicative of systems displaying a reduction in the number of dynamic interactions.Furthermore,this breakdown is not observed in healthy aging as there were no differences in CIobserved between healthy older and healthy younger individuals.8However,reductions in CIhave been observed among frail older individuals,26especially those with degradation of cutaneous sensation and vision.25A similar pattern of results is observed among individuals with MS,23such that larger reductions in postural complexity were observed among individuals with greater somatosensory impairment.Additionally,groups exhibiting lower complexity during baseline postural tasks appear to be most impacted by the addition of a cognitive stressor,suggesting that impaired basal postural performance is an indicator of a reduced ability to adapt to additional demands.26The complexity changes can be further impacted when tested under conditions other than during quiet standing tasks,such as during the performance of many common activities of daily living.Further study is needed to examine the effect that more challenging postures and postural manual interactions have on the complexity of postural fluctuations.
A major take-away message from the research on the complexity of postural fluctuations is that having an advanced age does not appear to reduce the complexity of postural fluctuations.8Rather,changes in postural complexity appear to be brought on by impairments in sensory-motor function,observed to different degrees in older individuals and those with disease-based impairment.22,23,32
An important distinction in the use of MSE compared to other single time scale analyses is the ability to gain insight both at and across a range of time scales that relate to the underlying physiological processes that govern the control of upright standing.Although the current analyses of MSE have focused on the CI,which integrates all time scales,this analysis provides SEmeasurements at each time scale of interest.In the ongoing effort to identify mechanisms that lead to balance impairments,future research may be able to leverage MSE analysis to focus on specific time scales and identify how these align with specific physiological deficits that accompany altered postural fluctuations.This approach is in agreement with the emergence of the systems biology perspective,which identifies that age related changes in behavior manifest by way of multilevel changes in the system,and if these changes are not occurring,then there is no reason to assume that changes in physiological performance should occur.32,33
Another important consideration in the study of postural control,especially among populations with sensory deficits,is that alterations in postural fluctuations may serve to enhance the identification of environmental information.This perspective is supported by the growing body of evidence where applying stochastic resonance to the soles of the feet improves boththe ability to detect external vibrations15,31and the complexity of postural fluctuations,27suggesting that enhancing perceptual processes has the benefit of improving the complexity of action processes.
4.Future considerations
MSE appears to be an effective tool for evaluating how CoP fluctuations are impacted by the breakdown of dynamical processes that are integrated in the control of posture.MSE analysis has not become widely adopted in the postural literature; however,the insights outlined here suggest that this may provide a powerful measure for quantifying clinically relevant dysfunction as well as assessing the efficacy of treatment methods intended to improve postural control.Additionally,as more work is done it may be possible,among relatable data sets,to develop guidelines for clinical levels of postural complexity at which individuals are at increased risk for falls.
Adopting a uniform reporting standard will clarify how methodological choices such as sampling rate and data collection times influence the results of MSE analysis.22We suggest here that it is more appropriate to report this information as a time scale (second or Hz) instead of scale factor (number).We propose this shift in notation as it reflects the degree of coarse graining,sample frequency,and m such that the time scale is represented by:

where m is distance of points in the time series to be compared and τ is the scale factor (Eq.3).This change in reporting will allow for systematic and clear comparisons between studies when evaluating complexity across a range of time scales.
Beyond identifying where differences arise in the time scales,MSE analysis has the potential to identify if individuals are able to exploit the complexity of the physiological systems to overcome deficits in one area in order to maintain a high level of overall adaptability in their sensory-motor outputs.This expansion of the MSE analysis technique is not directly related to the loss of complexity hypothesis but provides insight into how sensory reweighting can be used to maintain function.Additionally,the examination of tasks which place different demands on the system may reveal changes in complexity that reflect task requirements,i.e.,more difficult tasks will engage a greater number of degrees of freedom and this may increase CI.Further investigation into how the complexity of postural fluctuations is influenced by task difficulty may provide empirical evidence for the development of a unifying perspective,integrating both task difficulty and health status.
5.Conclusion
A variety of entropy techniques,intended to assess the nature of the point-to-point fluctuations in physiological signals,have been developed over the last 2 decades.These techniques vary in several important aspects,such as focusing on single (ApEn,SE,and CE) or multi-scale (MSE) analysis and assumptions of stationarity (ApEn,SE,MSE) versus allowing for non-stationary (CE) signals.While each of these provide insight into the underlying dynamics of physiological fluctuations,we discussed in detail the advantages of the multiscale approach for evaluating differences in postural fluctuations due to disease and age related physiological impairments.Specifically,the findings of studies that have used MSE in the examination of postural control suggest a breakdown of healthy physiological fluctuations with aging and disease.The observed changes in complexity suggest that individuals with elevated cutaneous sensory thresholds and impaired vision constrain their postural fluctuations in a manner that suggests a reduction in adaptability and may result in increased fall risk.Additionally,devices intended to improve postural function through the enhancement of cutaneous sensation appear well positioned to increase the multiscale complexity of postural fluctuations.These results suggest that treating the physiological mechanism underlying the changes in performance,in this case somatosensory impairment,may be a pathway for restoring adaptability and may lead to improved functional outcomes.Multiscale entropy is a promising technique that can quantify how changes in the physiological function that underlie the control of upright standing manifest in the multiscale complexity of postural sway patterns.The overall complexity index,CI,allows for the quantification of the integrated complexity of a biological signal across multiple time scales and provides an overall quantitative measure for evaluating the loss of complexity hypothesis.
Authors’contributions
MAB conceived the paper,contributed to the review of the literature and drafted the manuscript.RVE contributed to the design and execution of the paper and contributed to the drafting of the manuscript.Both authors have read and approved the final version of the manuscript,and agree with the order of presentation of the authors.
Competing interests
None of the authors declare competing financial interests.
References
1.Turvey MT,Fitch HL,Tuller B.The Berstein Perspective: I.The problems of degrees of freedom and context-conditioned variability.In: Kelso SJA,editor.Human motor behavior: an introduction.Hillsdale,NJ: Lawrence Erlbaum Associates,Inc.; 1982.p.239-51.
2.Bernstein NA.Dexterity and its development.Mahwah,NJ: Erlbaum; 1996.
3.Lipsitz LA.Dynamics of stability: the physiologic basis of functional health and frailty.J Gerontol A Biol Sci Med Sci 2002;57:B115-25.
4.Lipsitz LA,Goldberger AL.Loss of“Complexity”and aging.JAMA 1992;267:1806-9.
5.Kitano H.Systems biology: a brief overview.Science 2002;295:1662-4.
6.Reed ES.An outline of a theory of action systems.J Mot Behav 1982;14:98-134.
7.Gibson JJ.Observations on active touch.Psychol Rev 1962;69:477-91.
8.Duarte M,Sternad D.Complexity of human postural control in young and older adults during prolonged standing.Exp Brain Res 2008;191:265-76.
9.Shannon C.Communication in the presence of noise.P IRE 1949;37: 10-21.
10.Shannon C,Weaver W.The mathematical theory of information.Bell System Tech J 1948;27:623-56.
11.Kolmogorov AN.On the Shannon Theory of information transmission in the case of continuous signals.IRE Trans Inform Theory 1956;2:102-8.
12.Eckmann JP,Ruelle D.Ergodic theory of chaos and strange attractors.Rev Mod Phys 1985;57:617-56.
13.Pincus SM.Approximate entropy as a measure of system complexity.Proc Natl Acad Sci USA 1991;88:2297-301.
14.Richman JS,Moorman JR.Physiology time-series analysis using approximate entropy and sample entropy.Am J Physiol Heart Circ Physiol 2000;278:2039-49.
15.Wells C,Ward LM,Chua R,Inglis TJ.Touch noise increases vibrotactile sensitivity in old and young.Psychol Sci 2005;16:313-20.
16.Yentes JM,Hunt N,Schmid KK,Kaipust JP,McGrath D,Stergiou N.The appropriate use of approximate entropy and sample entropy with short data sets.Ann Biomed Eng 2013;41:349-65.
17.Bollt EM,Skufca JD,McGregor SJ.Control entropy: a complexity measure for nonstationary signals.Math Biosci Eng 2009;6:1-25.
18.McGregor SJ,Busa MA,Parshad RD,Yaggie JA,Bollt EM.Control entropy of gait: does running fitness affect complexity of walking? Clin Kines 2011;65:9-17.
19.McGregor SJ,Busa MA,Skufca JD,Yaggie J,Bollt EM.Control entropy identifies differential changes in complexity of walking and running gait patterns with increasing speed in highly trained runners.Chaos 2009;19:026109.
20.McGregor SJ,Busa MA,Yaggie JA,Bollt EM.High resolution MEMS accelerometers to estimate VO2and compare running mechanics between highly trained inter-collegiate and untrained runners.PLoS One 2009;4:e7355.doi:10.1371/journal.pone.0007355
21.Parshad RD,McGregor SJ,Busa MA,Skufca JD,Bollt E.A statistical approach to the use of control entropy identifies differences in constraints of gait in highly trained versus untrained runners.Math Biosci Eng 2012;9:125-48.
22.Gow B,Peng CK,Wayne P,Ahn A.Multiscale entropy analysis of center-of-pressure dynamics in human postural control: methodological considerations.Entropy 2015;17:7849.
23.Busa MA,Jones SL,Hamill J,Van Emmerik RE.Multiscale entropy identifies differences in complexity in postural control in multiple sclerosis.Gait Posture 2016;45:7-11.
24.Gruber AH,Busa MA,Gorton IIIGE,Van Emmerik RE,Masso PD,Hamill J.Time-to-contact and multiscale entropy identify differences in postural control in adolescent idiopathic scoliosis.Gait Posture 2011;34: 13-8.
25.Manor B,Costa MD,Hu K,Newton E,Starobinets O,Kang HG,et al.Physiological complexity and system adaptability: evidence from postural control dynamics of older adults.J Appl Physiol 2010;109:1786-91.
26.Kang HG,Costa MD,Priplata AA,Starobinets OV,Goldberger AL,Peng CK,et al.Frailty and the degradation of complex balance dynamics during a dual-task protocol.J Gerontol A Biol Sci Med Sci 2009;64: 1304-11.
27.Costa M,Priplata AA,Lipsitz LA,Wu Z,Huang NE,Goldberger AL,et al.Noise and poise: enhancement of postural complexity in the elderly with a stochastic-resonance-based therapy.Europhys Lett 2007;77: 68008.
28.Woollacott M,Shumway-Cook A.Attention and the control of posture and gait: a review of an emerging area of research.Gait Posture 2002;16: 1-14.
29.Fried LP,Tangen CM,Walston J,Newman AB,Hirsch C,Gottdiener J,et al.Frailty in older adults: evidence for a phenotype.J GerontolA Biol Sci Med Sci 2001;56:M146-57.
30.Benzi R,Sutera A,Vulpiani A.The mechanism of stochastic resonance.J Phys A Math Gen 1981;14:L453-7.
31.Collins JJ,Imhoff TT,Grigg P.Noise-enhanced tactile sensation.Nature 1996;383:770.
32.Van Emmerik RE,Remelius J,Johnson MB,Chung LH,Kent-Braun J.Postural control in women with multiple sclerosis: effects of task,vision and symptomatic fatigue.Gait Posture 2010;32:608-14.
33.West GB,Bergman A.Toward a systems biology framework for understanding aging and health span.J Gerontol A Biol Sci Med Sci 2009;64A:205-8.
杂志排行
Journal of Sport and Health Science的其它文章
- Non-linearity in the dynamic world of human movement
- Comparing dynamical systems concepts and techniques for biomechanical analysis
- Multi-scale interactions in interpersonal coordination
- Can coordination variability identify performance factors and skill level in competitive sport? The case of race walking
- A history of low back pain affects pelvis and trunk coordination during a sustained manual materials handling task
- Postural control deficits identify lingering post-concussion neurological deficits
