Determination of rhenium and osmium by ICP-MS for galena and sphalerite
2016-04-18YingyingLiuLiangQiJianfengGaoZhilongHuang
Yingying Liu·Liang Qi·Jianfeng Gao·Zhilong Huang
Determination of rhenium and osmium by ICP-MS for galena and sphalerite
Yingying Liu1,2·Liang Qi1·Jianfeng Gao1·Zhilong Huang1
DOI 10.1007/s11631-015-0076-0
Digestion with aqua regia in a Carius tube and separation of Re with anion exchange resin is commonly employed for Re-Os dating of molybdenite and pyrite. However,the recovery of Re is extremely low when this routine anion exchange method is applied to galena,causing difficulty in Re-Os dating of galena.In this study,we investigated the mechanism of Re loss during sample preparation and tested a revised procedure for Re-Os dating of galena and sphalerite.
Galena·Sphalerite·Rhenium and osmium· Anion exchange resin·ICP-MS
1 Introduction
Galena,sphalerite,and pyrite are the major ore minerals in Pb-Zn sulfide deposits.Most pyrites in these deposits have multiple origins(Brill 1989),making it difficult or impossible to determine the timing of Pb-Zn mineralization.Re-Os dating of galena and sphalerite yield more reliable geochronological constraints on the timing of ore formation(Stein et al.2000;Morelli et al.2004).However,Re recovery from galena is extremely low when the samples are processed as for molybdenite and pyrite,resulting in significant problems in Re-Os dating(Liu et al. 2015b).
Pre-concentration methods for Re in geological samples(Morgan et al.1991;Tagami and Uchida 2000)have been reported intensively.Anion exchange chromatography(Morgan et al.1991;Malinovsky et al.2002;Meisel et al.2003a;Qi et al.2007,2010)and extraction with organic solvent(Du et al.1994,2001;Birck et al.1997;Yang et al.2006;Li et al.2009)are commonly used for separation of Re.However,high concentrations of different matrix metallic ions in sulfides sometimes affect the recovery of Re.For example,the recovery of Re decreases with increasing concentration of Fe3+using anion exchange resin separation(Huang et al.2012). Although our previous study showed that HNO3-based anion exchange separation is more suitable for Re purification of galena and sphalerite than HCl-based anion exchange(Liu et al.2015b),how Pb2+and Zn2+affect the recovery and loss of Re during the HCl-based procedure is still unknown.
2 Experimental and analytical methods
2.1 Samples
The experimental galena sample was collected from the Fule Pb-Zn deposit and the sphalerite from the Laochang Pb-Zn deposit,both in Yunnan Province,southwest China(Liu et al.2015a,b).The Pb-Zn sulfide ores were mechanically crushed and washed by ultrapure water.After being sieved through size 20-40 meshes,sphalerite and galena separates were hand-picked under a binocular microscope,and then grinded to pass through about 200mesh.Concentrations of Re in these galena and sphalerite samples were less than 1.0 ng/g.
2.2 Instrumentation
The instrument used for analysis of Re in this study is an ELAN DRC-e inductively-coupled plasma mass spectrometry(ICP-MS)instrument(Perkin Elmer,USA)in the State Key Laboratory of Ore Deposit Geochemistry,Institute of Geochemistry,Chinese Academy of Sciences. Background counts for 3%HNO3solution are normally lower than 20 cps(counts per second)for Re.The sensitivity of the instrument was optimized to be more than 30,000 cps for 1 ng/mL of103Rh,in order to achieve the desired detection limits;the analytical uncertainty(RSD)was better than 3%.
Osmium was determined by a Bruker Aurora M90 ICPMS spectrometer.High sensitivity mode was used due to the low content of Os.In order to achieve the desired detection limits,the sensitivity of the instrument for high sensitivity mode was adjusted to more than 800,000 cps for 1 ng/mL of115In and 300,000 cps for 1 ng/mL of232Th. The RSD was better than 3%.
Total analytical blanks were less than 6.0 pg for Re and 2.5 pg for Os.
2.2.1 Reagents and solutions
Rhenium spike solutions(US Services Inc)were enriched in185Re.Abundances of185Re and187Re were 94.36 and 5.64%,respectively.
Re standard(std)solution 10 ng/mL(Perkin Elmer Pure Plus).
HCl was purified by sub-boiling distillation;HNO3was purified by bubbling clean air through the boiled HNO3with H2O2in a 3000-mL glass beaker to remove possible volatile OsO4,and then purified following routine subboiling distillation:
Ultrapure water was obtained from a Millipore purif ication system(18 MΩ cm-1).
Anion resin Bio-rad AG 1-X8(100-200 meshes).
Analytical reagents(ARs)FeCl3(99.8%)and Pb(NO3)2(99.0%).
Zn(NO3)2solution Concentration of Zn2+was about 0.1 g/mL,prepared by zinc metal powders(95.0%;AR)and sub-boiling HNO3.
Carius tubes used in this study are reusable(Qi et al. 2013;Liu et al.2014),and have an inner volume of about 200 mL,with a custom-made sealing system including a glass-lined PTFE stopper and a stainless steel screw cap. The reusable Carius tube is easy to clean,resulting in a lower Re blank.
2.3 Analytical procedure
The sulfides were precisely weighed and transferred into a reusable Carius tube with 6.0 ng Re spike and appropriate amount of reverse aqua regia(HNO3:HCl≈4:1).Each sealed Carius tube was placed into a stainless steel sheath and slowly heated to 150°C for 5 h and then 200°C for 12 h to dissolve the samples and reach isotopic exchange equilibrium.
After cooling down to about 40°C,the content in the tube was transferred to a 50-mL centrifuge tube for centrifuging.After centrifuging,the upper solution was decanted into a 50-mL glass beaker and evaporated to dryness. The residue was then dissolved by 12 mL of 2 mol/L HCl,transferred into a 15-mL centrifuge tube,and centrifuged for 5 min.The upper solution was used for anion exchange separation of Re.Next,12 mL of 2 mol/L HCl was used to wash the anion exchange resin and 15 mL of 9 mol/L HNO3was used to elute Re.The solution was evaporated to dryness and dissolved by 3 mL of 3%HNO3for ICPMS analysis(Qi et al.2007).
3 Results and discussion
3.1 Re loss during routine chemical treatment
As mentioned in our previous study(Liu et al.2015b),recovery of Re would be extremely low if we treated galena following the above described procedure(Table 1). For sphalerite,the rate of recovery of Re would decrease if the sample mass increased(Table 1).Low recovery of Re only occurred when large amounts of sphalerite(>1.0 g,15 mL)were dissolved and treated following the above described procedure(Table 1).However,using 2 mol/L HCl as a blank without any matrix did not show any effect on the recovery of Re(Table 1).We therefore conclude that 2 mol/L HCl is not suitable for loading the sample onto the anion exchange column for separating Re from galena and sphalerite,and the routine procedure is not suitable for precise Re-Os dating of galena and sphalerite. The loss of Re should be investigated during sample preparation in order to enhance the recovery of Re.
3.2 Effect of Pb2+and Zn2+for recovery of Re during anion exchange separation
We first evaluated the effect of Pb2+and Zn2+on anion exchange separation of Re.For Pb2+,0.2 to 1.0 g Pb(NO3)2was weighed and placed into a 50-mL glass beaker.Water was used to dissolve the powder,then 10 ng Re std solution and 2 mL HNO3were added to the beaker and mixed with the Pb(NO3)2.The HNO3was used tooxidize all Re to+7,making it exist as(ReO4)-in the solution.The mixture was evaporated to dryness.The residue was dissolved in 15 mL 2 mol/L HCl and centrifuged for 5 min.The upper solution was utilized for Re purification through anion exchange resin.For Zn2+,an appropriate amount of Zn(NO3)2solution was used as the matrix to evaluate the effect of Zn2+.
The results show that Re in a Pb(NO3)2matrix was quantitatively recovered(Table 2),indicating that Pb2+did not affect Re recovery for anion exchange separation and that low recovery of Re for galena samples might not relate to column separation.However,Zn2+showed different behavior from Pb2+during anion exchange separation. Large amounts of Zn2+(>1.0 g,15 mL)in the matrix severely impacted recovery of Re(Tables 1,3).Although Zn can form complex anions in an HCl medium(Harris et al.2003;Akinfiev and Tagirov 2014)and may occupy the anion resin,the recovery was high in this study when the total mass of Zn2+was less than 0.3 g(Table 3).That might be because Re has a higher affinity than Zn during anion resin exchange,and is preferentially exchanged.The low recovery of Re when large amounts of sphalerite sample were digested(>1.0 g,15 mL)may have been due to the matrix effect.
3.3 Behavior of Re during sample pretreatment
It is noted that abundant white precipitates of lead salt formed during both stages of dissolving and medium change(from HNO3to HCl).In the former dissolving stage,the precipitates were mostly PbSO4,Pb(NO3)2,and PbCl2(Fig.1a,b).During digestion of galena using reverse aqua regia,the PbSO4precipitate results from the formation of SO42-.Thus,the difference between Fig.1a,b is probably due to a stronger oxidability of 10 mL reverse aqua regia compared to 5 mL.However,the precipitates formed in the later medium change stage were dominantly PbSO4and ZnSO4·7H2O(Fig.1c,d).Therefore,it is necessary to distinguish the role of these precipitates in Re loss in galena samples.
As concentration of Re was as low as<1.0 ng/g in galena and sphalerite in this study,we added Re std solution into the sample to evaluate the recovery of Re following the procedure as follows:
Along with an appropriate amount of reverse aqua regia,0.5 g galena powder and 10 ng Re were accurately weighed and placed into a Carius tube.The tube was sealed and heated to 150°C for 5 h and then 200°C for 12 h. After cooling,the solution and white precipitate(Fig.1b)were transferred into a 15-mL centrifuge tube.The sample solution was centrifuged at 2500 rpm for 5 min.The upper solution was divided into three portions,which were transferred into three 50-mL beakers,and 6.0 ng185Re spike was added to each beaker.
After evaporating the solution to dryness in the first beaker,the residue was dissolved with 15 mL of 2 mol/L HCl and then transferred into a 15-mL centrifuge tube. New white precipitate formed during this stage(Fig.1c,d). After centrifuging,the upper solution was used for purif ication of Re through anion exchange resin.
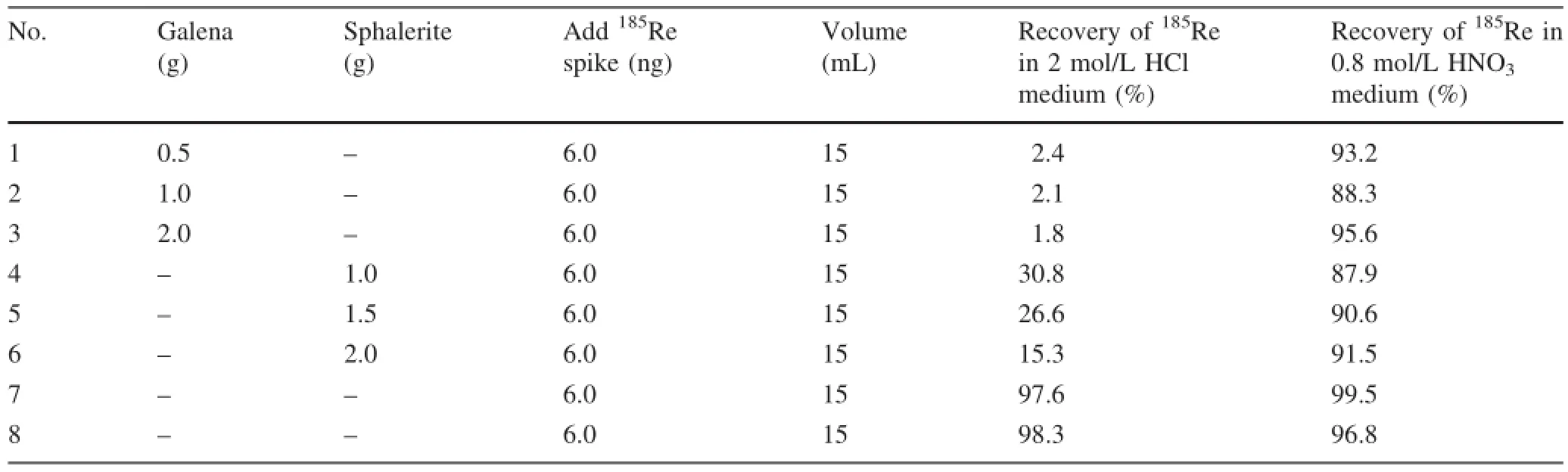
Table 1 Recovery of Re in galena and sphalerite during anion exchange

Table 2 Recovery of Re in Pb(NO3)2during anion exchange in 2 mol/L HCl medium

Table 3 Recovery of Re in Zn solutions during anion exchange in 2 mol/L HCl medium
As for the second beaker,1 mL of Zn(NO3)2solution(about 0.1 g Zn2+)was added and then evaporated to dry. New white precipitate also formed during the dissolution with 15 mL of 2 mol/L HCl,but was much less than in the fi rst procedure.
After evaporating the solution in the third beaker to dryness,about 15 mL of 0.8 mol/L HNO3was used to dissolve the residue(Tagami and Uchida 2000;Meisel et al.2003b;Chu et al.2007).Rhenium was separated and concentrated by anion exchange resin.In this procedure,only a few white precipitates formed.
Following the above procedures,the three eluted solutionsof15 mLof9 mol/L HNO3were evaporated to dryness and then dissolved by HNO3and diluted to about 3 mL.All solutions were measured by ICP-MS.Intensity of185Re and187Re of the first sample are both in the range of tens of cps, close to the background.Intensities of Re of the second and third samples are high enough to calculate the residual Re content in the dissolving solution by isotope dilution(ID). The recovery ofRe during the formerdissolving stage ranged from 84.0 to 89.1%(Table 4),indicating that the added Re remained in the dissolving solution(reverse aqua regia)of galena in abundance(Fig.1a,b).The precipitates of PbSO4and ZnSO4·7H2O(Fig.1c,d)thatformed atthe second stage might have caused the loss of Re.

Fig.1 X-ray powder diffraction(XRD)analysis spectra of white precipitates formed under different experimental conditions.a Precipitation formed under dissolving of 1.0 g galena by 10 ml reverse aqua regia(8 ml HNO3+2 ml HCl),b precipitation formed under dissolving of 0.5 g galena by 5 ml reverse aqua regia(4 ml HNO3+1 ml HCl),c precipitation formed when the dissolved solution of A is changed to HCl medium,d precipitation formed when the dissolved solution of B is changed to HCl medium
As a comparison,we used 0.1 g FeCl3instead of Zn(NO3)2to evaluate the role of existence of Zn2+in another set of parallel experiments(Table 4),in which 1.0 g galena was used.The recoveries of routine treatment,addition of FeCl3,and 0.8 mL HNO3medium were 4.6,96.1,and 100%,respectively,similar to the experiment using Zn(NO3)2.Thus,addition of Zn(NO3)2or FeCl3(0.5 g galena need about 0.1 g Zn(NO3)2or FeCl3)could significantly promote recovery of Re in HCl medium(Table 4).However,introduction of such reagents would enhance total procedure blank(Re concentrations of reagent FeCl3and zinc powders were 2.0 and 0.05 ng/g,respectively).Moreover,introducing a large amount of Fe3+(Huang et al.2012)and Zn2+(Tables 1,3)might also lower the recovery of Re.Thus,a relatively oxidized medium of 0.8 mol/L HNO3is more efficient for anion exchange of Re in galena(0.5-2.0 g)and sphalerite(>1.0 g)(Tables 1,4).Previous experimental studies suggest that Re is mobile in Cl-rich oxidizing f l uids(Xiong and Wood 1999,2000;Widom et al.2003),but immobile under reduced conditions(Colodner et al.1995;Xiong and Wood 2001).Results in this study also indicate that Re precipitation cannot form at the oxidizing medium.Thus,an oxidized solution is necessary with anion exchange separation of Re for galena,and Re should be released at a higher rate in a medium of 0.8 mol/L HNO3than of 2 mol/L HCl.On the other hand,although Os is also immobile under reduced environments(Xiong and Wood 2000;Widom etal. 2003),the matrix effect would not affect the in situ distillation of Os.

Table 4 Impact of the precipitation(formed at dissolving stage of galena)on recovery of Re
3.4 A revised procedure of Re-Os isotope dating of lead-zinc ores
Samples of 1.0-2.0 g of galena/sphalerite and appropriate185Re and190Os spikes were precisely weighed and placed into a Carius tube with 10-20 mL reverse aqua regia in ice water bath.The sealed Carius tube was placed into a stainless steel sheath and slowly heated to 150°C for 5 h and then 200°C for 12 h.After cooling down to about 40°C,the Carius tube was put into a refrigerator for 2 h for freezing.Then,20 mL of water was added and the tube was connected to the in situ distillation equipment for in situ distillation of Os(Qi et al.2010,2013).After that,the residual solution was transferred to a 50-mL beaker and evaporated to dryness.The residue was then dissolved by 15 mL of 0.8 mol/L HNO3and transferred into a 15-mL centrifuge tube.After 5-min centrifuging,the upper solution was used for anion exchange separation of Re.The anion exchange resin was washed with 12 mL 2 mol/L HNO3and 15 mL 9 mol/L HNO3was used to elute Re. The solution was evaporated to dryness and dissolved by 3 mL 3%HNO3for ICP-MS measurement.
3.5 Results of reference materials
Because of the lack of international reference materials of galena and sphalerite for Re-Os dating,we used molybdenite,JDC,and HLP as reference material.To monitor the actual samples of galena and sphalerite,0.1 g of JDC and HLP were added to 3.0 g of galena and sphalerite,respectively,as introduced by Qi et al.(2010).The same Pb-Zn ore samples were prepared as a blank without addition of molybdenite reference materials.The samples were digested following the procedure described in Sect.3.4.To obtain the measured values,the calculated results were deducted from the blank of galena/sphalerite. As shown in Table 5,the results for JDC and HLP are in fairly good agreement with the certified values,demonstrating that the proposed method is a reliable means of determining Re and Os in galena and sphalerite.We also analyzed a galena sample and a sphalerite sample following the procedure described above.Despite very low concentrations of Re and Os,the samples yielded excellent repeatability.The precision and accuracy for Re-and Ospoor Pb-Zn ores are significantly improved.
4 Conclusions
This study systematically investigated the controlling factors of low recoveries of Re through the routine analytical method for Re-Os dating of typical galena and sphalerite samples.Our results show the following conclusions.
Abundant Pb2+in galena matrix does not affect anion exchange of(ReO4)-.The white precipitates(PbSO4,Pb(NO3)2,and PbCl2)formed during reverse aqua regia dissolving of galena are not responsible for the loss of Re.The large amount of Zn2+in sphalerite matrix(>1.0 g)severely reduces the recovery of Re during anion exchange separation.HNO3-based anion exchange is more suitable for Re purification of galena and sphalerite,although the addition of Zn(NO3)2or FeCl3could also significantly promote the recovery of Re.The revised procedure is reliable and can be applied for Re-Os dating of galena and sphalerite.
Acknowledgments This study was supported by the 12th Five-Year Plan Project of State Key Laboratory of Ore Deposit Geochemistry,Chinese Academy of Sciences(SKLODG-ZY125-09;SKLODGZY125-02)and the National Natural Science Foundation of China(Nos.41373064;41430315).Thanks are given to Prof.Sun Yali for useful discussions and suggestions.
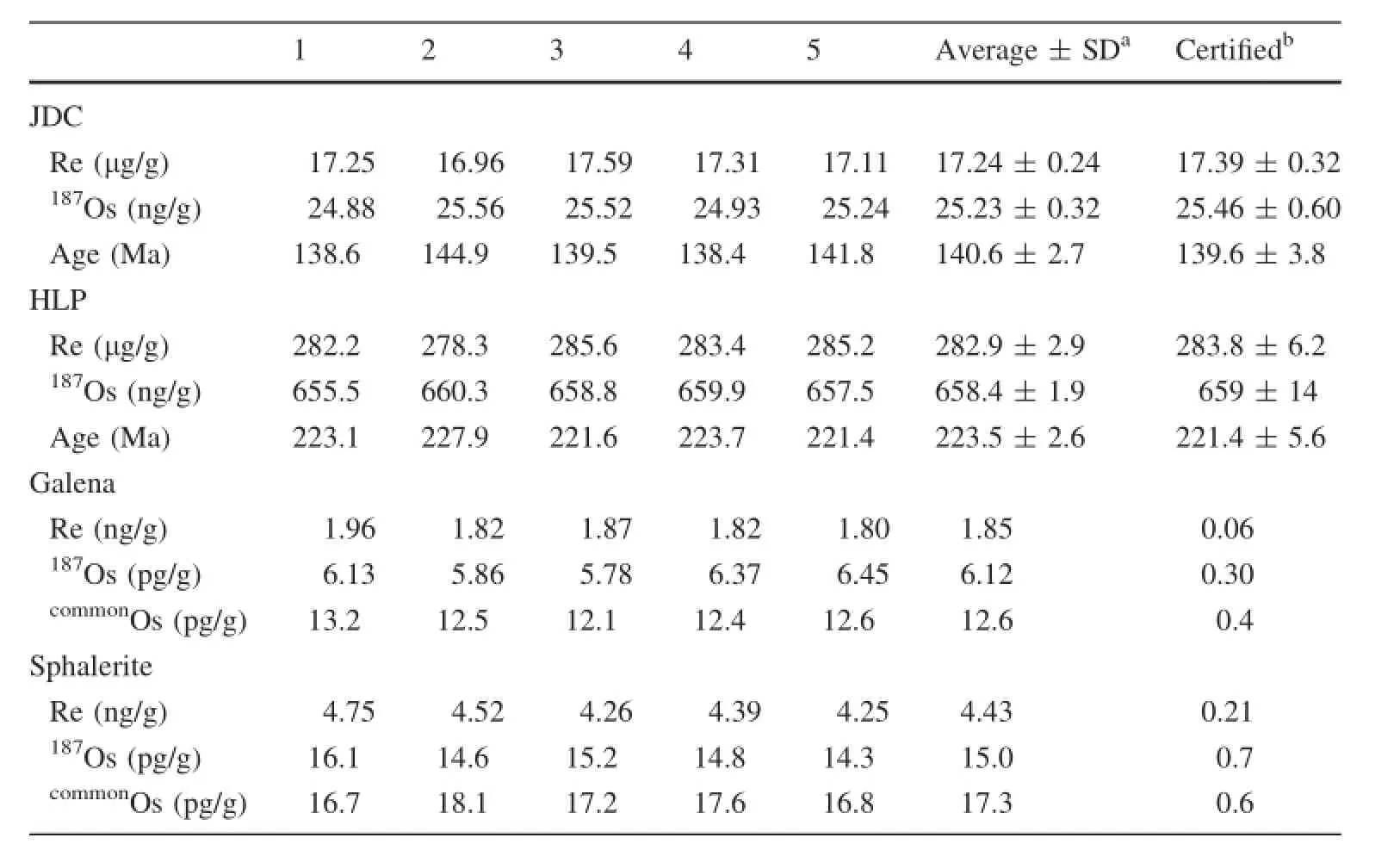
Table 5 Analytical results for molybdenites(HLP and JDC),galena and sphalerite
Akinfiev NN,Tagirov BR(2014)Zn in hydrothermal systems: thermodynamic description of hydroxide,chloride,and hydrosulfide complexes.Geochem Int 52(3):214-232
Birck JL,RoyBarman M,Capmas F(1997)Re-Os isotopic measurements at the femtomole level in natural samples. Geostand Geoanal Res 21(1):19-27
Brill BA(1989)Trace-elmenet contents and partitioning of elements in ore minerals from the CSA Cu-Pb-Zn deposit,Australia.Can Mineral 27:263-274
Chu ZY,Chen FK,Wang W(2007)High-precision measurement for the concentration and isotopic composition of rhenium and osmium in micro-amount of geological samples.Rock Min Anal 26(6):431-435
Colodner D,Edmond J,Boyle E(1995)Rhenium in the Black Sea: comparison with molybdenum and uranium.Earth Planet Sci Lett 131(1-2):1-15
Du AD,He HL,Yin NW(1994)A study on the rhenium-osmium geochronometry of molybdenites.Acta Geol Sin 68(4):339-347
Du AD,Zhao DM,Wang SX(2001)Precise Re-Os dating for molybdenite by ID-NTIMS with Carius tube sample preparation. Rock Miner Anal 20(14):247-252
Du AD,Wu SQ,Sun DZ(2004)Preparation and certification of Re-Os dating reference materials:molybdenites HLP and JDC. Geostand Geoanal Res 28(1):41-52
Harris DJ,Brodholt JP,Sherman DM(2003)Zinc complexation in hydrothermal chloride brines:results from ab initio molecular dynamics calculations.J Phys Chem 107:1050-1054
Huang XW,Qi L,Liu YY(2012)Preliminary study on samplepreparation for Re-Os isotopic dating of pyrite.Geochimica 41(4):380-386
Li C,Qu WJ,Du AD(2009)Comprehensive study on extraction of Rhenium with acetone in Re-Os isotopic dating.Rock Miner Anal 28(3):233-238
Liu YY,Qi L,Zhao Z(2014)Efficiency of a re-usable Carius tube for determination of platinum group elements in ultramafic rocks. Chin J Geochem 33:045-052
Liu YY,Huang ZL,Zhu CM(2015a)A high temperature and high pressure experimental study on Re-bearing capability of sulfide. J Earth Sci(in press)
Liu YY,Qi L,Gao JF(2015b)Re-Os dating of galena and sphalerite from lead-zinc sulfide deposits in Yunnan Province SW China. J Earth Sci 26(3):343-351
Malinovsky D,Rodushkin I,Baxter D(2002)Simplified method for the Re-Os dating of molybdenite using acid digestion and isotope dilution ICP-MS.Anal Chim Acta 463(1):111-124
Meisel T,Fellner N,Moser J(2003a)A simple procedure for the determination of platinum group elements and rhenium(Ru,Rh,Pd,Re,Os,Ir and Pt)using ID-ICP-MS with an inexpensive online matrix separation in geological and environmental materials. J Anal At Spectrom 18(7):720-726
Meisel T,Reisberg L,Moser J(2003b)Re-Os systematics of UB-N,a serpentinized peridotite reference material.Chem Geol 201(1-2):161-179
Morelli RM,Creaser RA,Selby D(2004)Re-Os sulfide geochronology of the red dog sediment-hosted Zn-Pb-Ag deposit,Brooks Range Alaska.Econ Geol 99(7):1569-1576
Morgan JW,Golightly DW,Dorrzapf AF(1991)Methods for the separation of rhenium,osmium and molybdenum applicable to isotope geochemistry.Talanta 38(3):259-265
Qi L,Zhou MF,Wang CY(2007)Evaluation of a technique for determining Re and PGEs in geological samples by ICP-MS coupled with a modified Carius tube digestion.Geochem J 41(6):407
Qi L,Zhou MF,Gao JF(2010)An improved Carius tube technique for determination of low concentrations of Re and Os in pyrites. J Anal At Spectrom 25(4):585
Qi L,Gao JF,Zhou MF(2013)The design of Re-usable Carius tubes for the determination of Rhenium,Osmium and Platinum-Group elements in geological samples.Geostand Geoanal Res 37(3):345-351
Stein HJ,Morgan JW,Schersten A(2000)Re-Os dating of low-level highly radiogenic(LLHR)sulfides:the Harnas gold deposit,southwest Sweden,records continental-scale tectonic events. Econ Geol 95(8):1657-1671
Tagami K,Uchida S(2000)Separation of rhenium by an extraction chromatographic resin for determination by inductively coupled plasma-mass spectrometry.Anal Chim Acta 405(1-2):227-229
Widom E,Kepezhinskas P,Defant M(2003)The nature of metasomatism in the sub-arc mantle wedge:evidence from Re-Os isotopes in Kamchatka peridotite xenoliths.Chem Geol 196:283-306
Xiong Y,Wood SA(1999)Experimental determination of the solubility of ReO2and the dominant oxidation state of rhenium in hydrothermal solutions.Chem Geol 158:245-256
Xiong Y,Wood SA(2000)Experimental quantification of hydrothermal solubility of platinum-group elements with special reference to porphyry copper environments.Mineral Petrol 68:1-28
Xiong Y,Wood SA(2001)Hydrothermal transport and deposition of rhenium under subcritical conditions(up to 200°C)in light of experimental studies.Econ Geol 96:1429-1444
Yang SH,Qu WJ,Du AD(2006)Determination of trace rhenium in geological samples using isootope dilution-inductively coupled plasma mass spectrometry.Rock Miner Anal 25(2):125-128
Received:25 May 2015/Revised:16 July 2015/Accepted:8 October 2015/Published online:30 November 2015
©Science Press,Institute of Geochemistry,CAS and Springer-Verlag Berlin Heidelberg 2015
✉Liang Qi qiliang@vip.gyig.ac.cn;qilianghku@hotmail.com
✉Zhilong Huang huangzhilong@vip.gyig.ac.cn
1State Key Laboratory of Ore Deposit Geochemistry,Institute of Geochemistry,Chinese Academy of Sciences,Guiyang 550180,China
2University of Chinese Academy of Sciences,Beijing 100049,China
杂志排行
Acta Geochimica的其它文章
- The Moon
- Equilibrium and kinetic Si isotope fractionation factors and their implications for Si isotope distributions in the Earth's surface environments
- Study of oxygen fugacity during magma evolution and ore genesis in the Hongge mafic-ultramafic intrusion,the Panxi region,SW China
- Oil-source correlation of Lower-Triassic oil seepages in Ni'erguan village,Southern Guizhou Depression,China
- The inf l uence of three mangrove species on the distribution of inorganic nitrogen and phosphorus in the Quanzhou Bay estuarine wetland soils
- Numerical simulation of groundwater under complex karstconditions and the prediction of roadway gushing in a coal mine: a case study in the Guang'an Longtan Reservoir in Sichuan Province,China
