Wnt3a对结肠癌SW480细胞增殖的影响及作用机制
2016-04-05马松林张姮廖宇圣徐丹吴杰
马松林,张姮,廖宇圣,徐丹,吴杰
(华中科技大学同济医学院附属武汉市中心医院,武汉430014)
Wnt3a对结肠癌SW480细胞增殖的影响及作用机制
马松林,张姮,廖宇圣,徐丹,吴杰
(华中科技大学同济医学院附属武汉市中心医院,武汉430014)
目的 探讨Wnt3a对结肠癌SW480细胞增殖的调控效应及其作用机制。方法 将培养好的结肠癌细胞SW480随机分为对照组和Wnt3a组,对照组仅给予DMEM培养基处理,Wnt3a组给予100 ng/mL Wnt3a培养。用MTT法检测细胞增殖情况,计算相对活细胞百分比;用平板克隆形成实验检测细胞克隆形成率(PE);用流式细胞术检测细胞周期分布;用Western blotting法检测细胞内醛脱氢酶1B1(ALDH1B1)蛋白表达,RT-PCR技术检测ALDH1B1 mRNA表达。结果 Wnt3a诱导24、48、72 h,Wnt3a组相对活细胞百分比高于对照组(P均<0.05)。Wnt3a诱导7 d,Wnt3a组PE高于对照组(P<0.05)。Wnt3a诱导24 h,Wnt3a组G1期细胞百分比低于对照组,G2期细胞百分比高于对照组(P均<0.05)。Wnt3a诱导48 h,Wnt3a组ALDH1B1蛋白、mRNA相对表达量高于对照组(P均<0.05)。结论 Wnt3a可能通过诱导ALDH1B1高表达促进结肠癌SW480细胞增殖。
结肠肿瘤;SW480细胞;Wnt3a;醛脱氢酶1B1;细胞增殖
结肠癌是最常见的恶性肿瘤之一。据调查,2014年美国有结肠癌新增病例136 830例,因结肠癌死亡50 310例[1]。结肠癌的发病与几种致瘤信号通路的异常激活密切相关。其中,Wnt/β-catenin信号通路被认为是结肠癌发病的重要原因之一[2]。Wnt/β-catenin信号的异常激活引导结肠正常细胞向癌细胞转变、促进结肠癌细胞向结肠基底细胞侵袭[2~4]。Wnt3a是Wnt信号蛋白的重要成员。研究表明,Wnt3a能促进结肠癌肿瘤组织血管形成[5,6],然而其对结肠癌细胞的增殖调控效应及其作用机制目前依然未知。2015年1月~2016年1月,本研究就Wnt3a对结肠癌细胞SW480增殖的调控作用及其分子机制进行了探讨。
1 材料与方法
1.1 材料 DMEM培养基、胎牛血清(FBS)、胰蛋白酶(购自美国Hyclone公司),TRIzol(美国Invitrogen公司),醛脱氢酶1B1抗体(anti-ALDH1B1)和anti-GAPDH(美国Santa Cruz公司),HRP标记的二抗(英国Abcam公司),人类结肠癌细胞SW480(美国ATCC细胞库)。
1.2 细胞培养及分组 结肠癌SW480细胞均采用DMEM培养基(添加10% FBS以及抗生素)于37 ℃、5%二氧化碳、饱和湿度的恒温细胞培养箱中培养。2~3 d传代1次,将处于对数生长期的细胞经消化分散后计数,制成细胞悬液。将SW480细胞随机分为对照组和Wnt3a组。对照组仅给予DMEM培养基处理,Wnt3a组给予100 ng/mL Wnt3a培养24 h。
1.3 细胞增殖情况检测 采用MTT法。Wnt3a诱导24、48、72 h,分别取SW480细胞按每孔10 000个接种于12孔板中,培养24 h 后添加5 mg/mL的MTT溶液40 μL,孵育4 h 后每孔加DMSO 200 μL。用酶标仪于波长490 nm处测吸光度值。计算相对活细胞百分比,相对活细胞百分比=实验组吸光度值/对照组吸光度值×100%。
1.4 细胞克隆形成率(PE)检测 采用平板克隆形成实验。Wnt3a诱导24 h,将细胞接种于12孔板中,每孔接种600个细胞。继续培养8 d,先去除培养液,用PBS清洗3遍,甲醇固定20 min,1%亚甲基蓝染色40 min,去离子水清洗两遍,晾干。显微镜下计数不少于50个细胞的克隆数,计算PE。PE=克隆数/接种细胞数×100%。
1.5 细胞周期检测 采用流式细胞术。Wnt3a诱导24 h,取细胞用PBS洗涤两遍,用70%乙醇固定,4 ℃保存过夜。PBS清洗1次,将细胞调整为1×106/mL,加入碘化丙啶染色液,染色30 min。采用流式细胞术检测细胞周期,计算各周期细胞百分比。
1.6 细胞内ALDH1B1表达检测 ①采用Western blotting法检测细胞内ALDH1B1蛋白表达。Wnt3a诱导48 h,根据文献[7]进行操作,使用anti-ALDH1B1一抗及anti-GAPDH一抗,浓度为1∶200,二抗浓度为1∶1 000。用Quantity One 1-D分析软件对蛋白质印迹条带进行分析。目的蛋白相对表达量=目的蛋白灰度值/内参蛋白灰度值。②采用RT-PCR技术检测细胞内ALDH1B1 mRNA表达。Wnt3a诱导48 h,按照TRIzol reagent说明书提取细胞总RNA,将所得的RNA使用ImPron-Ⅱ逆转录系统(Roche,USA)逆转录。使用SYBR Green PCR master mix试剂在ABI 7500 RT-PCR仪中进行RT-PCR。ALDH1B1 正向引物:5′-CCCATTCTGAACCCAGACATC-3′;反向引物:5′-AATGACCTCCCCGGTGGTA-3′;β-actin正向引物:5′-TGGCACCCAGCACAATGAA-3′;反向引物:3′-CTAAGTCATAGTCC- GCCTAGAAGCA-5′。反应条件:95 ℃ 5 min, 94 ℃变性30 s,60 ℃退火30 s,共进行40个循环。采用2-ΔΔCt法计算目的基因相对表达量。
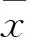
2 结果
2.1 两组细胞增殖情况比较 Wnt3a诱导24、48、72 h,Wnt3a组相对活细胞百分比分别为(240.63±27.34)%、(340.43±37.04)%、(410.23±45.21)%,对照组分别为(188.46±16.67)%、(236.75±26.68)%、(286.42±26.53)%;两组各时间点活细胞百分比比较差异有统计学意义(P均<0.05)。
2.2 两组PE比较 Wnt3a诱导7 d,Wnt3a组PE为(69.53±3.28)%,对照组为(27.36±2.57)%,两组PE比较差异有统计学意义(P<0.05)。
2.3 两组细胞周期分布情况比较 Wnt3a诱导24 h,Wnt3a组G1、G2期细胞百分比分别为(33.7±3.6)%、(21.2±2.5)%,对照组分别为(48.3±4.8)%、(10.8±1.4)%;两组细胞周期分布比较差异有统计学意义(P均<0.05)。
2.4 两组ALDH1B1表达比较 Wnt3a 诱导48 h,Wnt3a组ALDH1B1蛋白相对表达量为1.95±0.18,mRNA相对表达量为13.75±1.82,对照组分别为0.48±0.04、1.00±0.00;两组ALDH1B1蛋白、mRNA相对表达量比较差异有统计学意义(P均<0.05)。
3 讨论
近年来,对结肠癌分子机制的研究已经取得了显著进展。然而,目前为止,结肠癌依然是高度致死性的癌症之一[1]。研究表明,在结肠癌的发病过程中,Wnt/β-catenin信号通路出现异常,包括c-Myc和CyclinD1等在内Wnt/β-catenin信号依赖性分子转录失调,被认为是导致结肠癌发生的重要原因[5]。有催化活性的ALDH已被认定为多种肿瘤和肿瘤干细胞的生物标志物[5,6]。研究表明,在正常人类结肠,ALDH1B1仅在隐窝基底部及干细胞中表达。然而,该蛋白也在人结肠腺癌中高度表达[6,7]。有研究结果表明,ALDH1B1在结肠癌细胞的基因表达谱与Wnt/β-catenin信号活性具有某种同步性,因此,ALDH1B1被认为是结肠癌发生的原因之一[8]。Wnt3a是Wnt信号蛋白的重要成员,能促进结肠癌肿瘤组织血管形成[3]。然而,Wnt3a与ALDH1B1的相互作用依然未知。本研究着重探讨了Wnt3a对结肠癌细胞SW480的细胞增殖调控作用并进一步探讨了Wnt3a对ALDH1B1的基因表达调控效应。
ALDH1B1的生物学功能是通过代谢视黄醛而产生视黄酸(RA)[9]。RA作为一种维生素A类衍生物,是细胞增殖发育的必需成分[10]。RA能结合细胞视黄酸结合蛋白(CARBPⅡ)和脂肪酸结合蛋白5(FABP5)[11]。RA诱导CARBPⅡ和FABP5介导的视黄酸受体(RAR)或PPARβ/δ激活[12]。RAR的激活能刺激细胞分化,并抑制细胞增殖[13]。然而,PPARβ/δ的激活对PI3K/Akt介导的肿瘤生长有促进效应[14]。FABP5在结肠癌细胞中高表达,因此,推测RA能通过诱导FABP5激活PPARβ/δ从而促进细胞增殖、抗凋亡及促进肿瘤生长[15,16]。根据本研究的结果发现,Wnt3a促进ALDH1B1的基因表达,后者又可能通过激活PPARβ/δ,进而最终促进PI3K/Akt介导的肿瘤生长。
本研究利用Wnt3a蛋白诱导结肠癌细胞SW480,通过MTT活细胞计数、克隆形成实验及细胞周期分析发现,Wnt3a能显著促进SW480细胞生长、克隆形成及促使SW480细胞进入G2期。同时发现,Wnt3a能上调SW480细胞内ALDH1B1表达。ALDH1B1是一种重要的结肠癌细胞增殖促进因子[6],Wnt3a通过促进ALDH1B1的表达,从而促进结肠癌细胞的增殖。
[1] DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014[J]. CA Cancer J Clin, 2014,64(4):252-271.
[2] Reya T, Clevers H. Wnt signalling in stem cells and cancer[J]. Nature, 2005,434(7035):843-850.
[3] Morin PJ, Sparks AB, Korinek V, et al. Activation of β-catenin-Tcf signaling in colon cancer by mutations in β-catenin or APC[J]. Science, 1997,275(5307):1787-1790.
[4] Rubinfeld B, Albert I, Porfiri E, et al. Loss of β-catenin regulation by the APC tumor suppressor protein correlates with loss of structure due to common somatic mutations of the gene[J]. Cancer Res, 1997,57(20):4624-4630.
[5] You Z, Saims D, Chen S, et al. Wnt signaling promotes oncogenic transformation by inhibiting c-Myc-induced apoptosis[J]. J Cell Biol, 2002,157(3):429-440.
[6] Jackson BC, Holmes RS, Backos DS, et al. Comparative genomics, molecular evolution and computational modeling of ALDH1B1 and ALDH2[J]. Chem Biol Interact, 2013,202(1):11-21.
[7] Moreb JS, Mona M, Chang LJ, et al. The role of aldehyde dehydrogenase isoenzymes in cancer cell proliferation, migration and drug resistance[J]. Cancer Res, 2013,73(8 Supple):3762.
[8] Ida N, Hartmann T, Pantel J, et al. Analysis of heterogeneous βA4 peptides in human cerebrospinal fluid and blood by a newly developed sensitive Western blot assay[J]. J Biol Chem, 1996,271(37):22908-22914.
[9] Breitman TR, Selonick SE, Collins SJ. Induction of differentiation of the human promyelocytic leukemia cell line (HL-60) by retinoic acid[J]. Proc Natl Acad Sci U S A, 1980,77(5):2936-2940.
[10] Hertzel AV, Bernlohr DA. The mammalian fatty acid-binding protein multigene family: molecular and genetic insights into function[J]. Trends Endocrin Met, 2000,11(5):175-180.
[11] Furuhashi M, Hotamisligil GS. Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets[J]. Nat Rev Drug Discov, 2008,7(6):489-503.
[12] Schug TT, Berry DC, Toshkov IA, et al. Overcoming retinoic acid-resistance of mammary carcinomas by diverting retinoic acid from PPARβ/δ to RAR[J]. Proc Natl Acad Sci U S A, 2008,105(21):7546-7551.
[13] Berry DC, Noy N. All-trans-retinoic acid represses obesity and insulin resistance by activating both peroxisome proliferation-activated receptor β/δ and retinoic acid receptor[J]. Mol Cell Biol, 2009,29(12):3286-3296.
[14] Han S, Ritzenthaler JD, Zheng Y, et al. PPARβ/δ agonist stimulates human lung carcinoma cell growth through inhibition of PTEN expression: the involvement of PI3K and NF-κB signals[J]. Am J Physiol Lung Cell Mol Physiol, 2008,294(6):L1238-L1249.
[15] Koshiyama A, Ichibangase T, Imai K. Comprehensive fluorogenic derivatization-liquid chromatography/tandem mass spectrometry proteomic analysis of colorectal cancer cell to identify biomarker candidate[J]. Biomed Chromatogr, 2013,27(4):440-450.
[16] Peters JM, Foreman JE, Gonzalez FJ. Dissecting the role of peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) in colon, breast, and lung carcinogenesis[J]. Cancer Metast Rev, 2011,30(3-4):619-640.
Effect of Wnt3a on cell proliferation of colon cancer and its mechanism
MASonglin,ZHANGHeng,LIAOYusheng,XUDan,WUJie
(WuhanCentralHospitalAffiliatedtoHuazhongUniversityofScienceandTechnology,Wuhan430014,China)
Objective To investigate the potential role of Wnt3a in the regulation of colon cancer cell proliferation and its regulatory mechanism. Methods Colon carcinoma cell line SW480 was randomly divided into two groups, the control group treated with DMEM, and the Wnt3a group treated with 100 ng/mL Wnt3a. The proliferation ability was measured by MTT. The relative percentage of living cells was also counted. Cell clone formation rate (PE) was measured by plate clone formation assay. Cell cycle was analyzed by flow cytometry. RT-PCR was used to measure the expression level of ALDH1B1 mRNA. The expression level of aldehyde dehydrogenase (ALDH)1B1 protein was measured by Western blotting. Results After 24, 48 and 72 h of induction by Wnt3a, the relative percentage of living cells in the Wnt3a group was significantly higher than that of the control group (allP<0.05). After 7 d of induction by Wnt3a, the PE of the Wnt3a group was significantly higher than that of the control group (P<0.05). The cell percentage of G1phase in the Wnt3a group was significantly lower, and the cell percentage of G2phase was significantly higher than that of the control group after 24-hour treatment of Wnt3a (allP<0.05). After 48-hour treatment with Wnt3a, the expression level of ALDH1B1 protein and mRNA in the Wnt3a group was significantly higher than that of the control group (allP<0.05).Conclusion Wnt3a promotes colon cancer cell proliferation by inducing the high expression of ALDH1B1.
colonic neoplasms; SW480 cells; Wnt3a; aldehyde dehydrogenase1B1; cell proliferation
马松林(1979-),男,主治医师,硕士,主要研究方向为消化道肿瘤。E-mail:msl1002@126.com
简介:吴杰(1958-),男,主任医师,硕士生导师,主要研究方向为消化道肿瘤。E-mail:47343977@qq.com
10.3969/j.issn.1002-266X.2016.37.006
R735.35
A
1002-266X(2016)37-0018-03
2016-03-12)
