The inf l uence of three mangrove species on the distribution of inorganic nitrogen and phosphorus in the Quanzhou Bay estuarine wetland soils
2016-04-18GuiyaoZhouYanyouWuDekeXingMingmingZhangRuiYuWeiyiQiaoQaiserJaved
Guiyao Zhou·Yanyou Wu·Deke Xing·Mingming Zhang·Rui Yu· Weiyi Qiao·Qaiser Javed
The inf l uence of three mangrove species on the distribution of inorganic nitrogen and phosphorus in the Quanzhou Bay estuarine wetland soils
Guiyao Zhou1·Yanyou Wu2·Deke Xing1·Mingming Zhang1·Rui Yu1· Weiyi Qiao1·Qaiser Javed1
DOI 10.1007/s11631-015-0081-3
This study aims to investigate the effects of region and three regional dominated mangrove species(Avicennia marina,Aegiceras corniculatum and Kandelia candel)on the distribution of inorganic nitrogen and phosphorus.Measurement of the inorganic nitrogen and phosphorus and enzymatic activities was carried out in soils covered by three mangrove species in the Quanzhou Bay estuarine wetlands,a typical coastal wetland in China. Species with a higher biomass in upstream and midstream absorb more nitrogen from soils,and the retention of the available phosphorus in the soils of different regions causes the regional variation of phosphorus.In areas dominated by A.marina,nitrate nitrogen is lower while available phosphorus is higher.Meanwhile,nitrate nitrogen and available phosphorus are higher in the soils covered by K.candel. Moreover,all three species affect the elemental and enzymic stoichiometry.The mangrove species inf l uences the diversity of the elemental and enzymic stoichiometric relationship through differential microenvironments,which induce the biodiversity of wetland ecosystems.Thus,this study may facilitate a better understanding of the transformation ability of mangroves to nitrogen and phosphorus and will therefore be beneficial for providing a basis for the ecological restoration of estuarine wetlands.
Ecological restoration·Estuarine wetland· Mangrove·Nutritional elements
1 Introduction
Coastal areas make up only 4%of the earth's surface,but more than 1/3 of the world's population resides in these areas.The coastal ecosystem provides many important services for humanity,such as food,timber,raw chemical materials,and physical sites for tourism and education. However,human activities such as rapid industrialization,urbanization,and global warming largely reduce these areas and strongly disrupt coastal ecosystem functions(Comeaux et al.2012).In recent years,plenty of industrial and agricultural wastewater and sanitary sewage have been converged in estuary and gulf regions.This situation leads to large quantities of nitrogen and phosphorus entering coastal ecosystems,which is the main reason for the deterioration of water quality in coastal regions(Chatterjee et al.2009;Nobi et al.2010).The restoration of disturbed wetlands is an urgent issue concerning sustainable development of coastal ecosystems and has gained the attention of many researchers(Marchand et al.2006;Spencer and Harvey 2012;Yang et al.2008).Therefore,understanding the interaction mechanisms between plant and soils of the wetlands,especially in relation to nitrogen and phosphorus,has provided basic knowledge for us to protect and restore coastal ecosystems(Halpern et al.2007).
The mangrove ecosystem has a varied sediment composition,and different cover of mangrove plants results in different sediment conditions(Tam and Wong 1995). Mangrove plants absorb nutrient elements from sediment soil by rhizosphere for plant growth and metabolism.The distribution features of nutritional elements,such asammonium nitrogen,nitrate nitrogen,and available phosphorus in sediment soil,has an important inf l uence on nutrients'retention,transfer,and transformation.Low soil fertility leads to the slow growth of mangrove plants.One of the primary reasons for the high productivity of mangroves in nutrient poor sites(especially with low N)is the high nutrient use efficiency(Alongi et al.2005;Kao et al. 2001).Redox conditions,soil salinity,f l ood frequency,and soil aeration play important roles in determining the degree of soil fertility(Krauss et al.2006;Reef et al.2010;Ye et al.2001),and the distribution of mangrove plants has a close relationship with the distribution of nutrient elements in the soil sediment(Chen and Twilley 1999).
In natural wetlands,levels of nitrogen input and its distribution characteristics significantly inf l uence the cyclic process of the substance of the ecosystem.Surrounding nutritional elements such as inorganic nitrogen and phosphorus play key roles in driving plant distribution patterns and plant succession in wetlands(Crain et al. 2004;Li et al.2008).Soil enzymes catalyze soil biochemical reactions involved in almost all important metabolic processes of soils,thereby regulating the biogeochemical cyclic process of nitrogen and phosphorus in the ecosystem(Huang and Morris 2003;Jackson et al. 2009;Prenger and Reddy 2004;Robroek et al.2009;Turner 2010).Wetlands are a sensitive area in coastal regions where physicochemical and biological properties such as pH,salinity,root systems,and organic matters are easily affected by the surrounding environment(Sinsabaugh 2010;Williams et al.2000).
Therefore,in this study,we investigated the inf l uence of region and regional dominant mangrove species(Avicennia marina,Aegiceras corniculatum and Kandelia candel)on the distribution of ammonium nitrogen,nitrate nitrogen,and available phosphorus in the estuarine wetlands. Enzymatic activity is an important factor regulating distribution of nutrients in soils,and the differentiation of the microenvironment would improve the biodiversity of wetland ecosystems.Previous studies mainly focused on the speciation and ecological risk of heavy metals and the heterogeneity of elements such as salinity and pH in this region(Wang et al.2010;Yu et al.2008;Wu et al.2009). Until now,there was no report about the interaction between nutrients and enzymes in mangrove ecosystems in the Quanzhou Bay estuarine wetlands.Thus,to further understand how the interaction mechanism between nutrients and enzymes affects the diversity of nutritional mode and enzymic stoichiometry,we analyzed the correlation between ammonium nitrogen,nitrate nitrogen,available phosphorus,hydrolase and oxidases in the soils under different mangrove species.This study will facilitate a better understanding about the inf l uence and interaction mechanism regulating the elemental and enzymic stoichiometric relationship in the soils covered by mangrove species,and it is beneficial in providing a basis for ecological restoration in coastal districts.
2 Materials and methods
2.1 Study site and plant species
Quanzhou Bay,Fujian,China,is situated at 118°38′-118°52′E and 24°47′-24°58′and covers an area of 136.42 km2.The study site has a tidal f l at area of 568.5 hm2and has an area of 308.4 km2that is covered with water.It is dominated by a subtropical climate,with a mean annual temperature of 20.4°C.The lowest mean temperature of 11.9°Coccurs in January and the highest of28.3°Cin July. The mean annualprecipitation is 1095.4 mm,and the annual sunshine is at 2200 h,with alternating arid and humid seasons.Soil salinity and pH in downstream are higher than those in midstream and upstream(Wu and Liu 2011).A. marina,A.corniculatum and K.candel are the dominant plants in the estuarine wetlands.
2.2 Soil sampling and analysis
Three mangrove species were selected from typical sites where the experimental mangrove plant species grow for this study(Fig.1).These were Aegiceras corniculatum(Ac),Kandelia candel(Kc)and bared lands(Bl)in upstream;Aegiceras corniculatum(Ac),Avicennia marina(Am)and bared lands(Bl)in midstream;Aegiceras corniculatum(Ac),Kandelia candel(Kc),Avicennia marina(Am),and bared lands(Bl)in downstream.In each region,three similar soil cores were randomly selected with a bucket auger(5 cm inner diameter),and the sampling sites were kept at least 10 m away from each other.Soil samples were taken from 0 to 10,10 to 20,20 to 30,30 to 40,and 40 to 50 cm depths,respectively,on 15-17 April 2007(Spring),8-10 November 2007(Autumn),6-10 January 2008(Winter)and 1-3 August 2008(Summer).Plant debris and roots were removed from each sample.Some soil samples were collected,air-dried,ground,and sieved using a 2-mm mesh size.Part of fresh soil samples were also put into the refrigerator,prepared for the analysis of chemical compositions.
The concentration of nitrate nitrogen(NO3--N)and ammonium nitrogen(NH4+-N)were determined by extracting the samples with 0.01 M CaCl2at a 1:5 soil: extracting-solution ratio(w/v)followed by Continuous-Flow Analyzers(Auto Analyzer 3,Bran+Luebbe Co.,Germany).The concentration of the available phosphorus(A-P)of the soil samples was determined by extracting the samples with 0.5 M NaHCO3(pH 8.3)at a 1:20 soil:extracting-solution ratio(w/v)followed by Continuous-Flow Analyzers(Auto Analyzer 3,Bran+Luebbe Co.,Germany).Routine soil chemical parameters,including urease(U),phosphatase(P-P),polyphenol oxidase(PPO),and catalase(CAT)were analyzed using the methods suggested by Guan(1986).The urease activity(Eu)was represented as the amount of organic matter into the NH3in 100 g air-dried soil after incubation for 24 h and upon 30°C;Phosphatase activity(Epp)was represented as the number of milligrams of P2O5in 100 g air-dried soil after incubation for 24 h;Catalas activity(Ecat)was represented as the number of milliliters of 0.1 mol L-1KMnO4consumed by 1.00 g air-dried soil after incubation for 20 min;Polyphenol oxidase activity(Eppo)was described as the number of milliliters of gallic acid released by 1.0 g soil after incubation for 2 h.
Fig.1 Map of the study area
2.3 Statistical analysis
The mean and standard errors were calculated for each treatment.One-way ANOVA and pair-wise comparison tests(Turkey's test)were used to compare the contents of the nutritional elements of different mangrove plants. Relationships among nutritional elements,hydrolytic enzymes,and oxidizes were determined using correlation analysis.All statistical analyses were performed using SPSS 17.0(SPSS Inc.,192 Chicago,IL,USA).
3 Results
3.1 Regional variation in inorganic nitrogen and phosphate
The average concentration of ammonium nitrogen,nitrate nitrogen,and available phosphorus in upstream,midstream,and downstream is shown in Fig.2.It can be seen that there were no significant differences in the content of ammonium nitrogen among differentregionalsoils.The average content of nitrate nitrogen in downstream was higher than that in upstream and midstream,but there was no clear difference between upstream and midstream.The distribution of A-P followed the series midstream>downstream>upstream in the Quanzhou Bay wetland soils.

Fig.2 Effects of region on a ammonium nitrogen(NH4+-N),b nitrate nitrogen(NO3--N)and c available phosphorus(A-P)in Quanzhou Bay estuarine wetland soil.Letters above bars show significant differences among regions
3.2 Variations in inorganic nitrogen and phosphate of soils under different plant species
The content of ammonium nitrogen of soil under Ac and Am was relatively higher than that under Kc and Bl but had no significant difference among these three mangrove species(Fig.3).The average content of nitrate nitrogen of soils under Ac and Kc was higher than those under Am and Bl.Content of A-P of soils covered by mangrove plants was significant higher than those without plants,and the content of A-P followed the series Am>Kc>Ac.
3.3 Variations in enzyme activities of soils under different plant species
The U activity in the soils under vegetation was signif icantly higher than that in the soils without vegetation with no significant difference among the three species.Similar to the U activity,the P-P activity in the soil under mangrove species was significantly higher than those without soil,and P-P activity in the soil under Ac was significantly higher than those in the soils under the other two species. The CAT activity in the soil under Am was significantly higher than that in the soils under the other two species and in the soil without species.No significant difference in PPO activity was observed among the soils under the three mangrove species(Table 1).
3.4 Available N/P ratio of soils under different plant species
Mangrove species significantly modified the available N/P ratio,and this ratio relationship varied from species to species(Table 2).The available N/P ratio in the soil without vegetation was the highest,with a value of 1.05,compared with Ac,Kc and Am.Soil under Kc acquired the highest available N/P ratio of 0.93,significantly higher than that of the soil under Ac(0.86)and Am(0.74).
3.5 Correlation among nutritional elements under different plant species
There was no significant relationship between NO3--N and A-P in the soils covered by mangrove species,but a positive correlation between these two elements was found in Bl(Table 3).NO3--N had a significant positive correlationwith A-P in the soil without mangrove species and no significant correlation pattern between these two factors in the soils covered by Ac,Kc and Am.

Fig.3 Effects of specie on a ammonium nitrogen(NH4+-N),b nitrate nitrogen(NO3--N)and c available phosphorus(A-P)in Quanzhou Bay estuarine wetland soil.Letters above bars show significant differences among species
3.6 Correlation between nutritional elements,hydrolytic enzymes,and oxidases under different plant species
The relationship between nutritional elements and enzymes in the test soils was unique for each mangrove species(Table 4).A significant correlation was observed between NH4+-N and P-P,PPO and CAT,NO3--N and U,A-P and U,and CAT in Bl.However,the mangrove species modifi ed the relationship between PPO and NH4+-N in the soils under Kc and Am,between U and NO3--N in the soils under Ac and Kc,and between A-P and P-P in the soils under Ac,Kc and Am.These results showed that mangrove species contributed to the differential distribution of nutritional elements and enzymes.
4 Discussion
4.1 Effect of regions on the distribution of nutrients
The pH and salinity play important roles on the utilization strategy of plants for NH4+-N(Clarkson and Warne 1979;Lavoie et al.1992).However,as shown in Fig.2,the present study demonstrated that the difference of NH4+-N was insignificant in different districts.This suggests that salinity and pH were not the main factors inf l uencing the regional variation of NH4+-N but high heterogeneity of pH and salinity among upstream,midstream and downstreamin the wetland was reported in our previous studies(Wu and Liu 2011).The average content of NO3--N in downstream was higher than those in upstream and midstream. The difference in concentration may result from the difference exogenous input or output quantity in different regions,and the absorption difference among species is also a possible reason to induce the variation.The plant with the highest biomass in midstream is about 2/3,and in downstream it is about 1/2 that in upstream.Species with higher biomass in upstream and midstream absorb more nitrogen,which decreases the amount of nitrogen retention in soils.Similarly,the content of A-P in soils depends on the retention and the absorption of phosphorus by plants or sedimentary soils(Chu et al.2000;Ramose et al.2007). The present study demonstrated that soil A-P in upstream was significantly lower than those in midstream and downstream,suggesting that the effect of retention was higher than that of the absorption in the Quanzhou Bay estuarine wetlands.The low content of soil A-P in upstream resulted from relative shorter time for retention and more absorption on phosphorus by plants.

Table 1 Activities of urease(U),phosphatase(P-P),polyphenol oxidase(PPO),and catalase(CAT)in soils under different mangrove species

Table 3 Correlation coefficients(Pearson)among nutritional elements in soils under different mangrove species
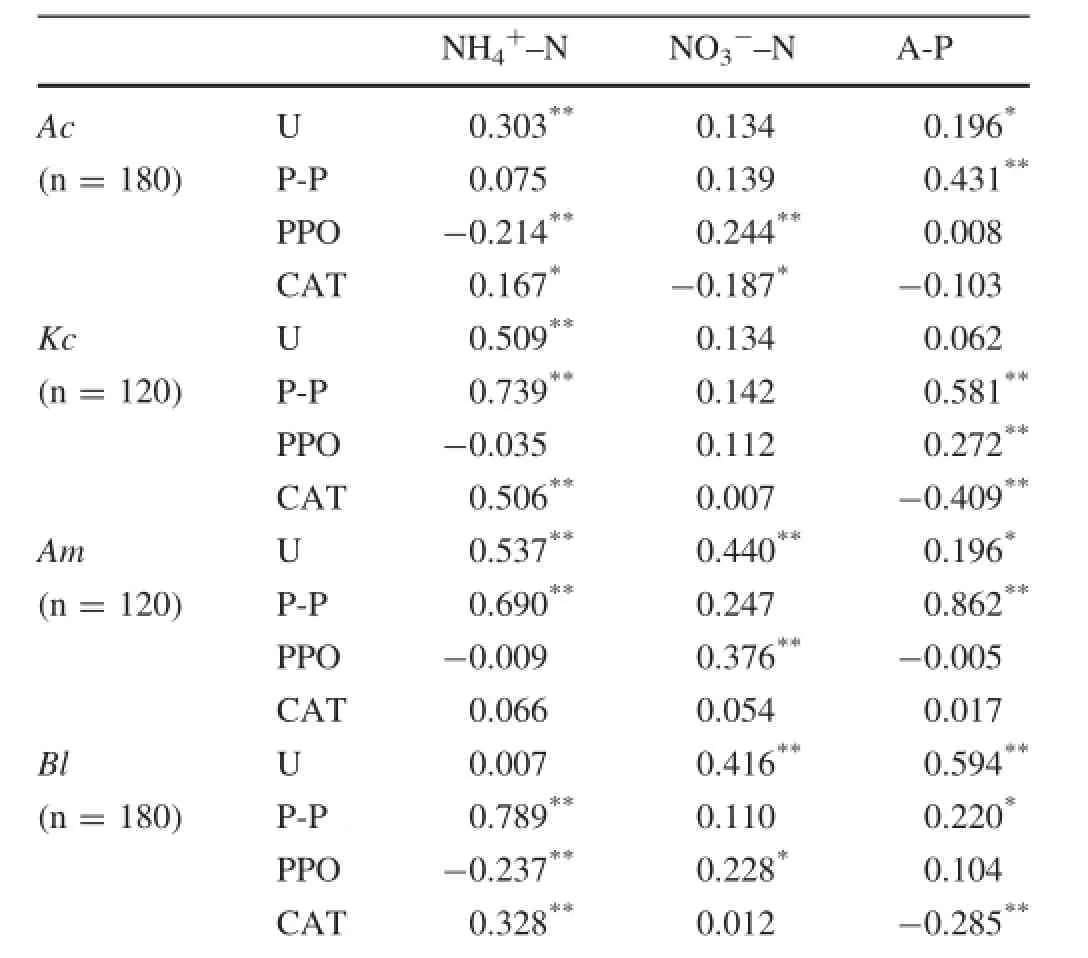
Table 4 Correlation coefficients(Pearson)between nutritional elements,hydrolytic enzymes,and oxidases in soils under different mangrove species

Table 2 Available N/P ratio in soils under different mangrove species
4.2 Effect of species on the distribution of nutrients
Biological species difference affects the stoichiometry of nutrient elements in an ecosystem(Klausmeier et al.2008;Price 2005).In the present study,the average content of NO3--N and A-P varied from species to species.The difference in absorption may account for the variation of NO3--N.Kc belongs to a fast-growing modelin plantheight with a rate of62.00 cm/year,while Ac and Am were 9.70 cm/ year and 8.27 cm/year.Similar to plant height parameters,Kc also has the largest crown diameter growth rate with 20.05 cm/year,compared with 3.43 cm/year under Ac and 1.81 cm/yearunder Am in thisregion(Wu and Liu 2011).Am wasplanted in 1970s,Ac in the end ofthe 20th century,while Kc has the shortest planting time,as it was planted in this region in 2005.Due to the difference in planting density and planting time,the biomass of Kc,Ac,and Am was estimated to be about 160,300,and 450 kg/acre,respectively.Compared with Ac and Kc,Am has a longer planting time in this region and has a better developed root system,making Am absorb more nitrogen from wetland soils than the other two species.While bare land was easily disturbed by the activities of humans and animals,the microbial biomass in these soils is lowerthan those covered with vegetation(Chaudhuri et al.2009),therefore,low levels of nitrifying bacteria led to the low content of NO3--N.Biomass contributes to improving phosphatase activity(Pancholy and Rice 1973)due to supplying enough nutritionalelements and because of the good ventilation caused by the developed root system,which promotes metabolism and respiration rate of microorganisms(Taylor et al.2002).Thus,the content of A-P in the soilunder Am was higherthan thatunderthe other two species because ofits developed root system and greater biomass.As for the content of A-P in the soil under Kc,although it had a shorter planting time,its rapid growth rate could decrease the biomass effect on the distribution of A-P. Interestingly,average content of A-P in the soils covered by mangrove plants was significantly higher than those without mangrove species.This may be due to the root exudates produced by mangrove plants,which have the ability to facilitate the activation of available phosphorus.
4.3 Nutritional mode and stoichiometry
The variations of available N/P ratio among species may result from the difference of biomass.This ratio under the soils without vegetation reached 1.05,while that in soils covered by Am was only 0.74,indicating that the utilization of mangrove species for nitrogen was greater than phosphorus.Biomass difference among species induced by plant time regulated the stoichiometry of nutrients.With a relatively longer plant time,Ac and Am had a greater biomass,making available N/P ratio in the soil under these two vegetation low.The recently grown Kc,with a lower biomass,makes available N/P ratio under it higher;therefore,the expanded acreage and prolonged growth time may contribute to aggravating the degree of restriction for nitrogen. Biomass is an important factor regulating the urease and phosphatase activity(Pancholy and Rice 1973).The difference ofbiomass among Ac,Kc and Am led to the variation of the urease and phosphatase activity,affecting the content difference of NH4+-N and A-P in soils under these vegetations,which modified the available N/P ratio pattern among species.Compared with the soils covered by mangrove species,the contentof A-P in the soilwithoutvegetation was the lowest(Fig.3),making ithave the highestavailable N/P. Greater biomass induced by longer plant time disproportionately increased the content of NH4+-N and A-P in soils covered by Am,which led to the low available N/P ratio in it. The shortest plant time resulted in the lower biomass of Kc than in the other two species,yielding a lower content of NH4+-Nand A-P,which isregulated by soilenzymesrelated to biomass.However,the rapid growth rate significantly increased the content of NO3--N(Fig.3),and therefore,variations of NO3--N were the major reason for making the available N/P ratio highest.
The present study demonstrated that nutritional mode and stoichiometry under the inf l uence of plant species in this district was an interactive process.Biomass increases urease activity in wetland soils(Pancholy and Rice 1973). Accordingly,mangrove plants modified the relationship pattern between NH4+-N and urease in soils covered by mangrove species.A broad rhizosphere environment formed by mangrove species also can increase phosphataseactivity through boosting the soil microbial community(Song et al.1990).The present study showed that the relationship between A-P and P-P in the soils under mangrove species was significantly different,compared with that in soil without vegetation,suggesting that plants play an important role in the transformation of soil inorganic phosphorus(Table 4).Drying-wetting alternation environments increase the phosphatase activity through signif icantly stimulating the activity of the soil microbial community(Song et al.2007),and the aerobic environment difference induced by the root system changes the stoichiometric relationship(Liang et al.2003).Salinity is an important factor affecting the catalase activity(Sinsabaugh 2010),and the average value of salinity in the soils in this region was 14.15 mS/cm under Ac,13.98 mS/cm under Kc,and 14.72 mS/cm under Am,which had been investigated in our previous work(Liu and Wu 2011).Therefore,relative high activities of CAT modified the stoichiometric relationship between CAT and NH4+-N in the soils under Ac and Am.The high pH in the rhizosphere environment can increase the polyphenol oxidase activity(Williams et al.2000).In our previous study,we have learned that the average value of pH in soils was 7.06 under Ac,6.96 under Kc and 6.87 under Am in this region(Wu and Liu 2011). Therefore,the variable pH of soils under different mangrove species modified the relationship between PPO and P-P.The difference in root systems among mangrove species led to the diversification of species in response to the surrounding environments.Thus,the interactive pattern between nutrients and enzymes varied from species to species,and the pattern in the soils under mangrove species was significantly different from that without plants.
As mentioned above,in this study,differential microenvironments formed by different mangrove species,which inf l uenced the distribution of nutrients and enzymes,had a distinctive difference,characterized by Ac with high pH,a developed root system,middle biomass,and salinity;Kc with middle pH,an undeveloped root system,low biomass,and salinity and;Am with low pH,a developed root system,high biomass,and salinity.Therefore,these variations among species led to diverse interactive patterns among elemental and enzymic stoichiometric relationships. These patterns and relationships eventually induced the biodiversity of the wetlands ecosystem.
5 Conclusion
Distribution of inorganic nitrogen and phosphorus under the inf l uence of the region and three regional dominant mangrove species(A.corniculatum,K.cande and A. marina)were studied in the Quanzhou Bay for the first time.High biomass of mangrove plants in upstream and midstream could contribute to lowering the content of nitrate nitrogen.Mangroves regulate the stoichiometry of nutrients in this estuarine wetlands ecosystem.The inf l uence of region and mangrove species on the distribution of ammonium nitrogen was not significant.However,the inf l uence on the distribution of nitrate nitrogen and available phosphorus depended on the mangrove species.Differential microenvironment controlled by mangrove species and the surrounding environment resulted in the diversity of the elemental and enzymic stoichiometry,which induced the biodiversity of the wetlands ecosystem. This study contributes to the understanding of long-term management and conservation of wetlands,especially the treatment of nitrogen and phosphorus pollution in coastal regions.
Acknowledgments The authors thank the two anonymous reviewers for their insightful comments.Acknowledgments are made to Asante Eric Amoah,who works at the Mechanical Engineering Department of Koforidua Technical University of Ghana,for his kindly suggestion on writing this manuscript in English.We also thank the financial support for this project provided by National Science and Technology Support Program(2009BADB2B04-03)and‘‘Hundred Talents Program''of Chinese Academy of Sciences.
Alongi DM,Clough BF,Robertson AI(2005)Nutrient-use efficiency in arid-zone forests of the mangroves Rhizophora stylosa and Avicennia marina.Aquat Bot 82:121-131
Chatterjee M,Massolo S,Sarkar SK,Bhattacharya AK,Bhattacharya BD,Satpath KK,Saha S(2009)An assessment of trace element contamination in intertidal sediment cores of Sunderban mangrove wetland,India for evaluating sediment quality guidelines. Environ Monit Assess 150:307-322
Chaudhuri S,Dinesh GR,Sheeja TE,Raja R,Jeykumar V,Srivastava RC(2009)Physico-chemical,biochemical and microbial characteristics of soils of mangroves of the Andamans:a posttsunami analysis.Curr Sci 97:98-102
Chen RH,Twilley RR(1999)Patterns of mangrove forest structure and soil nutrient dynamics along the Shark River estuary,Florida.Estuaries 22:955-970
Chu HY,Tam NFY,Lam SKS,WongY S(2000)Retention of pollutants by mangrove soil and the effects of pollutants on Kandelia candel.Environ Technol 21:755-764
Clarkson DT,Warne AJ(1979)Relationships between root temperature and the transport of ammonium and nitrate ions by Italian and perennial ryegrass(Lolium multiftorum and Lolium perenne).Plant Physiol 64:557-561
Comeaux RS,Allison MA,Bianchi TS(2012)Mangrove expansion in the Gulf of Mexico with climate change:implications for wetland health and resistance to rising sea levels.Estuar Coast Shelf Sci 96:81-95
Crain CM,Silliman BR,Bertness SL,Bertness MD(2004)Physical and biotic drivers of plant distribution across estuarine salinity gradients.Ecology 85:2539-2549
Guan SY(1986)Soil enzyme and study method.Agricultural Press,Beijing
Halpern BS,SillimanB R,Olden JD,Bruno JP,Bertness MD(2007)Incorporating positive interactions in aquatic restoration and conservation.Front Ecol Environ 5:153-160
Huang X,Morris JT(2003)Trends in phosphatase activity along a successional gradient of tidal freshwater marshes on the Cooper River South Carolina.Estuaries Coasts 26:1281-1290
Jackson CR,Liew KC,Yule CM(2009)Structural and functional changes with depth in microbial communities in a tropical Malaysian Peat Swamp Forest.Microb Ecol 57:402-412
Kao WY,Tsai HC,Tsai TT(2001)Effect of NaCl and nitrogen availability on growth and photosynthesis of seedlings of a mangrove species,Kandelia candel(L.)Druce.J Plant Physiol 158:841-846
Klausmeier CA,Litchman E,Daufresne T,Levin SA(2008)Phytoplankton stoichiometry.Ecol Res 23:479-485
Krauss KW,Doyle TW,Twilley RR,Rivera-Monroy VH,Sullivan JK(2006)Evaluating the relative contributions of hydroperiod and soil fertility on growth of south Florida mangroves. Hydrobiologia 569:311-324
Lavoie N,Ve´zina LP,Margolis HA(1992)Absorption and assimilation of nitrate and ammonium ions by jack pine seedlings. Tree Physiol 11:171-183
Li WQ,Liu XJ,Khan MA,Gul BQ(2008)Relationship between soil characteristics and halophytic vegetation in coastal region of North China.Pak J Bot 40:1081-1090
Liang C,Das KC,McClendon RW(2003)The inf l uence of temperature and moisture contents regimes on the aerobic microbial activity of abiosolids composting blend.Bioresour Technol 86:131-137
Liu RC,Wu YY(2011)The dynamic growth and development information of several plant species in Quanzhou Bay estuarine wetland.Adv Mater Res 185:13-17
Marchand C,Lallier-Verge`s E,Baltzer F,Albe`ric P,Cossa D,Baillif P(2006)Heavy metals distribution in mangrove sediments along the mobile coastline of French Guiana.Mar Chem 98:1-17
Nobi EP,Dilipan E,Thangaradjou T,Sivakumar K,Kannan L(2010)Geochemical and geo-statistical assessment of heavy metal concentration in the sediments of different coastal ecosystems of Andaman Islands,India.Estuar Coast Shelf Sci 87:253-264
Pancholy SK,Rice EL(1973)Soil enzymes in relation to old field succession:amylase,cellulose,invertase,dehydrogenase and urease.Soil Sci Soc Am Proc 37:47-50
Prenger JP,Reddy KR(2004)Microbial enzyme activities in a freshwater marsh after cessation of nutrient loading.Soil Sci Soc Am J 68:1796-1804
Price NM(2005)The elemental stoichiometry and composition of an iron-limited diatom.Limnol Oceanogr 50:1159-1171
Ramose SC,Oliveira S,Re´go R,Mozeto A(2007)Dynamics of phosphorus and nitrogen through litter fall and decomposition in a tropical mangrove forest.Mar Environ Res 64:524-534
Reef R,Feller IC,Lovelock CE(2010)Nutrition of mangroves.Tree Physiol 30:1148-1160
Robroek BJM,Adema EB,Venterink HO,Leonardson L,Wassen MJ(2009)How nitrogen and sulphur addition,and a single drought event affect root phosphatase activity in Phalaris arundinacea. Sci Total Environ 407:2342-2348
Sinsabaugh RL(2010)Phenol oxidase,peroxidase and organic matter dynamics of soil.Soil Biol Biochem 42:391-404
Song JM,Li Y,Zhu ZB(1990)Relationship between Eh value and redox environment in marine sediments.Mar Sci Bull 9:30-33
Song KY,Zoh KD,Kang H(2007)Release of phosphate in a wetland by changes in hydrological regime.Sci Total Environ 380:13-18
Spencer KL,Harvey GL(2012)Understanding system disturbance and ecosystem services in restored salt marshes:integrating physical and biogeochemical processes.Estuar Coast Shelf Sci 106:23-32
Tam NFY,Wong YS(1995)Spatial and temporal variations of heavy metal contamination in sediments of a mangrove swamp in Hong Kong.Mar Pollut Bull 31:254-261
Taylor JP,Wilson B,Mills MS,Burns RG(2002)Comparison of microbial numbers and enzymatic activities in surface and subsoil using various technique.Soil Biol Biochem 3:387-401
Turner BL(2010)Variation in pH optima of hydrolytic enzyme activities in tropical rain forest soils.Appl Environ Microbiol 76:6485-6493
Wang L,Yu R,Hu G,Tu X(2010)Speciation and assessment of heavy metals in surface sediments of Jinjiang River tidal reach,southeast of China.Environ Monit Assess 165:491-499
Williams CJ,Shingara EA,Yavitt JB(2000)Phenol oxidase activity in peat lands in New York State:response to summer drought and peat type.Wetlands 20:416-421
Wu YY,Liu RC(2011)The plants'adaptability to environment of Quanzhou bay estuary wetland.Science Press,Beijing
Wu YY,Liu RC,Liang Z(2009)The distribution of salinity and pH in the coastal estuarine mangrove wetland soil.Geochemica et Cosmochimica Acta.73:A1455
Yang Q,Tam NFY,Wong YS(2008)Potential use of mangroves as constructed wetland for municipal sewage treatment in Futian,Shenzhen,China.Mar Pollut Bull 57:735-743
Ye Y,Tam NFY,Wong YS(2001)Livestock wastewater treatment by a mangrove pot-cultivation system and the effect of salinity on the nutrient removal efficiency.Mar Pollut Bull 42:513-521
Yu RL,Yuan X,Zhao YH,Hu GR,Tu XL(2008)Heavy metal pollution in intertidal sediments from Quanzhou Bay,China. J Environ Sci 20:664-669
Received:28 October 2014/Revised:17 June 2015/Accepted:10 November 2015/Published online:17 November 2015
©Science Press,Institute of Geochemistry,CAS and Springer-Verlag Berlin Heidelberg 2015
✉Yanyou Wu wuyanyou@mail.gyig.ac.cn
1Key Laboratory of Modern Agricultural Equipment and Technology,The Ministry of Education of the People's Republic of China,Institute of Agricultural Engineering,Jiangsu University,Zhenjiang 212013,China
2Research Center for Environmental Bio-Science and Technology,State Key Laboratory of Environmental Geochemistry,Institute of Geochemistry,Chinese Academy of Sciences,Guiyang 550081,China
杂志排行
Acta Geochimica的其它文章
- The Moon
- Equilibrium and kinetic Si isotope fractionation factors and their implications for Si isotope distributions in the Earth's surface environments
- Study of oxygen fugacity during magma evolution and ore genesis in the Hongge mafic-ultramafic intrusion,the Panxi region,SW China
- Determination of rhenium and osmium by ICP-MS for galena and sphalerite
- Oil-source correlation of Lower-Triassic oil seepages in Ni'erguan village,Southern Guizhou Depression,China
- Numerical simulation of groundwater under complex karstconditions and the prediction of roadway gushing in a coal mine: a case study in the Guang'an Longtan Reservoir in Sichuan Province,China
