土壤中汞胁迫对牧草生理生化的影响
2016-03-21王道涵杜俊楠李玉娥初世敬
王道涵,杜俊楠,李玉娥,初世敬
(辽宁工程技术大学 环境科学与工程学院,阜新 123000)
土壤中汞胁迫对牧草生理生化的影响
王道涵,杜俊楠,李玉娥,初世敬
(辽宁工程技术大学 环境科学与工程学院,阜新 123000)
研究紫花苜蓿、披碱草、高羊茅三种牧草,在10个浓度梯度汞胁迫条件下发芽率、植株高度、过氧化物酶与过氧化氢酶活性、丙二醛与脯氨酸含量、可溶性蛋白及可溶性糖含量生理生化指标的变化情况。旨在探索土壤中汞对牧草的影响机理,反映土壤中汞含量富集过多后对草原的影响。
土壤;汞;牧草;生理生化
自然界土壤中汞的来源主要通过大气沉降、燃煤、火山爆发等形式释放到环境中,在迁移转化过程中,通过干、湿沉降的形式进入土壤(王起超等,1999)。煤炭资源作为内蒙古的支柱性矿产资源(党民团和刘娟,2005),2012年以来,内蒙古部分大型煤炭企业融合电力行业,大力发展燃煤电厂,促进煤电一体化形式的发展。这同时预示着内蒙古地区煤碳消耗量将有所增长。内蒙古煤炭中平均汞含量为0.28 mg · kg-1,煤炭燃烧向大气中排放的汞对环境的污染具有严重的影响(张静静等,2014)。
污染源释放出来的汞,一小部分依附于颗粒,数天内沉降在离释放源数公里的范围内。美国EPA估计,美国燃煤电厂烟尘所含的汞约75%会进入高层大气循环。HgO和GEM是大气汞的主要存在形态,占大气总汞的95%—99%,可在大气中停留0.5—2年,最终通过水-气等界面间HgO的交换和大气干、湿沉降进入地表冰雪和湖泊等介质或其他陆地生态系统,在土壤中与其他化合物形成络合物(Gbor et al,2007;Lai et al,2007)。汞在土壤环境中由于受pH、有机配体、无机配体及Eh等因素的影响(汪霞等,2010),在微生物作用下,土壤汞的存在形态相互转化,外源汞的不同形态汞向惰性汞转化。陈丽萍和胡恭任(2009)提出连续提取汞相态分类法,将汞的形态分为可交换态、碳酸盐结合态、铁锰氧化物结合态、有机物结合态和残渣态,应用最为广泛。Leticia et al(2006)将土壤中汞分为可交换态汞、碳酸汞、铁锰结合态汞、有机汞和残留汞。相关研究(Bravo,2014;Meng,2014;Stolle et al,2014)表明在外源汞加入的条件下,种植植物后土壤中水溶解态的汞、腐植酸结合态汞的含量显著减少,而有机质结合态明显增加,随着外源汞加入量的增加,土壤中汞的残渣态、有机质结合态的含量减少,而交换态、碳酸盐、铁锰氧化态的含量相对增加(陈礼洪等,2015)。汞属于非降解型有毒元素,毒性效应是不可降解的。土壤中汞达到一定量时会影响植物的形态结构和生理生化过程(付学吾等,2005)。土壤中汞被植物吸收后可通过多种途径对植物产生影响,影响生物大分子的合成与功能,进而导致组织功能异常、影响光合作用效率、影响植物生长发育、破坏植物体内元素平衡等。汞可以影响植物细胞的分裂,导致植物细胞膜脂过氧化程度升高,从而抑制植物正常生长发育。
汞可以通过土壤-植物的吸收、累积和富集效应,通过食物链的作用,进而流向人类种群,并随着食物链的逐级放大,逐步增大富集量,对人类健康产生危害。随着草原燃煤电厂的发展建设,燃煤烟气中汞的排放,将随时间的推移在草原土壤中逐步累积。
本文通过模拟汞沉降后土壤对三种牧草生理生化特性的影响,研究汞胁迫下牧草生理生化特性,为揭示草原土壤累积汞后对牧草影响提供理论依据。
1 材料与方法
1.1 室内盆栽试验
试验材料为紫花苜蓿(Medicago sativa)、披碱草(Elymus dahuricus Turcz. ex Griseb.)、高羊茅(Festuca elata Keng ex E. Alexeev),牧草种子供试种子由中国科学院内蒙古东乌珠穆沁旗草原站提供。试验土壤采自中国科学院内蒙古东乌珠穆沁旗草原站草场。试验土壤基本理化性质及汞含量见表1。

表1 土壤理化参数及汞含量表Tab.1 Physicochemical statistics parameters and mercury contents of soil
采用盆栽控制实验,土样风干、研磨后过2 mm尼龙筛后混匀,每盆7 cm × 7 cm × 7 cm(高),装土150 g(干重)。根据国家土壤环境质量标准三级标准结合土壤汞安全临界值(黄玉芬,2011;洪曾纯,2012),依照等比法设置处理水平间隔,模拟大气汞沉降进入本底土壤后影响,HgCl2处理设置10个水平添加外源汞T1—T10(0、0.5 mg · kg-1、1.0 mg · kg-1、1.5 mg · kg-1、2.0 mg · kg-1、4.0 mg · kg-1、8.0 mg · kg-1、16.0 mg · kg-1、32.0 mg · kg-1、64.0 mg · kg-1),3次重复,土样混匀,稳定7天。供试种子筛选出的饱满籽粒的种子(紫花苜蓿、披碱草、高羊茅)浸泡在蒸馏水中催芽,露白后选择长势均一的种子种花盆中,每盆种入15颗。日常以去离子水维持适宜水分,采用自然光照。培养7周后取样进行测定(胡宗英,2014)。
1.2 测定方法
过氧化物酶(POD)活性采用愈创木酚法测定(薛亮,2013)。过氧化氢酶(CAT)活性采用紫外吸收法测定(宋云华等,2007)。丙二醛(MDA)采用硫代巴比妥酸比色法测定(潘瑞炽,1994)。脯氨酸采用磺基水杨酸法测定(华东师范大学生物系植物生理教研室,1980)。可溶性蛋白采用考马斯亮蓝染色法测定。可溶性糖含量采用蒽酮法测定(北京师范大学生物系生化教研室,1982)。
1.3 数据分析
试验数据采用SPSS19.0软件对所测数据统计分析,用平均值和标准误差表示测定结果;采用WPS 2016制图。
2 结果与分析
2.1 汞对牧草种子发芽率、植株高度的影响
以第7天统计发芽植株数量与播种总量之比计算发芽率,可反映植株生长状况。在最终收取植株样品过程中,用刻度尺对植株高度进行测量,记录最终生长状况。
根据对植株生长情况的监测记录,整理并分析可知(表2),高浓度Hg2+抑制了三种牧草的种子萌发及植株高度。土壤中Hg2+浓度低于8.0 mg · kg-1时,紫花苜蓿种子发芽率比对照组有所提高,依照Hg2+浓度,分别提高了4.32%,16.70%,25.14%,37.52%,8.44%和8.44%,影响程度先增高后降低。当Hg2+浓度大于8.0 mg · kg-1时,种子发芽率降低,降低随Hg2+浓度升高而增强。披碱草和高羊茅种子发芽率随土壤中Hg2+浓度升高而逐步降低,分别由降低2.36%至44.93%和6.85%至52.25%,Hg2+浓度越高对发芽率影响幅度越大。这一现象的出现原因可能由于,汞对紫花苜蓿种子萌芽初期有短暂的促进作用(高大翔等,2008),但高浓度则表现为抑制作用。
随Hg2+浓度升高,三种牧草植株高度比对照组始终呈降低趋势,降低幅度分别由9.50%至52.99%,3.69%至27.95%,10.11%至48.57%。这与潘瑞炽曾指出的汞抑制植物根系正常发育,使植株变矮的这一理论相符(潘瑞炽,1994)。
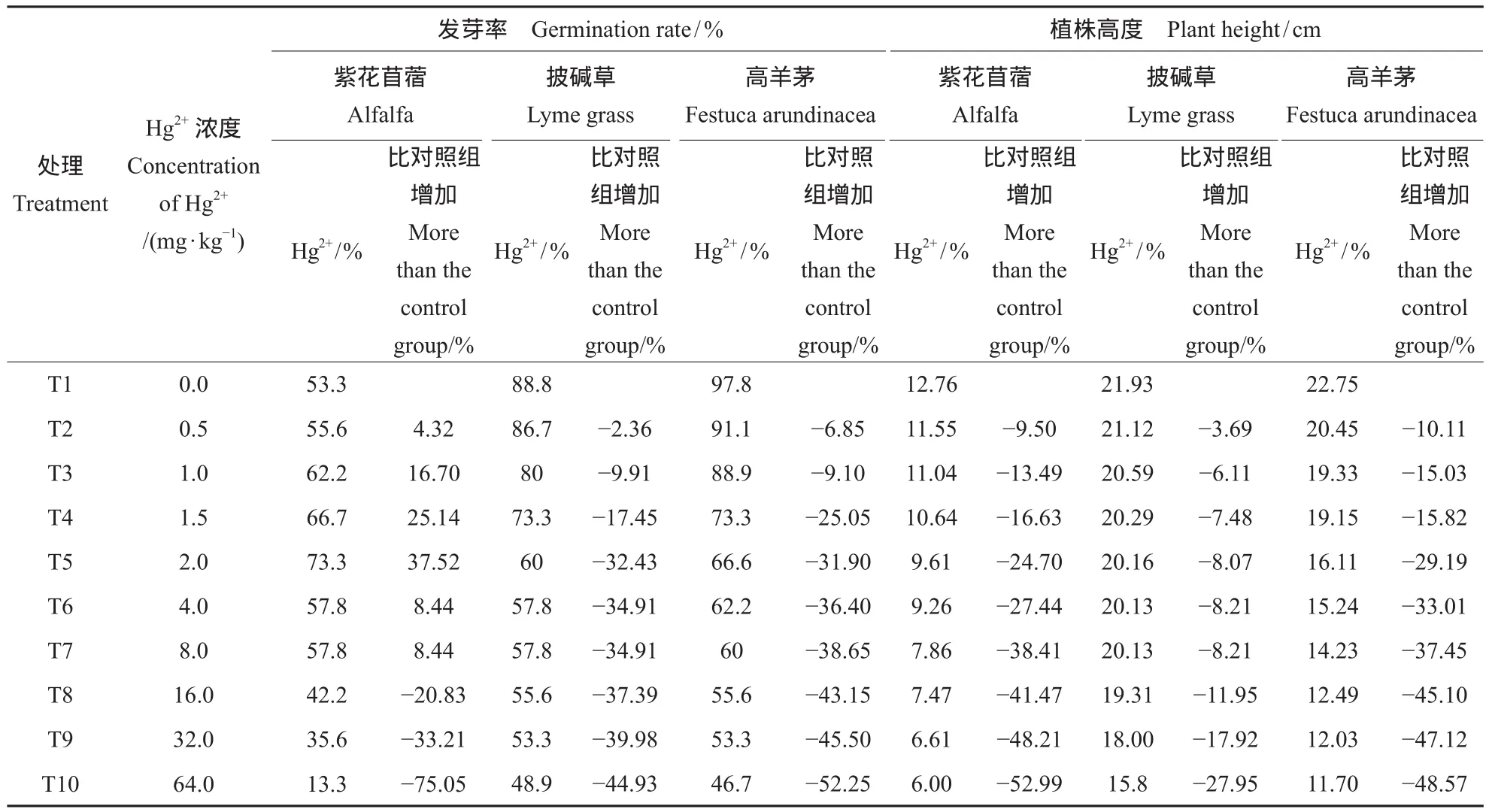
表2 Hg2+对牧草发芽率及植株高度影响Tab.2 Impact on grass germination rate and plant height of Hg2+
2.2 汞对牧草过氧化物酶(POD)、过氧化氢酶(CAT)活性的影响
过氧化物酶(POD)是普遍存在于植物体内的较高活性酶。植物生长发育中,其活性不断发生变化,与呼吸作用、光合作用及生长素的氧化等紧密相关。POD在生物体内可以消除对氧自由基、过氧化物,抑制自由基对膜脂的过氧化作用,防止对膜造成损伤和破坏,同时POD还参与膜外活性氧的产生。由于植物对汞害的敏感性与POD酶变化间存在着一定的关系,因此可将POD酶带的变化视为一种生化指标(母波等,2007),来检测植物受汞胁迫后的损害程度并筛选耐汞植物品种。过氧化氢酶(CAT)可以减少植物中的H2O2,降低H2O2在体内积累而限制潜在的氧伤害。植物细胞具有产生和清除自由基两个过程,二者协调一致,自由基才能保持在低含量水平,防止细胞受自由基毒害。汞能引起植物产生O-和H2O2等自由基,并随着汞处理浓度的增加而上升(刘建新,2005)。CAT、POD是植物对膜脂过氧化的酶促防御系统的保护酶。
本文研究表明(图1),在土壤中Hg2+胁迫下,Hg2+浓度低于1.5 mg · kg-1时,紫花苜蓿POD活性呈上升趋势,当Hg2+浓度大于1.5 mg · kg-1时,紫花苜蓿POD活性呈下降趋势。披碱草POD活性整体呈上升趋势。高羊茅POD活性平稳。说明三种牧草相比较,披碱草抑制自由基对膜脂过氧化作用能力最强。
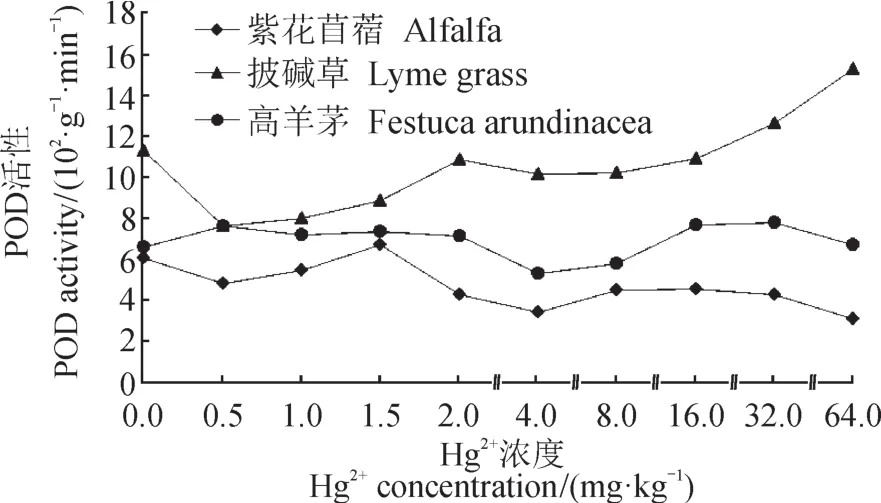
图1 Hg2+胁迫对牧草POD活性的影响Fig.1 The effects of Hg2+stress on the POD in pasture
在土壤中Hg2+胁迫下,Hg2+浓度低于2.0 mg · kg-1时,三种牧草CAT活性均呈上升趋势,当Hg2+浓度高于2.0 mg · kg-1时,皆出现降低趋势,其中披碱草在Hg2+浓度高于32.0 mg · kg-1时活性升高;高羊茅在Hg2+浓度高于16.0 mg · kg-1时活性持续升高(图2)。数据表明,三种牧草在Hg2+浓度较低时,通过提升CAT活性,清除植物中的H2O2,避免细胞受自由基毒害。当Hg2+浓度过高时CAT酶表达受到抑制,导致CAT活性降低。
2.3 汞对牧草丙二醛、脯氨酸含量的影响
丙二醛(MDA)是膜质过氧化的产物, MDA含量可以反映膜质过氧化程度,反映植物遭受逆境伤害的程度。细胞毒性物质破坏膜结构,并与膜结构上的酶和蛋白质发生作用并使细胞失去固有活性,通过与蛋白质分子发生聚合,类囊体膨胀变形、排列顺序改变,最终导致细胞基粒消失等叶绿体超微结构发生改变(梁胜伟等,2009)。MDA可使叶绿素含量减少,导致光合作用减弱。汞通过作用于细胞膜上磷脂,使细胞膜透性改变。植物在汞胁迫下,细胞内活性自由基含量增加,膜中不饱和脂肪酸产生过氧化反应,从而使膜的结构和功能遭到破坏。
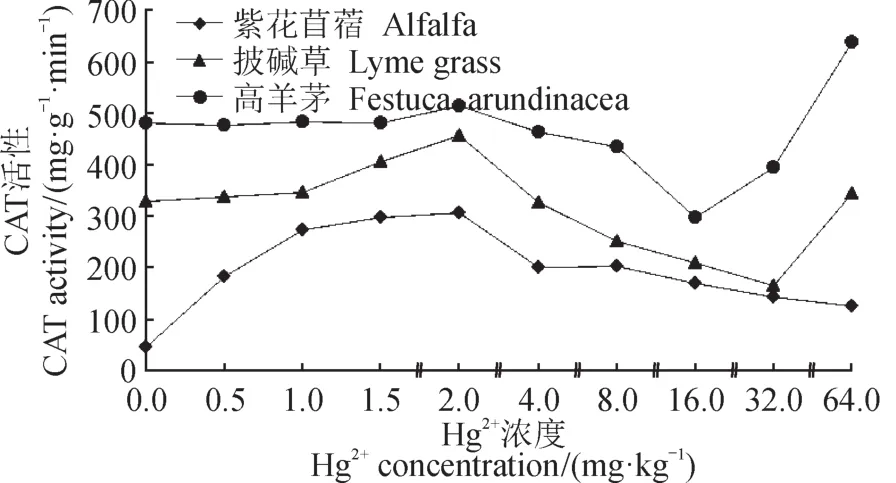
图2 Hg2+胁迫对牧草CAT活性的影响Fig.2 The effects of Hg2+stress on the CAT in pasture
脯氨酸(Pro)作为植物蛋白质的组分可以以游离态广泛存在于植物体中。脯氨酸一方面作为植物细胞质内渗透调节物质,另一方面可以稳定生物大分子结构、降低细胞酸性、解除氨毒以及调节细胞氧化还原势(马成仓和洪法水,1998)。植物内脯氨酸含量可以代表植物抗逆性,脯氨酸含量高则抗逆性强。同时脯氨酸还有降低细胞内水势的作用,从而能较高效的对于各种酶系以及复合体蛋白的四级结构起到保护作用,维持细胞系统在逆境中的稳定性,降低膜脂过氧化。脯氨酸的合成主要在线粒体中进行,主要分布于线粒体及细胞质基质中,从而提高细胞质的渗透压,对细胞质与液泡间的渗透压差调节,使在渗透胁迫下的叶绿体和线粒体仍能维持较好的水分状况,保障光合作用和呼吸作用的运行。
在土壤中Hg2+胁迫下,紫花苜蓿丙二醛含量持续降低;披碱草丙二醛含量亦呈下降趋势,至Hg2+浓度高于32.0 mg · kg-1后出现较前一处理浓度下,其含量升高;高羊茅丙二醛含量则呈先降低后升高的趋势,当土壤Hg2+浓度高于1.5 mg · kg-1时,丙二醛含量逐渐升高(图3)。说明在土壤中Hg2+胁迫下,高浓度时,高羊茅膜脂过氧化程度高,植物细胞膜受Hg2+胁迫影响大,而紫花苜蓿和披碱草膜脂过氧化程度相对较轻。

图3 Hg2+胁迫对牧草丙二醛含量的影响Fig.3 The effects of Hg2+stress on the contents of MDA in pasture
同时,紫花苜蓿脯氨酸含量较无胁迫状态有明显下降,并保持平稳趋势;披碱草脯氨酸含量较无胁迫状态有明显升高,且当土壤Hg2+浓度达到64.0 mg · kg-1时,含量增加幅度较大,比对照组增加252.78%;高羊茅脯氨酸含量呈升高趋势,且土壤Hg2+浓度高于8.0 mg · kg-1时,含量急剧升高(图4)。说明三种牧草中,高羊茅对于Hg2+胁迫,抗逆性最强。

图4 Hg2+胁迫对牧草脯氨酸含量的影响Fig.4 The effects of Hg2+stress on the contents of proline in pasture
2.4 汞对牧草可溶性蛋白、可溶性糖含量的影响
植物细胞中可溶性蛋白含量直接可以反映细胞内蛋白质合成、变性及降解等状态(徐小蓉等,2011)。Hg2+能增加细胞内核糖体,核糖体亚基及多聚核糖体的数量,促进蛋白质合成。由于低浓度的Hg2+作用DNA后,刺激了DNA的活性,促进了有关基因的表达。可溶性糖属于植物的逆境指标。可溶性糖越多,植物抗性越强 ,呈正比关系。在作物的碳素营养中,可溶性糖作为营养物质可以合成纤维素并组成细胞壁;转化并组成其他有机物如核苷酸、核酸等;分解产物是其他许多有机物合成的原料,为作物的各种合成过程和各种生命活动提供了所需的能量。
在土壤中Hg2+胁迫下,三种牧草可溶性蛋白质含量在较低浓度下,皆高于无胁迫状态,随着胁迫浓度升高,逐步降低,至高浓度胁迫状态下又出现升高(图5)。三种牧草出现含量变化趋势改变的浓度不同,表明三种牧草DNA活性受Hg2+胁迫影响程度不同:Hg2+胁迫浓度低于8 mg · kg-1或高于16 mg · kg-1时会促进紫花苜蓿蛋白质的合成;Hg2+胁迫浓度低于16 mg · kg-1或高于32 mg · kg-1时会促进披碱草蛋白质的合成;Hg2+胁迫始终促进高羊茅蛋白质的合成。同时,分析可溶性糖含量(图6),Hg2+胁迫下紫花苜蓿可溶性糖含量降低,高羊茅在汞浓度高于32 mg · kg-1时含量升高,披碱草在Hg2+胁迫状态下可溶性糖含量始终高于无胁迫状态。说明在积累糖类物质中,披碱草有较明显优势。
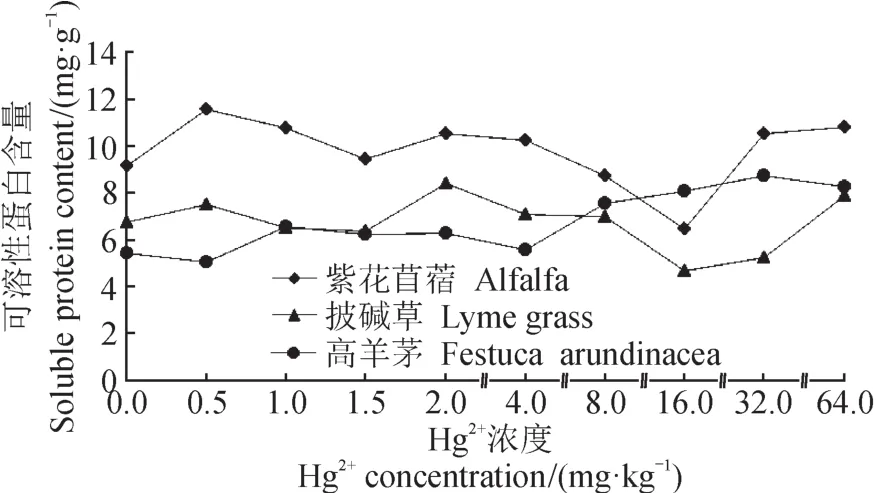
图5 Hg2+胁迫对牧草可溶性蛋白含量的影响Fig.5 The effects of Hg2+stress on the contents of soluble protein in pasture

图6 Hg2+胁迫对牧草可溶性糖含量的影响Fig.6 The effects of Hg2+stress on the contents of soluble sugar in pasture
3 讨论与结论
土壤中汞胁迫对紫花苜蓿、披碱草、高羊茅三种牧草生理生化特征影响如下:(1)发芽率:低浓度Hg2+胁迫下,会促进紫花苜蓿种子发芽率,高浓度则抑制;Hg2+胁迫会降低披碱草和高羊茅种子发芽率,而且浓度越高,发芽率越低。(2)植株高度:Hg2+胁迫下,三种植株高度皆降低。(3)过氧化物酶活性:随着Hg2+浓度升高,紫花苜蓿POD活性先上升后降低;披碱草POD活性上升;高羊茅POD活性保持平稳。(4)过氧化氢酶活性:三种牧草CAT活性,随着Hg2+浓度升高,先上升后降低。(5)丙二醛含量:随着Hg2+浓度升高,紫花苜蓿丙二醛含量降低;披碱草和高羊茅丙二醛含量先降低后升高。(6)脯氨酸含量:Hg2+胁迫下,紫花苜蓿中脯氨酸含量下降,并保持平稳;披碱草和高羊茅脯氨酸含量上升。(7)可溶性蛋白含量:三种牧草皆表现为先升高后降低,高浓度胁迫下再升高的变化规律。(8)可溶性糖含量:在Hg2+胁迫下,紫花苜蓿可溶性糖含量降低,披碱草和高羊茅含量上升。
北京师范大学生物系生化教研室. 1982. 基础生物化学实验 [M]. 北京:人民教育出版社: 142 – 162. [Beijing Normal University. Department of Biology. Biochemistry Teaching and Research Section. 1982. Foundation biochemistry experiment [M]. Beijing: People's Education Press: 142 – 162.]
陈礼洪,赵康平,蒋柱武,等. 2015. 土壤汞污染对油菜的氧化胁迫效应[J].环境化学, 34(2): 241 – 246. [Chen L H, Zhao K P, Jiang Z W, et al. 2015. Oxidative stress induced by mercury contaminated soil onBrassica juncea[J].Environmental Chemistry, 34(2): 241 – 246.]
陈丽萍, 胡恭任. 2009. 土壤和沉积物中汞的提取与检测方法研究进展[J].环境与健康杂志, 26(6): 557 – 560. [Chen L P, Hu G R. 2009. The extraction and determination of mercury in soil and sediment are reviewed [J].Environment and Health, 26(6): 557 – 560.]
党民团,刘 娟. 2005. 中国汞污染的现状及防止对策[J].应用化工, 34(7): 394 – 396. [Dang M T, Liu J. 2005. The current situation of mercury pollution in China and preventing countermeasures [J].Applied Chemical Industry, 34(7): 394 – 396.]
付学吾,冯新斌,王少锋, 等. 2005. 植物中汞的研究进展[J].矿物岩石地球化学通报, 3(24): 232 – 238. [Fu X W, Feng X B, Wang S F, et al. 2005. The research progress of mercury in plants [J].Bulletin of Mineralogy Petrology and Geochemistry, 3(24): 232 – 238.]
高大翔,郝建朝,李子芳,等. 2008. 汞胁迫对水稻生长及幼苗生理生化的影响[J].农业环境科学学报, 27(1): 58 – 61. [Gao D X, Hao J C, Li Z F, et al. 2008. Mercury stress on rice growth and physiological and biochemical effects [J].Journal of Agro-Environment Science, 27(1): 58 – 61.]
洪曾纯. 2012. 福建16种蔬菜土壤汞安全临界值的研究[D].福州: 福建农林大学. [Hong Z C. 2012. Research of soil mercury safety threshold 16 kinds of vegetables in Fujian [D]. Fuzhou: Fujian Agriculture and Forestry University.]
胡宗英. 2014. 不同盐碱胁迫对披碱草和紫花苜蓿种子萌发的影响[D]. 长春: 吉林农业大学. [Hu Z Y. 2014. Different salinity-alkalinity stress on alkali grass and the infl uence of alfalfa seed germination [D]. Changchun: Jilin Agricultural University.]
华东师范大学生物系植物生理教研室. 1980. 植物生理学实验指导[M]. 北京:高等教育出版社: 88. [East China Normal University. Department of Biology. Plant Physiology Teaching-research Section. 1980. Plant physiology experiment instruction [M]. Beijing: Higher Education Press: 88.]
黄玉芬. 2011. 土壤汞对作物的毒害及临界值研究[D]. 福州:福建农林大学. [Huang Y F. 2011. Mercury poisoning crops and soil research [D]. Fuzhou: Fujian Agriculture and Forestry University.]
梁胜伟,胡新文,段瑞军,等. 2009. 海马齿对无机汞的耐性和吸附积累[J].植物生态学报, 33(4): 638 – 645. [Liang S W, Hu X W, Duan R J, et al. 2009. Hippocampal tooth on patience and adsorption of inorganic mercury accumulation [J].Chinese Journal of Plant Ecology, 33(4): 638 – 645.]
刘建新. 2005. Hg2+对豌豆幼苗生长和生理代谢的影响[J].宁夏大学学报(自然科学版), 26(1): 67 – 70. [Liu J X. Hg2+effects on pea seedling growth and physiological metabolism [J].Journal of Ningxia University (Natural Science Edition), 26(1): 67 – 70.]
马成仓,洪法水. 1988. 汞对小麦种子萌发和幼苗生长作用机制初探[J].植物生态学报, 22(4): 373 – 378. [Ma C C, Hong F S. 1988. Mercury on mechanism of a preliminarystudy on the seed germination and seedling growth of wheat [J].Chinese Journal of Plant Ecology, 22(4): 373 – 378. ]
母 波,韩善华,张英慧, 等. 2007. 汞对植物生理生化的影响[J].中国微生态学杂志, 12(19): 582 – 583. [Mu B, Han S H, Zhang Y H, et al. 2007. The infl uence of mercury on plant physiological and biochemical [J].Chinese Journal of Microecology, 12(19): 582 – 583.]
潘瑞炽. 1994. 植物生理学上册 [M].第二版. 北京:高等教育出版社: 57. [Pan R C. 1994. Plant Physiology (Ⅰ) [M]. Second Edition. Beijing: Higher Education Press: 57.]
宋云华,钟建明,马琼媛, 等. 2007. 不同紫花苜蓿种子处理技术的比较研究[J].牧草科学, 3(136): 26 – 27. [Song Y H, Zhong J M, Ma Q Y, et al. 2007. A comparative study of different alfalfa seed treatment technology [J].Grass Science, 3(136): 26 – 27.]
汪 霞,南忠仁,武文飞,等. 2010. 干旱区绿洲土壤中重金属的形态分布及生物有效性研究[J].生态环境学报, 19(7): 1663 – 1667. [Wang X, Nan Z R, Wu W F, et al. 2010. The formation distribution of heavy metals in soil oases in arid areas and biological effectiveness research [J].Ecology and Environmental Sciences, 19(7): 1663 – 1667.]
王起超,沈文国,麻壮伟. 1999. 中国燃煤汞排放量估算[J].中国环境科学, 19(4): 318 – 321. [Wang Q C, Shen W G, Ma Z W. 1999. China coal mercury emissions estimates [J].China Environmental Science, 19(4): 318 – 321.]
徐小蓉,张习敏,杨立昌, 等. 2011. 汞胁迫对蜈蚣草生理特征的影响[J].安徽农业科学, 39(24): 14772 – 14774. [Xu X R, Zhang X M, Yang L C, et al. 2011. The infl uence of mercury stress on the centipede grass physical characteristics [J].Journal of Anhui Agricultural Sciences, 39(24): 14772 – 14774.]
薛 亮. 2013. 锑矿区植物重金属积累特征及其耐锑机理研究[D]. 北京: 中国林业科学研究院. [Xue L. 2013. Antimony and antimony resistance mechanism of plant heavy metal accumulation characteristics research [D]. Beijing: Chinese Academy of Forestry Sciences. ]
张静静, 郑 娜, 周秋红, 等. 2014. 内蒙古自治区原煤中汞含量分布及燃煤大气汞排放量估算[J].环境化学, 33(9): 1613 – 1614. [Zhang J J, Zheng N, Zhou Q H, et al. 2014. The Inner Mongolia autonomous region distribution of mercury content in the raw coal and coal to estimate atmospheric mercury emissions [J].Environmental Chemistry, 33(9): 1613 – 1614.]
Bravo A G, Cosio C, Amouroux D. 2014. Extremely elevated methyl mercury levels in water, sediment and organisms in a Romanian reservoir affected by release of mercury from a chlor-alkali plant [J].Water Research, 49: 391 – 405.
Gbor P K, Wen D, Meng F, et al. 2007. Modeling of mercury emission, transport and deposition in North America [J].Atmospheric Environment, 41(6): 1135 – 1149.
Lai S O, Holsen T M, Han Y J, et al. 2007. Estimation of mercury in sediment loadings to Lake Ontario: Results from the Lake Ontario atmospheric deposition study (LOADS) [J].Atmospheric Environment, 41(37): 8205 – 8218.
Leticia G R, Mercedes V R, Martn E J. 2006. Geochemistry of mercury in sediment of oyster areas in Sonora, Mexico [J].Marine Pollution Bulletin, 52(4): 453 – 458.
Meng M, Li B, Shao J J, et al. 2014. Accumulation of total mercury and methylmercury in rice plant [J].Environmental Pollution, 184: 179 – 186.
Stolle R, Koeser H, Gutberlet H. 2014. Oxidation and reduction of mercury by SCR DeNOx catalysts under flue gas conditions in coal fi red power plants [J].Applied Catalysis B: Environmental, 144: 486 – 497.
The infl uence of mercury in the soil stress on physiological characteristics of forage
WANG Daohan, DU Junnan, LI Yu'e, CHU Shijing
(Engineering and Environmental Research School, Liaoning Technical University, Fuxin 123000, China)
Background, aim, and scopeParts of large-scale coal enterprises in Inner Mongolia have been striving to develop coal-fired power plants and promoting the integration of coal and electricity, which indicates the rapid increase of coal consumption in the region of Inner Mongolia. During the coal combustion, the phenomenon of mercury emissions into the atmosphere appears, which has a serious impact on environmental pollution. This paper aims to explore the mechanism of how mercury in soil influences the growth of forage, and the effect to grassland caused by high mercury content in soil.Materials and methodsIn this study, test materials for alfalfa, cover with alkali grass, festuca arundinacea, grass seed were seeds from East Wuzhu Muqinqi grassland station in Inner Mongolia of Chinese Academy of Sciences. Test the soil collected from East Wuzhu Muqinqi grassland station in Inner Mongolia of Chinese Academy of Sciences. Peroxidase (POD) activity of guaiacol method is used to determine. Catalase (CAT) activity using ultraviolet absorption spectrometry. Malondialdehyde (MDA) using glucosinolates barbituric acid colorimetric method. Proline using sulfosalicylic acid method. Soluble protein using coomassie brilliant blue staining method. Soluble sugar content determination by anthrone method.ResultsAlkali grass and festuca arundinacea seed germination rate gradually reduced with Hg2+concentration in the soil, briefl y in the early years of alfalfa seed germination, but high concentration of inhibition. In this paper, research has shown that, under the stress of Hg2+in soil, Hg2+concentration is lower than 1.5 mg · kg-1, alfalfa POD activity is on the rise, when the Hg2+concentration is greater than 1.5 mg · kg-1, alfalfa POD activity falling. Cover with alkali grass POD activity as a whole is on the rise. Tall fescue POD activity smoothly. Under the stress of Hg2+in soil, Hg2+concentration is lower than 2.0 mg · kg-1, the three kinds of forage grass CAT are on the rise, and when the concentration of Hg2+ishigher than 2.0 mg · kg-1, all show the tendency of lower. The duration of alfalfa malondialdehyde content reduced; cover with alkali grass malondialdehyde content also is on the decline, to Hg2+concentration is higher than 32.0 mg · kg-1after the previous deal with higher concentration of content; festuca arundinacea MAD content tends to reduce the rising trend, after first when soil Hg2+concentration is higher than 1.5 mg · kg-1, malondialdehyde content increased. Three kinds of grass under the low concentration, soluble protein content were higher than that of no stress state, with increasing stress concentration, reduce gradually, appeared rise to high levels of stress condition. Three kinds of forage grass appears content change trend of the change of different concentrations of DNA showed that three kinds of forage grass active Hg2+stress influence degree is different. Analysis of soluble sugar content, soluble sugar content of alfalfa under Hg2+stress reduction, tall fescue in mercury concentration higher than 32 mg · kg-1with elevated levels, cover with alkali grass in Hg2+stress state soluble sugar content is higher than no stress state. Instructions in the accumulation of soluble sugars, alkali grass has more obvious advantages.DiscussionThe normal development of mercury inhibition of plant roots and make plant becomes short. POD in organism can eliminate oxygen free radical, superoxide, inhibit free radicals on membrane lipid peroxidation, preventing damage to membrane damage and, at the same time outside the POD also participated in the fi lm the generation. CAT can be reduced by H2O2in plants, and reduced H2O2accumulation in the body and limiting oxygen damage potential. CAT, POD is enzymatic defense system of plants to membrane lipid peroxidation of protective enzyme. Mercury by acting on the cell membrane phospholipids, make the cell membrane permeability change. Plants under the stress of mercury, active free radicals content in cells, membrane peroxidation of unsaturated fatty acids, which makes the structure and function of membrane is destroyed. MDA content can refl ect the degree of membranous peroxide, reflect the extent to which plants suffer adversity harm. Plants can represent proline content in plant resistance, high proline content, strong resistance. Soluble protein content in plant cells can directly refl ect the state of intracellular protein synthesis, denaturation and degradation. Proline and reduce the effect of the water in the cell, which can be more effi cient for a variety of enzymes and complex protein quaternary structure protection, system stability in the adversity of cell and reduce the membrane lipid peroxidation. Soluble sugar of plant adversity. The more soluble sugar, the stronger the resistance of plants, there was a positive relationship.ConclusionsUnder Hg2+stress, inhibition of grass seed germination rate, and the higher the concentration of, the lower the germination rate, affect the plant height. Peroxidase activity and CAT activity and MDA content, proline content and soluble protein content, soluble sugar content changes under the stress of mercury, and the higher the concentration, the greater the long-term accumulated after mercury will have an impact on the prairie grasses.Recommendations and perspectivesCurrent grassland mercury accumulation quantity is less, but should attach importance to the control of mercury from coal-fi red power plants, to prevent the accumulation of prairie grass pollution, prevent the mercury in coal from entering the food chain, ecological health.
soil; mercury; grass; physiological and biochemical
WANG Daohan, E-mail: wangdaohan@sina.com
10.7515/JEE201606008
2016-04-01;录用日期:2016-05-06
Received Date:2016-04-01;Accepted Date:2016-05-06
(美国)国家地理空气与水保护基金资助项目(GEFC30-14)
Foundation Item:National Geographic Air and Water Protection of Funded Projects, US (GEFC30-14)
王道涵,E-mail: wangdaohan@sina.com
