海洋链霉菌来源的天然产物*
2016-03-15梅显贵朱伟明
王 聪 梅显贵 朱伟明
(中国海洋大学医药学院 海洋药物教育部重点实验室, 青岛 266003)
海洋链霉菌来源的天然产物*
王 聪 梅显贵 朱伟明①
(中国海洋大学医药学院 海洋药物教育部重点实验室, 青岛 266003)
海洋链霉菌(Streptomyces spp.)由于其独特的生理和代谢功能, 成为海洋微生物活性物质的主要来源。对2010~2013年初的海洋放线菌天然产物的统计表明, 研究最多的放线菌是链霉菌, 占海洋放线菌新天然产物的 60%。本文综述了自 1976年第一个海洋链霉菌天然产物到2016年6月的40年间报道的547个海洋链霉菌天然产物的结构、生物活性及其微生物来源。其结构包括含氮化合物(如生物碱)、聚酮、萜类、甾体等, 其中含氮化合物是其主要类型, 占海洋链霉菌天然产物总数的 61%; 而 67%的海洋链霉菌天然产物表现出细胞毒、抑菌、抗疟和抗寄生虫等生物活性。
海洋放线菌; 链霉菌; 天然产物; 来源; 结构; 生物活性
海洋链霉菌由于其独特的生理和代谢功能, 成为海洋微生物活性天然产物的重要来源。本实验室对2010~2013年初海洋放线菌新天然产物的统计表明, 海洋放线菌研究最多的是链霉菌(Streptomyces), 占海洋放线菌新天然产物的60%(赵成英等, 2013)。海洋链霉菌新天然产物的研究始于1976年, Okami 课题组报道了首例海洋链霉菌天然产物 aplasmomycins A-C (150~152)(Okami et al., 1976); 截至2016年6月的40年时间里, 已报道了547个海洋链霉菌新天然产物。这些天然产物的结构类型包括生物碱、聚酮、萜类、甾体、卤代物、聚醚类等; 并具有多种生物活性, 包括抗肿瘤、抗菌、抗疟和抗寄生虫等。因此, 海洋链霉菌天然产物可能是发现药物先导化合物的重要的资源宝库, 本文将从海洋链霉菌的来源出发, 综述这些天然产物的结构及其生物活性。
1 海洋动物来源的海洋链霉菌天然产物
1.1 海绵来源的海洋链霉菌天然产物
海绵来源的(下同)链霉菌Streptomyces sp. Ni-80代谢产生urauchimycins A(1)和B(2), 两个化合物在 10µg/mL时具有抑制白色念珠菌(Canidia albicans)活性(Imamura et al., 1993)。Streptomyces sp. KM86-9B代谢产生具有拓扑异构酶 I抑制活性的化合物 3~9 (Lee et al., 1998)。环肽 dehydroxynocardamine (10)和desmethylenylnocardamine (11)来自Streptomyces sp. M1087, 具有微弱的重组酶sortase B抑制活性, EC50分别为88.3µg/mL和126.4µg/mL (Lee et al., 2005)。Streptomyces sp. HB202代谢产生吩嗪类化合物streptophenazines A–H (12~19), 化合物12、14~16和19对枯草芽胞杆菌(Bacillus subtilis)的最小抑菌浓度(minimum inhibitory concentration, MIC)分别为46.9µg/mL、15.6µg/mL、62.5µg/mL、62.5µg/mL和15.6µg/mL, 化合物 12~14、17和 18对金黄色葡萄球菌(Staphylococcus aureus)的 MIC分别为62.5µg/mL、62.5µg/mL、46.9µg/mL、62.5µg/mL和62.5µg/mL(Mitova et al., 2008)。Streptomyces sp. SpC080624SC-11代谢产生3个吩嗪类化合物20~22(Izumikawa et al., 2010b), Streptomyces sp. Sp080513GE-23代谢产生 2个含有氯代吲哚单元的二肽类化合物 23和24(Motohashi et al, 2010c), Streptomyces sp. NBRC105896代谢产生化合物 25、对人宫颈癌 Hela细胞和恶性胸膜间皮瘤细胞 ACCMESO-1的IC50分别为49μmol/L和88μmol/L (Izumikawa et al., 2010a), Streptomyces sp. Sp080513GE-26产生 2个新的蒽环类化合物26和27、化合物26对Hela细胞和HL-60细胞的 IC50分别为 120μmol/L和 210μmol/L (Motohashi et al., 2010a)。Streptomyces sp. SpD081030ME-02代谢产生水杨酰胺衍生物28, 对 Hela细胞的 IC50为 28μmol/L(Ueda et al., 2010)。联苯蒽糖苷29来自Streptomyces sp. HB202(Schneemann et al., 2010), 化合物29对HepG2、HT-29、GXF251L、LXF529L等8种人肿瘤细胞的 IC50为 0.13~0.33μmol/L, 对鼠成纤维细胞NIH-3T3的IC50为0.22μmol/L, 且对包括耐甲氧西林金黄色葡萄球菌、表皮葡萄球菌(Staphylococcus epidermidis)、人皮肤杆菌和痤疮丙酸杆菌(Propionibacterium acnes)等9种细菌的IC50为2.5~8.4μmol/L。Tetromycins化合物30~33产自链霉菌S. axinellae Pol001T,可以抑制布氏锥虫(Trypanosoma bruce) (Pimentel-Elardo et al., 2011)。化合物Lobophorins C (34)和D (35)产自肉质链霉菌S. carnosus AZS17,化合物34和35对人肝癌细胞株7402的IC50分别为0.6μg/mL和723.1μg/mL、对人乳腺癌细胞株MDA-MB-435的IC50分别为61.8μmol/L和7.5μmol/L(Wei et al., 2011)。细黄链霉菌(S. microflavus)代谢产生 3-乙酰基-5-甲基-2′-脱氧尿嘧啶 36(Li et al., 2011); Streptomyces sp. SpD081030SC-03代谢产生小肽JBIR-56 (37)和JBIR-57 (38) (Motohashi et al., 2011)。吲哚生物碱 streptomycindole (39)产自链霉菌Streptomyces sp. DA22, 对 HL-60、结肠癌HCT-116、卵巢上皮性癌HO-8910和人肝癌细胞HepG2没有活性(Huang et al., 2011)。3个曲古抑菌素 40~42是链霉菌 Streptomyces sp. RM72的代谢产物, 对组蛋白脱乙酰化酶HDAC1的 IC50分别为 48μmol/L、74μmol/L和 57μmol/L(Hosoya et al., 2012)。环脂肽43~46产自Streptomyces sp. RV15 (Abdelmohsen et al., 2012); 苯并蒽醌糖苷 47~49产自Streptomyces sp. BCC45596 (Supong et al., 2012),其对疟原虫的IC50为0.053μg/mL、0.142μg/mL和 2.93μg/mL, 对结核杆菌的 MIC分别为3.13μg/mL、12.50μg/mL和6.25μg/mL, 化合物47对KB、MCF-7、NCl-H187和Vero细胞的IC50分别为0.179μg/mL、0.196μg/mL、0.092μg/mL和 1.71μg/mL, 化合物 48对 KB、MCF-7、NCl-H187和 Vero细胞的 IC50分别为0.324μg/mL、0.45μg/mL、0.242μg/mL和3.05μg/mL,化合物49对KB、MCF-7、NCl-H187和Vero细胞的 IC50分别为 6.96μg/mL、3.41μg/mL、3.97μg/mL和10.07μg/mL。链霉菌S. tateyamensis代谢产生化合物 JBIR-107(50)(Izumikawa et al., 2013)。吩嗪类化合物streptophenazines I-K(51~53)来自链霉菌Streptomyces HB202, 化合物52对磷酸二酯酶的IC50为12.0μmol/L, 化合物53对枯草芽胞杆菌、表皮葡萄球菌、磷酸二酯酶的 IC50分别为 21.6μmol/L、14.5μmol/L和12.2μmol/L (Kunz et al., 2014)。环圈链霉菌S. anulatus S71代谢产生化合物 54(Sun et al., 2014b)。Streptomyces sp. M7_15代谢产生monacyclinones A-F (55~60), 化合物 57、59和60对横纹肌肉瘤X细胞SJCRH30的IC50分别为 160μmol/L、270μmol/L和 0.73μmol/L (Vicente et al., 2015)。二肽xestostreptin (61)产自Streptomyces sp. S.4, 其对恶性疟原虫的 IC50为50μmol/L(Rakotondraibe et al, 2015)。化合物 quinomycin G (62)和环二肽 63来源于Streptomyces sp. LS298, 化合物63对ACHN、786-O、U87 MG和Jurkat肿瘤细胞的IC50分别为 0.552μmol/L、0.721μmol/L、0.627μmol/L和0.414μmol/L(Zhen et al., 2015)。Streptomyces sp. SBT345代谢产生氯代化合物 ageloline A (64), 对沙眼衣原体的 IC50为 9.54μmol/L (Cheng et al., 2016)。
1.2 珊瑚来源的海洋链霉菌天然产物
珊瑚来源的(下同)的链霉菌Streptornyces sp.代谢产生octalactins A (65)和B (66), 化合物65对 B-16-FI0和 HCT-116细胞的 IC50分别为0.0072μg/mL和0.5μg/mL (Tapiolas et al., 1991)。多氯代聚酮 strepchloritides A (67)和B (68)产自Streptomyces sp. OUCMDZ-1703, 其对MCF-7 细胞的IC50分别为9.9μmol/L和20.2μmol/L(Fu et al., 2013)。Axinelline A (69)来自链霉菌 S. axinellae SCSIO02208, 对环氧化酶 COX-2的 IC50为2.8mmol/L(Ai et al., 2014)。Nahuoicacids 70~73来自 Streptomyces sp. SCSGAA 0027 (Nong et al., 2016)(图1)。

图1 化合物1~73的结构Fig.1 Structures of compounds 1~73
1.3 其他动物来源的链霉菌天然产物
其他海洋动物来源的(下同)链霉菌 S. hygroscopicus代谢产生 salinamides A(74)和B(75), 其对肺炎链球菌Streptococcus pneumoniae的 MIC 均为 4μg/mL、对酿脓葡萄球菌(Staphylococcus pyrogenes)的 MIC 分别为4μg/mL和2μg/mL; 化合物74和75还有抗炎活性, 浓度为59μg/mL时, 其对佛波醇酯诱导的小鼠耳水肿的抑制率分别为 84%和 83% (Trischman et al., 1994)。Halichomycin(76)来自链霉菌 S. hygroscopicus, 对小鼠白血病 P388细胞的 ED50为 0.13μg/mL(Takahashi et al., 1994)。大环内酰胺aburatubolactam A (77)产自Streptomyces sp. SCRC-A20, 可抑制TPA诱导的超氧化阴离子的产生(Bae et al., 1996)。吲哚衍生物78~80产自Streptomyces sp. BL-49-58-005 (Sánchez et al., 2003), 化合物78对白血病K562细胞的GI50为8.46μmol/L; 化合物79对不同肿瘤细胞株表现出中等细胞毒活性, GI50在微摩尔级, 并且无明显选择性; 化合物80对所测试的肿瘤细胞株没有活性。粉蝶霉素piericidins C7(81)and C8(82)来自Streptomyces sp. YM14-060, 可抑制小鼠神经母细胞瘤细胞 Neuro-2a增殖而不杀死细胞, IC50分别为0.83 nmol/L 和 0.2nmol/L (Hayakawa et al., 2007)。Streptomyces sp. JP90 代谢产生cinnamoylphosphoramide (83), 其对乙酰胆碱酯酶的 IC50为 250μmol/L(Quitschau et al., 2008)。Streptomyces sp. CP32的代谢产生噻唑类衍生物84~88, 化合物84和87可选择性地结合 5-HT2B受体(Ki分别为 0.50μmol/L和1.54μmol/L) (Lin et al., 2010)。化合物89产自Streptomyces sp. BOSC-022A(Lorente et al., 2010)。Nobilamides A-E(90~94)和F-H(95~97)分别产自链霉菌HQ696493和HQ696492, 化合物 91具有拮抗 TRPV1作用(Lin et al., 2011)。化合物 98产自 Streptomyces sp., 对P388细胞有细胞毒活性(Mahyudin et al., 2012)。Streptomyces sp. 1053U.I.1a.1b的代谢产生化合物99及其糖苷100(Lin et al., 2012)。化合物 violapyrones H (101)和 I (102)来自Streptomyces sp. 112CH148, 化合物 101对HCT-15细胞的活性最高, GI50为 1.10μg/mL (Shin et al., 2014)。聚酮类lobophorins H (103)和 I (104)产自 Streptomyces sp. 1053U.I.1a.3b,化合物 104对结核分枝杆菌和枯草杆菌的MIC分别为2.6mmol/L和10.6mmol/L(Lin et al., 2014)。Hyaluromycin (105)来自Streptomyces sp. MB-PO13, 化合物 105对透明质酸酶的 IC50为14μmol/L(Harunari et al., 2014)。大环内酯类化合物 PM100117 (106) 和PM100118 (107) 产自链霉菌 S. caniferus GUA-06-05-006A, 其对 A549细胞的 GI50分别为1.3μmol/L和0.83μmol/L、对MDAMB-231细胞的 GI50分别为 2.7μmol/L和1.7μmol/L、对人结肠癌细胞 HT29 的 GI50分别为3.8μmol/L和9.2μmol/L (Pérez et al., 2016)。
2 海洋植物来源的海洋链霉菌天然产物
2.1 海藻来源的海洋链霉菌天然产物
海藻链霉菌Streptomyces sp. #N1-78-1代谢产生蒽醌类化合物 108~110, 3个化合物对金黄色葡萄球菌的 IC50分别为 0.15μmol/L、0.36μmol/L和31μmol/L(Socha et al., 2006)。化合物streptobactin (111)、dibenarthin (112)和tribenarthin (113)产自海藻链霉菌Streptomyces sp. YM5-799(Matsuo et al., 2011)。
2.2 红树林来源的海洋链霉菌天然产物
红树植物来源的链霉菌(下同) Streptomyces sp. GT2002/1503代谢产生吲哚并倍半萜烯xiamycin B (114)、indosespene (115)和sespenine (116), 化合物 114具有选择性抑制 HIV活性(Ding et al., 2010)(图2)。

图2 化合物74~115的结构Fig.2 Structures of compounds 74~115
Streptomyces sp. HK10552代谢产生化合物 117~120(Wang et al., 2010); 吲哚生物碱121和吲哚生物碱 122产自 Streptomyces sp. GT2002/1503, 121和 122对 HT-29、GXF 251L、LXFA 629L等 12种人癌细胞的平均IC50值分别为大于 30μmol/L和 10.1μmol/L;化合物121选择性地抑制HIV病毒的R5受体活性(Ding et al., 2010)。化合物 divergolides A-D (123~126)产自Streptomyces sp. HKI0576,化合物123对肺癌细胞LXFA 629L、胰腺癌细胞PANC-1、肾癌细胞RXF 486L和恶性毒瘤 Saos-2的 IC50值为 1.0~2.0μmol/L(Ding et al., 2011)。化合物divergolides A-D (127~130)也产自该菌(Xu et al., 2014)。链霉菌 S. lusitanus XM52代谢产生131~132, 其中132对金黄色葡萄球菌、枯草杆菌的MIC分别为32μg/mL和8μg/mL(Han et al., 2012)。倍半萜类化合物133~137产自放线菌Streptomyces sp. HKI0595, 对 12种人肿瘤细胞均无细胞毒活性, 对枯草杆菌和牛分支杆菌有较弱的抑制活性(Ding et al., 2012)。
2.3 其他植物来源的链霉菌天然产物
其他海洋植物来源的链霉菌Streptomyces sp. CANU Fox 21-2-6代谢产生化合物138~140, 对小鼠白血病细胞P388的IC50值为0.4~0.06µg/mL(Phipps et al., 2004)。来自草绿盐角草(Salicornia herbacea)的链霉菌Streptomyces sp. FX-58代谢产生化合物141 (Huang et al., 2006a)和化合物142~143 (Huang et al., 2006b),化合物143对HL-60、BCTC-823和MDA-MB-435细胞的IC50值分别为6.83μg/mL、82.2μg/mL和 56.59μg/mL。黄酮类化合物 144产自Streptomyces sp. MA-12, 浓度为 0.25mmol/L时, 对香蕉炭疽病菌、小麦赤霉病菌和桔青霉3种植物病原菌有抑制作用(Ding et al., 2013)。不饱和脂肪酸 145产自链霉菌 S. violans HTTA-F04129, 对 A2780细胞的 IC50值为4.36μmol/L(Huang et al., 2014)。Streptomyces sp. LC6代谢产生juanlimycins A (146)和B (147), 可抑制沙门氏菌毒力岛-1的分泌(Zhang et al., 2014a)。来源于浒苔的链霉菌Streptomyces sp. OUCMDZ- 3434代谢产生结构新颖的二聚体wailupemycins H (148)和I (149), 其对α-糖苷酶的Ki值分别为16.8μmol/L和6.0μmol/L、IC50值分别为19.7μmol/L和8.3μmol/L (Chen et al., 2016)。
3 海泥和海水来源的链霉菌天然产物
3.1 海泥来源的链霉菌天然产物
海泥来源的链霉菌(下同)S. griseus SS-20代谢产生aplasmomycins A-C (150~152), 能抑制多种革兰氏阳性细菌(包括分枝细菌), 同时具有抗疟原虫的活性。感染了疟原虫的小鼠口服后, 疟原虫大大减少, 且小鼠全部存活, 而未经过治疗的小鼠在8d内全部死亡(Okami et al., 1976; Nakamura et al., 1977)。链霉菌S. tenjimariensis SS-939代谢产生氨基糖苷类化合物istamycins A和istamycins B (153和154),对革兰氏阳性菌和阴性菌都有很好的抑制作用(Okami et al., 1979)。生物碱altemicidin(155)产自S. sioyaensis SA-1758, 具有抗肿瘤和杀螨活性(Okami et al., 1989)。4个罕见的吩嗪生物碱类化合物 156~159来自 Streptomyces sp. CNB-253, 化合物 156 对嗜血杆菌属流感的IC50值可达到 1μg/mL、对梭菌属产气荚膜杆菌 IC50值可达到 4μg/mL(Pathirana et al., 1992)。N-乙酰-β-D-氨基葡糖苷酶抑制剂pyrostatins A (160)和B (161)产自Streptomyces sp. SA-3501(Aoyama et al., 1995)。二酮哌嗪maremycins A (162)和B (163)产自Streptomyces sp. B 9173 (Balk-Bindseil et al., 1995)。Streptomyces sp. BD-26T(20)代谢产生 wailupemycins A-C (164~166)和 3-epideoxyenterocin (167), 化合物167浓度在1mg/6mm disk时, 对金黄色葡萄球菌的抑菌圈为 18mm; 化合物 164浓度在0.1mg/6mm disk时, 对大肠杆菌E. coli 的抑菌圈为15mm (Sitachitta et al., 1996)。多色霉素 类 抗 生 素 δ-indomycinone 168 产 自Streptomyces sp. B 8300, 对枯草芽孢杆菌 B. subtilis的 MIC 为 100µg/mL(Biabani et al., 1997)。Actinoflavoside (169)产自Streptomyces sp. CNB-689, 对革兰氏阳性菌肺炎链球菌(S. pneumonia)、酿脓葡萄球菌(S. pyrogenes)、金黄色葡萄球菌和藤黄微球菌(M. luteus)的MIC均为64µg/mL(Jiang et al., 1997)。Streptomyces sp. B 8251代谢产生生物碱170, 具有微弱的抑制大肠杆菌(E. coli)和枯草芽孢杆菌(B. subtilis)的活性(Pusecker et al., 1997)。Anthranilamide (171)产自Streptomyces sp. B7747, 浓度为100μg/disk,对淡水小球藻(C. sorokiniana)的抑制圈直径为11mm、对普通小球藻(C. vulgaris)的抑制圈直径为 13mm、对栅藻(S. subspicatus)的抑制圈直径为 12mm(Biabani et al., 1998)。Streptomyces sp. BD-18T(41)代谢产生多环苯醌类化合物 halawanones A-D(172~175)(Ford et al., 1998)。两个弱的抗菌活性的芳香族胺类化合物 lornemides A(176)和 B(177)产自Streptomyces sp. MSTMA190, 176对枯草杆菌(B.subtilitis)的 LD99为 50μg/mL(Capon et al., 2000)。化合物178~181来自Streptomyces sp. B5632和B3497(Mukku et al., 2000)。化合物182和183分别来自Streptomyces sp. SS99BA-2 (Hernandez et al., 2000)和 Streptomyces sp. 1010(Ivanova et al., 2001), 化合物183对藤黄微球菌(M. luteus)和枯草芽孢杆菌(B. subtilis)的MIC分别为15µg/mL和50µg/mL, 而对白色念珠菌(C. albicans)和大肠杆菌(E. coli)没有抑制活性。Streptomyces sp. M02750代谢产生化合物 184~189, 无抗白色念珠菌(C. albicans)活性(Cho et al., 2001)。大环内酯chalcomycin B (190)产自Streptomyces sp. B7064, 其在浓度为10μg/9mm disk时, 对金黄色葡萄球菌、大肠杆菌(E. coli)、枯草杆菌(B. subtilis)的抑菌圈直径分别为23mm、28mm和21mm (Asolkar et al., 2002)。化合物 parimycin(191)来自Streptomyces sp. B8652, 其对GXF251L、H460、LXFA629L、LXFL529L、MCF-7、MAXF401NL、MEXF462NL和 MEXF 514L细胞的 IC70为0.9~6.7μg/mL(Maskey et al., 2002)。化合物gutingimycin(192)也来源于该链霉菌(Maskey et al., 2004)。链霉菌Streptomyces sp. BD21-2代谢产生bonactin (193), 在测试浓度为1mg/mL时,对金黄色葡萄球菌、芽孢杆菌(B. megaterium)和酿酒酵母菌(S. cerevisiae)的抑菌圈直径分别为7mm、8mm和7.5mm (Schumacher et al., 2003)。Komodoquinone A (194)产自Streptomyces sp. KS3, 在1µg/mL时, 即可诱导神经母细胞瘤细胞株 Neuro 2A 的形态变化(Itoh et al., 2003)。内酯类化合物195~196和大环内酰胺类化合物197分别产自Streptomyces sp. B6007 (Stritzke et al., 2004) 和S. aureoverticillatus NPS001583(Mitchell et al., 2004), 对HT-29、B16-F10和人外周白血病细胞的EC50分别为3.6μmol/L、2.2μmol/L和2.3μmol/L。
Lajollamycin (198)产自链霉菌S. nodosus NPS007994, 对耐青霉素肺炎双球菌(S. pneumonia)和耐甲氧西林葡萄球菌(S. aureus)的MIC分别为1.5µg/mL和5µg/mL, 对小鼠黑色素瘤细胞系B16-F10的EC50为9.6μmol/L (Manam et al., 2005)。氯代手霉素衍生物chinikomycins A (199)和B (200)来自Streptomyces sp. M045, 化合物 199对乳腺癌细胞 MAXF 401NL、黑色素瘤细胞MEXF 462NL和肾癌细胞 RXF 944L的 IC50分别为 2.41µg/mL、 4.15µg/mL 和 4.02µg/mL, 化合物 200对MAXF 401NL的 IC50为 3.04µg/ml(Li et al., 2005)。Streptomyces sp. NPS008187代谢产生glaciapyrroles A-C (201~203), 其中201对结直肠腺癌细胞HT-29和黑色素瘤细胞B16-F10的IC50均为180μmol/L(Macherla et al., 2005)。拒霉素衍生物204产自Streptomyces sp. B8005,抑制大肠杆菌(E. coli)、金黄色葡萄球菌和绿色产色链霉菌(S. viridochromogenes)的 MIC大于40µg/mL(Kock et al., 2005)。Actinofuranones A (205)和 B(206)产自 Streptomyces sp. CNQ766 (Cho et al., 2006a), 该菌株还代谢产生azamerone (207)(Cho et al., 2006b)。Daryamides A-C (208~210)、化合物211来自Streptomyces sp. CNQ-085, 化合物208对HCT-116细胞的IC50为3.15μg/mL, 化合物208~209对白色念珠菌的 MIC分别为 62.5μg/mL和 125μg/mL (Asolkar et al., 2006) (图3)。

图3 化合物116~211的结构Fig.3 Structures of compounds 116~211
星孢菌素 N-carboxamidostaurosporine (212)、倍半萜类 213和生物碱类214来自链霉菌Streptomyces sp. QD518, 化合物214对膀胱、中枢神经系统、结肠、胃、头、颈、肺、乳房、胰腺、前列腺, 以及黑色素瘤等 37个人癌细胞的平均 IC50和 IC70值分别为0.016μg/mL和 0.17μg/mL, 化合物 214在40μg/disc时对金黄色葡萄球菌的抑菌圈直径为11mm(Wu et al., 2006)。Streptomyces sp. CNQ-583代谢产生吡咯生物碱 bohemamine B (215)、bohemamine C (216)和 5-chlorobohemamine C(217)(Bugni et al., 2006)。化合物urauchimycin C (218)来自Streptomyces sp. B1751(Yao et al., 2006)。Streptokordin (219)产自Streptomyces sp. KORDI- 3238, 对细胞MDA-MB-231、HCT15、PC-3、NCI-H23、ACHN、LOX-IMVI、K-562的IC50值分别为7.5μg/mL、7.8μg/mL、3.2μg/mL、3.5μg/mL、4.7μg/mL、7.4μg/mL和 8.6μg/mL(Jeong et al., 2006)。Streptomyces sp. B8000代谢产生化合物 220和 221, 化合物 220在 40μg/disc时, 对金黄色葡萄球菌和绿色链霉菌的抑菌圈直径为分别为14mm和12mm(Poumale et al., 2006)。化合物octalactin C (222)来自Streptomyces sp. V5(陈光英等, 2007)。Streptomyces sp. M491代谢产生倍半萜化合物 223~225(Wu et al., 2007)和226~231(Ding et al., 2009), 化合物 226对多种肿瘤细胞的平均 IC50为6.7µg/mL。Streptomyces sp. CNH990代谢产生醌类化合物 marmycins A (232)和 marmycins B(233), 化合物232对人结肠癌细胞HCT-116的IC50为60.5nmol/L, 是其氯代同系物227的18倍(IC50为1.09 μmol/L), 232还具有G1期阻滞 活 性 (Martin et al., 2007)。 环 六 肽piperazimycins A-C (234~236)产自链霉菌Streptomyces sp. CNQ-593, 其对人结肠癌细胞HCT-116的平均GI50均为76ng/mL (Miller et al., 2007)。吡咯生物碱 marinopyrroles A (237)和 B (238)来自链霉菌 Streptomyces sp. CNQ-418, 其对耐甲氧西林金黄色葡萄球菌(MRSA)的 MIC90分别为 0.61μmol/L 和1.1μmol/L、对 HCT-11细胞的 IC50分别为8.8μmol/L和9.0μmol/L(Hughes et al., 2008); 对该菌株优化后又得到 marinopyrroles C-F (239~245), 对抑制人结肠癌 HCT-116细胞的IC50为1~5μg/mL, 化合物239抑制MRSA的MIC 小于 1μg/mL(Hughes et al., 2010)。Streptomyces sp. MS239代谢产生吲哚类化合物 243和萜类化合物 244, 化合物 243对枯草杆菌 ATCC6633有弱的抑菌活性(Motohashi et al., 2008)。化合物essramycin (245)来自于Streptomyces sp. Merv8102, 其对革兰阳性菌和革兰阴性菌的 MIC 为1.0~8.0μg/ml。对大肠杆菌(ATCC 10536)、铜绿假单胞菌(ATCC 10145)、枯草杆菌(ATCC 6051)、金黄色葡萄球菌(ATCC 6538)和藤黄微球菌(ATCC 9341)的 MIC分别为 8μg/mL、3.5μg/mL、1μg/mL、1μg/mL和1.5μg/mL (El-Gendy et al., 2008)。
Streptomyces sp H668代谢产生聚醚类化合物246, 有抗疟疾活性, IC50为100~200ng/mL (Na et al., 2008)。有机酸 247和 248来自Streptomyces sp. Act8015 (Shaaban et al., 2008)。聚酮phaechromycins F-H (249~251)产自Streptomyces sp. DSS-18(Li et al., 2008)。Streptomyces sp. CNQ-617代谢产生marineosins A和 B(252~253), 其对人结肠癌细胞 HCT-116的 IC50分别为 0.5μmol/L 和 46μmol/L (Boonlarppradab et al., 2008); 对marineosins的生物合成研究, 又从该链霉菌的代谢产物中获得了化合物254~257, 其中256对HepG2细胞的IC50为4.17μmol/L; 化合物256对氯喹敏感(CQS)、氯喹拮抗, 以及采用氯喹处理过的菌株P. falciparum的IC50分别为2.3nmol/L、12nmol/L和1.5nmol/L (Salem et al., 2014)。三环聚丙酸酯类indoxamycins A-F (258~263)产自 Streptomyces sp. NPS-643, 化合物 258和 263对人结肠癌细胞 HT-29的 IC50为0.3~3μmol/L(Sato et al., 2009)。氯代生物碱ammosamides A (264)和B (265)产自链霉菌Streptomyces sp. CNR-698, 264暴露在空气中可逐渐转化为265, 其对人结肠癌HCT-116细胞的IC50均为320nmol/L, 作用靶点为肌球蛋白(Hughes et al., 2009)。化合物splenocins A-J (266~275)产自 Streptomyces sp. CNQ431, 抑制脾脏细胞因子的 IC50为 2~50nmol/L, 且对哺乳动物细胞的细胞毒性较小, 具有治疗哮喘的潜力(Strangman et al., 2009)。Streptomyces sp. NTK 227代谢产生albidopyrone A (276),具有蛋白酪氨酸磷酸酶 B(PTP1B)抑制活性, IC50为128μg/ml(Hohmann et al., 2009)。细胞毒活性的 mansouramycins A-D (277~280)和tartrolon D (281)分别产自 Streptomyces sp. Mei37(Hawas et al., 2009)和Streptomyces sp. MDG-04-17-069(Pérez et al., 2009), 化合物281对A549、HT29和MDA-MB-231的GI50分别为0.16μmol/L、0.31μmol/L和0.79μmol/L。二酮哌嗪naseseazines A (282)和B (283)产自Streptomyces sp. CMB-MQ030 (Raju et al., 2009)。Lorneic acids A (284)和B (285)产自Streptomyces sp. NPS554, 其抑制人血小板磷酸二酯酶5(PDE5)的IC50分别为12.6μmol/L和87.1μmol/L(Iwata et al., 2009); 多环聚酮类化合物akaeolide (286)也产自该菌, 其对大鼠成纤维细胞3Y1的IC50为8.5μmol/L(Igarashi et al., 2013)。Tirandamycins C (287)和D (288)产自Streptomyces sp. 307-9, 抑制耐万古霉素肠球菌(VRE)的 MIC分别为 110μmol/L和大于9μmol/L(Carlson et al., 2009)。化合物289来自S. xiamenensis 318, 具有治疗肺纤维化的潜力(Xu et al., 2012)。肿大链霉菌(S. tumescens) YM23-260产生环肽类化合物290和291, 化合物290在浓度为100μmol/L时, 具有抑制阿尔兹海默病活性(Motohashi et al., 2010b)。环肽类化合物 292 产自 Streptomyces sp. MWW064, 具有抑制鼠肿瘤细胞 26-L5的转移作用(Igarashi et al., 2010)。Streptomyces sp. CMB-M0406产生heronamides A-C (293~295)(Raju et al., 2010a)和 8-deoxyheronamide C (296)(Sugiyama et al., 2014), 296对裂殖酵母细胞的MIC可达5.8μmol/L。化合物297~299为Streptomyces sp. CMB-M0423的代谢产物,对革兰阳性菌的IC50为0.6~6.5μmol/L(Raju et al., 2010b)(图4)。 MDA-MB-231、HT-29和A549的GI50为0.24~
图4 化合物212~298的结构Fig.4 Structures of compounds 212~298
化合物 300~304产自 Streptomycetaceae CNQ-509, 化合物300、302和303对HCT-116细胞的 IC50分别为 31.1μmol/L、31.0μmol/L和5.7μmol/L(Kwon et al., 2010); Streptomyces sp. B6219代谢产生化合物305(Abdalla et al., 2010)。吡喃酮类化合物306~309产自S. albus POR-04-15-053, 化合物 306和 309对0.69μmol/L(Schleissner et al., 2011)。抗霉素antimycins A19和A20(310~311)产自S. antibioticus H74-18, 其抑制白色念珠菌(C. albicans)的MIC为5~10μg/mL(Xu et al., 2011)。Streptomyces. sp CNQ-027代谢产生 actinoramides A-C (312~314)(Nam et al., 2011)和 actinoranone (315)(Nam et al., 2013), 化合物 315 对HCT-116细胞的LD50为2.0μg/mL。Streptomyces sp. RJA2928代谢产生 pandanamide B (316) (Williams et al., 2011)、nahuoic acid A (317) (Williams et al., 2013)和 nahuoic acids D-E (318~319)(Williams et al., 2016), 化合物316抑制 T淋巴细胞细胞的 IC50为 20μg/mL、化合物317和319对组蛋白甲基化酶的IC50分别6.5μmol/L和13μmol/L。缩酚酸肽fijimycins A-C (320~322)来自Streptomyces sp. CNS-575,其对 MRSA (ATCC33591)、Sanger 252和UAMS1182的MIC100为4~16μg/mL(Sun et al., 2011)。Streptomyces sp. 211726代谢产生化合物323~324(Yuan et al., 2011)和325~331(Yuanet al., 2013), 对白色念珠菌(C. albicans)和人结肠癌细胞HCT-116有抑制活性。化合物332产自 Streptomyces sp. 061316 (Chen et al, 2011)。安沙霉素类化合物ansalactam A (333)来自 Streptomyces sp. CNH-189(Wilson et al., 2011); 该菌还代谢产生混源萜 334~337 (Kaysser et al., 2012)和ansalactams B-D (338~ 340)(Le et al., 2016), 化合物338~340对MRSA的 MIC 分别为 31.2μg/mL、31.2μg/mL 和62.5μg/mL。化合物 benzoxacystol (341)来自Streptomyces sp. NTK 935, 抑制糖原合成酶激酶GSK-3β的IC50为1.35μmol/L, 对小鼠成纤维细胞NIH- 3T3有弱抑制活性(Nachtigall et al., 2011)。Streptomyces sp. B8112代谢产生glucopiericidin C (342), 有抗米黑毛霉菌活性和细胞毒活性 (Shaaban et al., 2011)。Streptomyces sp. MST- MA568代谢产生螺环缩酮 reveromycin E (343)(Fremlin et al., 2011)。吡喃酮A-C (344~346)产自Streptomyces sp. CNQ-301 (Fukuda et al, 2011)。Streptomyces sp. WuXin代谢产生化合物 347和 348, 对HL-60有细胞毒活性(Li et al., 2011); 化合物usabamycin A-C (349~351)产自Streptomyces sp. NPS853, 对人宫颈癌HeLa细胞有弱细胞毒活性并可选择性抑制对 5-羟色胺的摄取(Sato et al., 2011)。Streptomyces sp. W007代谢产生化合物352, 对A549细胞有细胞毒活性(Zhang et al., 2011)(图5)。

图5 化合物299~352的结构Fig.5 Structures of compounds 299~352
化合物 353 产自杆菌状链霉菌 S. bacillaris SNB-019(Hu and MacMillan, 2012)。对弗氏链霉菌S. fradiae 007进行紫外照射和亚硝基胍诱变, 得到突变株 S. fradiae 007M135, 该突变株代谢产生吲哚咔唑生物碱 354~356, 其对 HL-60、K562、A549、BEL-7402细胞均有抑制活性, 对蛋白激酶 C (PKC-α)的IC50值为0.001~4.6μmol/L(Fu et al., 2012a)。糖苷类化合物 357~361来自 S. lusitanus SCSIO LR32, 357~360对多个肿瘤细胞的 IC50值为 1.1~31μmol/L (Huang et al., 2012)。Streptomyces sp. CMB-M0392代谢产生化合物 362, 对枯草杆菌 ATCC6052和ATCC6633的IC50分别为8μmol/L和14μmol/L (Raju et al., 2012)。化合物 363和 364产自Streptomyces sp. CNQ343, 化合物363对白色念珠菌有较强的抑制活性(Kim et al., 2012)。Streptomyces sp. SCSIO 03032代谢产生吲哚生物碱365~368(Zhang et al., 2012)、indimicins A-E (369~373)、lynamicin F (374)和lynamicin G (375)(Chen et al., 2014), 以及piericidin A1 (376)(Zhang et al., 2014a)、heronamides D-F (377~379)(Zhang et al., 2014b), 化合物366~ 368对多种肿瘤细胞的 IC50为 4~15μmol/L、370对MCF-7的IC50为10.0μmol/L。吩嗪类化合物380为Streptomyces sp. LB173的代谢产物, 有微弱的抗菌活性, 是一种乙酰胆碱酯酶抑制剂(Ohlendorf et al., 2012)。化合物381产自Streptomyces sp. M268(Xie et al., 2012);化合物382~385产自Streptomyces sp. SCSIO 02999(Zhang et al., 2012), 4个化合物对致病菌的MIC分别为E.coli ATCC 25922(8μg/mL、8μg/mL、16μg/mL 和 4μg/mL)、S.aureus ATCC29 213(8μg/mL、16μg/mL、16μg/mL、8μg/mL)、B.subtilis SCSIO BS01(64μg/mL、128μg/mL、大于 128μg/mL、64μg/mL)和 B. thuringiensis SCSIO BT01(64μg/mL、64μg/mL、大于128μg/mL、大于128μg/mL)。化合物386产自Streptomyces sp. CP13-10, 对酵母Sir2p基因的MIC为2.5mmol/L、对人类SIRT1和 SIRT2基因的 IC50分别为 3.7 mmol/L和4.5mmol/L(Amagata et al., 2012)。
S. variabilis SNA-020代谢产生生物碱387, 387对胰腺细胞 MIA PaCa-2的 IC50为3.2μmol/L(Pan et al., 2012)。化合物388产自Streptomyces sp. TP-A0879, 对小鼠结肠癌细胞26-L5的IC50为0.25μmol/L(Igarashi et al., 2012)。吲哚咔唑生物碱389和390产自Streptomyces sp. FMA, 化合物 390 对HL-60、A549和 Hela细胞的 IC50值分别为1.4μmol/L、5.0μmol/L和34.5μmol/L(Fu et al., 2012b)。Streptomyces sp. CNT-179代谢产生cyanosporasides D-F (391~393)(Lane et al., 2013); S. antibioticus PTZ0016 代谢产生394~396, 化合物 394~396对金黄色葡萄球菌ATCC6538的 MIC 分别为 6.0mg/mL、6.0mg/mL 和 8.0mg/mL(Lian and Zhang, 2013)。生物碱nitrosporeusines A (397)和B (398)产自S. nitrosporeus CQT1424, 具有抑制甲型H1N1流感病毒活性(Yang et al., 2013a)。S. niveus SCSIO 3406代谢产生倍半萜marfuraquinocins A-D (399~402)、phenaziterpenes A (403)和B (404), 化合物399和401对肺癌细胞 NCI-H460的 IC50值分别为 3.7μmol/L和4.4μmol/L, 化合物399、401和402对金黄色葡萄球菌 ATCC292138的 MIC值均为8μg/mL; 化合物401和402对耐甲氧西表皮葡萄球菌shhs-E1的MIC值为8μg/mL(Song et al., 2013)。Streptomyces sp. CNH-287代谢产生chlorizidine A (405), 其对HCT-116细胞的IC50为3.2~4.9μmol/L (Alvarez-Mico et al., 2013)。脱氢二酮哌嗪406~410产自Streptomyces sp. FXJ7.328, 化合物407抑制H1N1病毒的IC50为 41.5μmol/L(Wang et al., 2013)。Streptomyces sp. MS100061 代 谢 产 生lobophorin G1 (411), 其对枯草芽胞杆菌和结核分支杆菌H37Rv的MIC分别为3.1μg/mL和32μg/mL(Chen et al., 2013)(图6)。

图6 化合物353~410的结构Fig.6 Structures of compounds 353~410
Streptomyces sp. SNJ013代谢产生化合物sungsanpin (412)(Um et al., 2013a)。Streptomyces sp. CNQ-329 代谢 产 生 napyradiomycins A-E(413~417), 化合物 413、416和 417对HCT-116细胞的IC50为4.2μg/mL、16.1μg/mL和4.8μg/mL, 化合物413和414对MRSA的MIC分别为26μg/mL和64μg/mL(Cheng et al., 2013)。 Streptomyces sp. CNH-070 产 生napyradiomycin F (418), 其对HCT-116细胞的IC50为9.42μg/mL(Cheng et al., 2013)。Streptomyces sp. SCSIO 10428代谢产生化合物419~421, 化合物419对金黄色葡萄球菌ATCC29213、枯草芽胞杆菌SCSIO BS01和苏云金杆菌SCSIO BT01的 MIC分别为 4μg/mL、4μg/mL和 8μg/mL, 化合物 420对金黄色葡萄球菌ATCC29213、枯草芽胞杆菌SCSIO BS01和苏云金杆菌 SCSIO BT01的 MIC分别为0.5μg/mL、1μg/mL和1μg/mL, 化合物421对枯草芽胞杆菌 SCSIO BS01和苏云金杆菌SCSIO BT01的 MIC分别为 8μg/mL和16μg/mL (Wu et al., 2013a)。化合物anthracimycin (422) 来自Streptomyces sp. CNH365, 对炭疽芽胞杆菌 UM23C1-1、金黄色葡萄球菌ATCC 13709、粪肠球菌 ATCC 29212、肺炎链球菌ATCC 51916、流感嗜血杆菌ATCC 31517KO的MIC分别为0.03125μg/mL、0.0625μg/mL、0.125μg/mL、0.25μg/mL和4μg/mL(Jang et al., 2013)。环肽surugamides A-E (423~427)来自
Streptomyces sp. JAMM992, 对牛组织蛋白酶B 的 IC50分别为 21μmol/L、27μmol/L、36μmol/L、18μmol/L和16μmol/L(Takada et al., 2013)。Streptomyces sp. SCSIO 03219代谢产生萜类化合物 03219A (428) (Zhang et al., 2013)。Streptomyces sp. Eg25 代谢产生maroxazinone (429), 对 MCF7、HEPG2和HCT116细胞的 IC50分别为 4.32μg/mL、2.90μg/mL和8.51μg/mL(Abdelfattah, 2013)。Streptomyces sp. CNT-372代谢产生farnesides A (430) 和B (431), 430对恶性疟原虫的IC50为69.3μmol/L(Zafrir et al., 2013)。Streptomyces sp. 7-145代谢产生化合物432和433, 对耐甲氧西林金黄色葡萄球菌和耐万古霉素肠球菌有很好的抗菌活性(Wu et al., 2013b)。环肽ohmyungsamycin A(434)和 ohmyungsamycin B(435)产自链霉菌 Streptomyces sp. SNJ042,化合物434对枯草芽胞杆菌ATCC6633、藤黄微球菌NBRC12708和变形杆菌NBRC3851的MIC 分别为 4.28μmol/L、1.07μmol/L 和2.14μmol/L, 化合物 435 对藤黄微球菌NBRC12708的MIC为8.5μmol/L, 化合物434对HCT116、A549、SNU-638、MDAMB-231和SKHEP-1细胞的IC50分别为0.359μmol/L、0.551μmol/L、0.532μmol/L、0.688μmol/L和0.816μmol/L(Um et al., 2013b)。Separacenes A-D(436~439) 产自Streptomyces sp. SNJ210,化合物436对白色念珠菌异柠檬酸裂解酶、人癌细胞HCT-116和A549有弱活性(Bae et al., 2013)。Streptomyces sp. 12A35代谢产生lobophorins H (440)和I (441), 化合物440对金黄色葡萄球菌ATCC29213和枯草芽胞杆菌CMCC63501的 MIC分别为 50μg/mL和1.57μg/mL(Pan et al., 2013)。Strepsesquitriol (442)来自 Streptomyces sp. SCSIO 10355, 具有抑制 TNF-α的活性(Yang et al., 2013b)。Mollemycin A (443) 产自 Streptomyces sp. CMBM0244, 对金黄色葡萄球菌 ATCC 25293和 ATCC 9144、表皮葡萄球菌ATCC12228、枯草芽胞杆菌 ATCC 6051和ATCC 6633、大肠杆菌ATCC 25922、绿脓杆菌 ATCC 27853和牛结核杆菌的IC50值分别为50nmol/L、10nmol/L、50nmol/L、10nmol/L、10nmol/L、10nmol/L、50nmol/L和3200nmol/L;对药敏型和多药耐药的恶性疟原虫的 IC50分别为 9nmol/L和 7nmol/L, 活性远大于氯喹,与青蒿素相当(Raju et al., 2014)。S. drozdowiczii SCSIO 10141代谢产生 marformycins A-F (444~449), 化合物 444~448对藤黄微球菌的MIC 分 别 为 0.25mg/mL、 4.0mg/mL、0.25mg/mL、0.063mg/mL和4.0mg/mL (Zhou et al., 2014)。S. nitrosporeus YBH10-5代谢产生nitrosporeunols A-G (450~456)(Liu et al., 2014)。S. scopuliridis SCSIO ZJ46代谢产生化合物 457~459, 457对金黄色葡萄球菌ATCC 29213、肺炎双球菌NCTC 7466、耐甲氧西林表皮葡萄球菌的 MIC 值分别为16.0μg/mL、12.5μg/mL和32.0μg/mL (Song et al., 2014)(图7)。

图7 化合物411~459的结构Fig.7 Structures of compounds 411~459
Streptomyces sp. LZ35DgdmAI代谢产生echosides A-E (460~464), 化合物460有很明显的拓扑异构酶Ⅰ活性, 化合物462有拓扑异构酶 IIa活性(Deng et al., 2014)。化合物465~468产自Streptomycetaceae CNQ525, 可以诱导 HCT-116细胞凋亡(Farnaes et al., 2014)。Streptomyces sp. ACT232代谢产生ahpatinin Ac (469)和ahpatinin Pr (470), 对胃蛋白酶的IC50为11~50nmol/L (Sun et al., 2014a)。Carpatamides A-C(471~473)来自Streptomyces sp. SNE-011, 化合物471和473对NSCLC、HCC366、A549和 HCC44细胞的 IC50为2.2~8.4 μmol/L (Fu et al., 2014)。化合物anmindenols A (474) 和 B (475) 产自Streptomyces sp. CMDD10D111, 抑制NO产生的 IC50值为 23μmol/L和 19μmol/L (Lee et al., 2014)。Arcticoside (476)和 C-1027 chromophore-V (477)产自 Streptomyces sp. ART5, 化合物476和477对白色念珠菌异柠檬酸(裂合)酶的 IC50分别为 30.4μmol/L和37.9μmol/L; 化合物 477对癌细胞 MDAMB231和HCT-116的IC50分别为0.9μmol/L和2.7μmol/L(Moon et al., 2014)。Lajollamycins B−D (478~480)产自链霉菌 Streptomyces sp. SMC72, 对白色念珠菌异柠檬酸裂解酶的IC50分别为40μmol/L、50μmol/L和120μmol/L (Ko et al., 2014)。Streptomyces sp. SNM55代谢产生mohangamides A (481)和B (482)(Bae et al., 2015b)、hormaomycins B (483) 和C (484) (Bae et al., 2015a), 化合物481和482对白色念珠菌 ICL 的 IC50分别为 4.4μmol/L 和20.5μmol/L, 化合物 483/484对金黄色葡萄球菌ATCC 25923、枯草芽胞杆菌 ATCC 6633、藤黄微球菌 NBRC 12708、化脓性链球菌ATCC 19615、肠道沙门菌ATCC 14028和P.hauseri NBRC 3851的MIC分别为7/7μmol/L、14/56μmol/L、0.4/0.23μmol/L、14/8μmol/L、29/114μmol/L和29/14μmol/L。Streptomyces sp. IFM 11440代谢产生nubosins A-C (485~487),化合物486具有Ngn2基因启动子的活性、促进神经干细胞分化基因有关的mRNA的表达(Arai et al., 2015)。Streptomyces sp. SCSIO 11594代谢产生marangucyclines A (488)和B (489), 化合物489对A549、CNE2、MCF-7、HepG2和HL7702细胞的IC50分别为0.45μmol/L、0.56μmol/L、0.24μmol/L、0.43μmol/L和3.67μmol/L (Song et al., 2015)。Suncheonosides A-D (490~ 493)来自 Streptomyces sp. SSC21, 化合物490、491和493能促进脂联素的产生, 具有降糖的潜力(Shin et al., 2015)。二酮哌嗪494产自 Streptomyces sp. SCSIO 04496(Luo et al., 2016)。Rocheicoside A (495)和rocheicoside 496产自S. rochei 06CM016, 化合物495对大肠杆菌O157: H7 RSKK 234、耐甲氧西林金黄色葡萄球菌 DSM 11729 和人白色念珠菌 DSM 5817的 MIC分别为 16μg/mL、8μg/mL和4μg/mL; 化合物496对这3株致病菌的MIC分别为16μg/mL、16μg/mL和8μg/mL (Aksoy et al., 2016)。Streptomyces sp. IFM11490代谢产生elmonin (497)、elmenols A (498)和elmenols B (499), 化合物497和499对AGS细胞有弱细胞毒活性(Yixizhuoma et al., 2015)。Streptomyces sp. SNB-048代谢产生spinoxazines A (500)和spinoxazines B (501)、bohemamine D-I (502~ 507)(Fu et al., 2016)。Streptomyces sp. CHQ-64代谢产生 drimentine I (508), 对 Hela的 IC50为16.7μmol/L(Che et al., 2016)。Streptomyces sp. 10A085代谢产生anithiactins A-C (509~ 511), 其对乙酰胆碱酯酶的 IC50分别为63μmol/L、53μmol/L和68μmol/L(Kim et al., 2014)(图8)。
多环蒽醌512和513来自Streptomyces sp. 182SMLY, 对C6、U251、U87-MG和SHG-44c 4种胶质瘤细胞的IC50为0.5~7.3μmol/L; 化合物512对S. aureus的MIC为20.0μmol/L(Liang et al., 2016b); 该菌株还代谢产生吩嗪类化合物 514~516(Liang et al., 2016a)。Mohangic acids A-E (517~521)来自 Streptomyces sp. SNM31, 化合物 521对醌还原酶具有良好的活性, 当浓度为 20μmol/L时, 活性是阳性药β-萘黄酮的2.1倍(Bae et al., 2016)。Actinoquinolines A (522)和 B (523)产自 Streptomyces sp. CNP975, 对环氧化酶 COX-1的 IC50分别为7.6μmol/L和2.13μmol/L, 对环氧化酶COX-2的 IC50分别为 24.9μmol/L 和 1.42μmol/L (Hassan et al., 2016)。麦角脂醇类化合物ananstreps A-C (524~526)来自 S. anandii H41-59, 其中化合物 526对 SF-268、MCF-7和 NCI-H460的 IC50分别为 13.0μg/mL、18.1μg/mL和23.5μg/mL (Zhang et al., 2016)。
3.2 海水来源的链霉菌天然产物
海水来源的链霉菌(下同)Streptomyces sp. Z00045代谢产生cycloheximide acid A (527) (Xu et al., 2013)。Streptomyces sp. F001代谢产生diazaquinomycins E-G (528~530), 528对卵巢癌 OVCAR5细胞的 IC50为 9.0μmol/L (Mullowney et al., 2014)。Streptomyces sp. TPU1236A 代谢产生 streptcytosines A-E (531~535), 531对耻垢分枝杆菌的 MIC为32µg/mL (Bu et al., 2014)。一株gntR基因突变的链霉菌代谢产生化合物536~538 (Liu et al., 2015)。
4 未知来源的海洋链霉菌天然产物
来源未知的链霉菌(下同)Streptomyces sp. GWS-BW-H5产生内酯类化合物 539~544 (Dickschat et al., 2005)。降倍半萜 545来自Streptomyces sp. 0616208, 对肝瘤细胞 SMMC-7721有细胞毒活性(Xie et al., 2006)。吩嗪类化合物 546和 547产自 Streptomyces sp. CNS284, 其对TNF-α介导的NFκB有抑制作用, IC50分别为4.1μmol/L和24.2μmol/L; 抑制LPS介导的NO的IC50分别为48.6μmol/L和15.1μmol/L; 对细胞生长和调节因子——前列腺素 E2(PGE2)有抑制作用, IC50分别是7.5μmol/L 和 0.89μmol/L(Kondratyuk et al., 2012)(图9)。

图8 化合物460~502的结构Fig.8 Structures of compounds 460~502
综上所述, 从 1976年报道的首例海洋链霉菌来源的aplasmomycins A-C(Okami et al., 1976)到2016年6月的40年时间里, 一共报道了547个海洋链霉菌新天然产物(表1)。其结构类型呈现多样性, 涉及含氮化合物, 如生物碱、聚酮、萜类、甾体、糖苷、聚醚类及其卤代物等, 且67.3%的化合物表现出肿瘤细胞毒、抗菌、抗疟、抗寄生虫, 以及糖苷酶抑制等生物活性(表 1), 是发现海洋活性化合物的重要资源。
(1) 从海洋链霉菌NPs的数量看, 新化合物的数量呈现年递增的趋势。从2006年开始,新化合物递增的趋势越来越显著, 2014年最多, 报道了82个(图10)。
(2) 从海洋链霉菌的样品来源看, 产生新化合物最多的海洋链霉菌的来源依次是海泥(377个)、海绵(64个)和其他海洋动物(34个), 分别占68.9%、11.7%和6.2%(图11)。
(3) 从化合物的结构类型看, 化合物最多的类型依次是含氮(336个)和聚酮(196个), 分别占海洋链霉菌 NPs总数的 61.4%和 35.8% (图12)。
(4) 约 67.3%的海洋链霉菌 NPs(368个)表现出肿瘤细胞毒、抗菌、抗疟和抗寄生虫等生物活性, 而肿瘤细胞毒活性(141个)和抑菌活性(117个)是主要的活性类型, 分别占活性化合物总数的38.3%和31.8%(图13)。
(5) 欧美、中国和其他亚洲国家是海洋链霉菌NPs的主要发现者, 其发表化合物的数量分别为 256个、163个和 112个, 我国学者贡献了29.8%的海洋链霉菌 NPs(图14见文后彩图); 欧美、中国和其他亚洲国家的学者分别发表了 93篇、63篇和52篇文章, 其他国家发表了9篇相关文章, 分别占42.9%、29.0%、24.0%和4.1%。

图9 化合物503~547的结构Fig.9 Structures of compounds 503~547
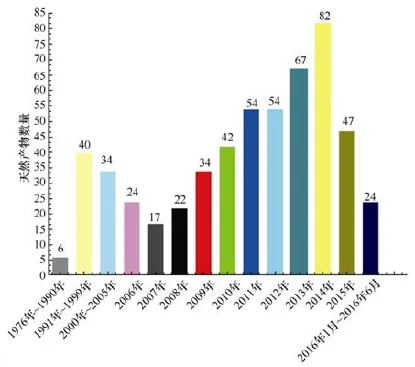
图10 每年从海洋链霉菌的代谢产物中分离的天然产物的数量Fig.10 Annual number of marine-derived Streptomyces actinomycetes MNPs since 1976

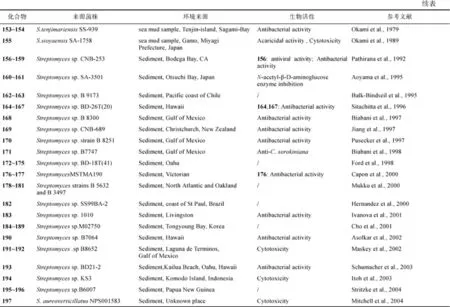
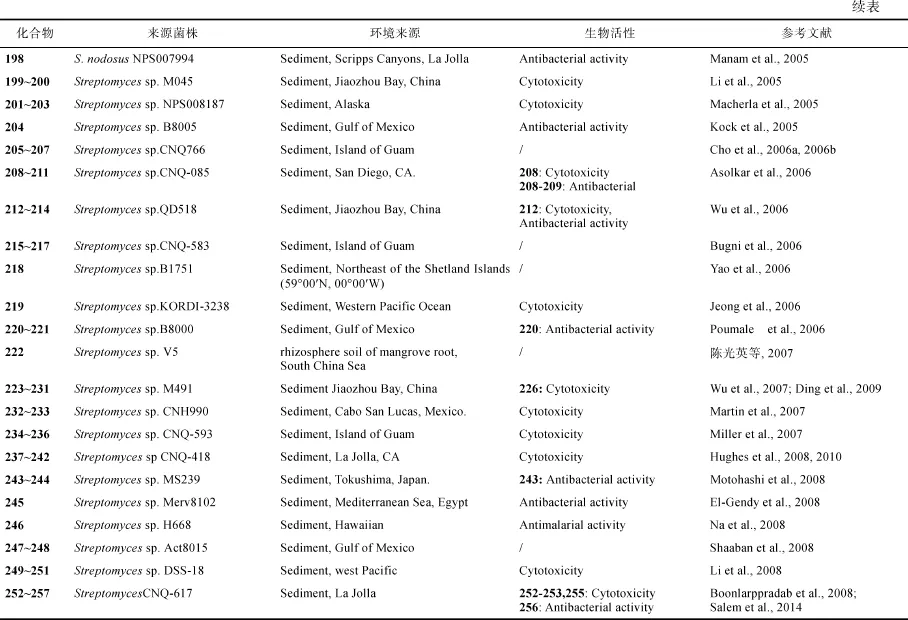
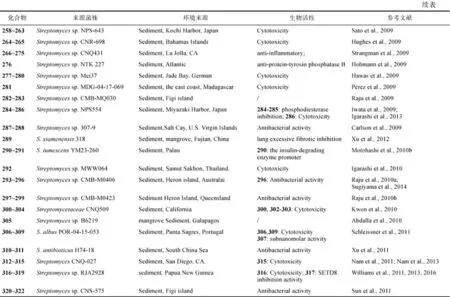
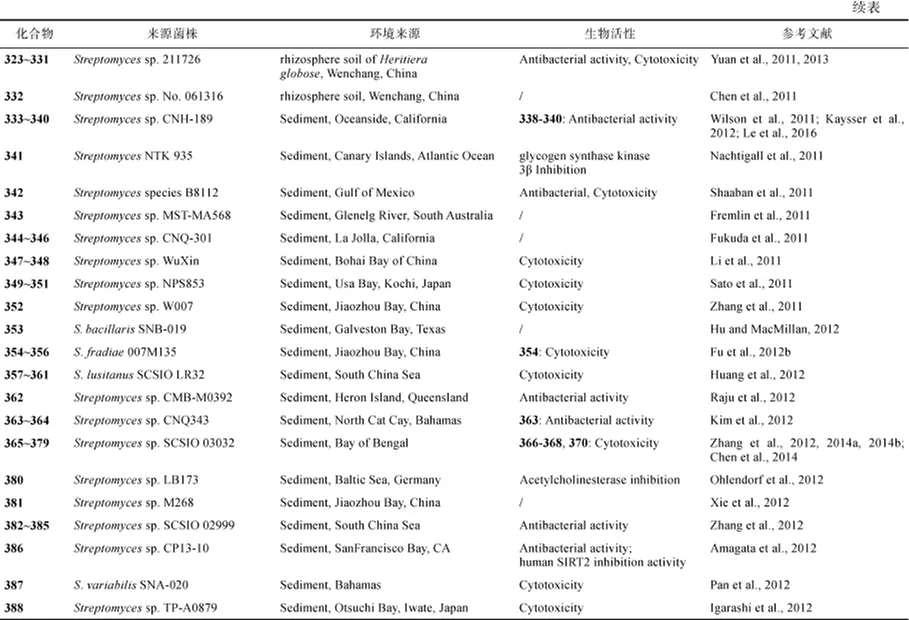
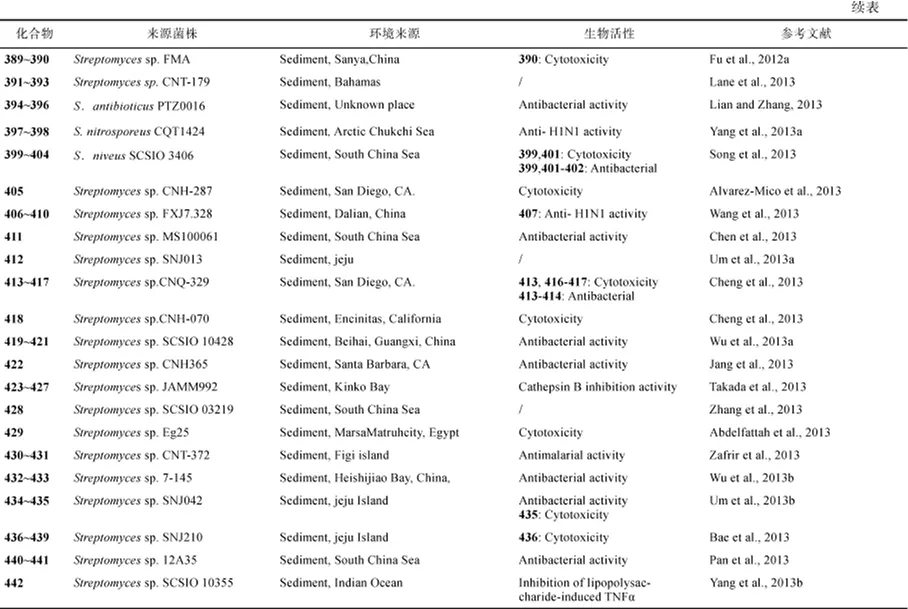
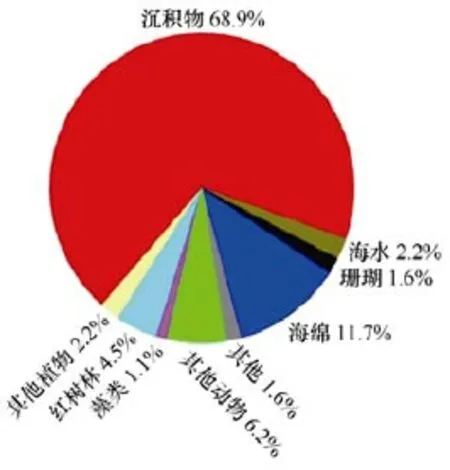
图11 海洋链霉菌天然产物的来源Fig.11 Origin categoriesof marine-derived Streptomyces actinomycetes MNPs
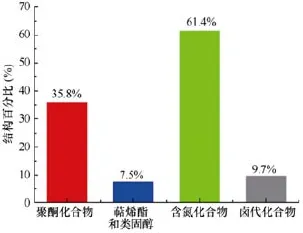
图12 海洋链霉菌天然产物的结构分类Fig.12 The main structure types of marine-derived Streptomyces actinomycetes MNPs

图13 海洋链霉菌天然产物的活性分类Fig.13 Bioactive categories of marine-derived Streptomyces actinomycetes MNPs

图14 海洋链霉菌天然产物的主要发现者国别Fig.14 Country categories of the main discoverers on Streptomyces MNPs
(6) 我国学者在 63篇学术文章中贡献了163个海洋链霉菌NPs, 在天然产物化学类、有机化学类和海洋药物类的主流杂志, 如 J Nat Prod、Org Lett、J Org Chem和Mar Drugs的学术文章分别有11篇、7篇、1篇和13篇,占其文章总数的17.5%、11.1%、1.6%和20.6% (图15)。

图15 中国学者发表论文的期刊分类Fig.15 Journal categories of Chinese scholars for publishing Streptomyces MNPs
陈光英, 朱峰, 林永成. 2007. 海洋放线菌 Streptomyces sp. V5产生的一个新的八元环内酯. 有机化学, 27(9): 1159-1161
赵成英, 朱统汉, 朱伟明. 2013. 2010-2013之海洋微生物新天然产物. 有机化学, 33(6): 1195-1234
Abdalla M A, Helmke E, Laatsch H. 2010. Fujianmycin C, A bioactive angucyclinone from a marine derived Streptomyces sp. B6219. Nat Prod Commun, 5(12): 1917-1920
Abdelfattah M S. 2013. A new bioactive aminophenoxazinone alkaloid from a marine-derived actinomycete. Nat Prod Res, 27(22): 2126-2131
Abdelmohsen U R, Zhang G L, Philippe A, et al. 2012. Cyclodysidins A-D, cyclic lipopeptides from the marine sponge-derived Streptomyces strain RV15. Tetrahedron Lett, 53(1): 23-29
Ai W, Lin X P, Tu Z C, et al. 2014. Axinelline A, a new COX-2 inhibitor from Streptomyces axinellae SCSIO02208. Nat Prod Res, 28(16): 1219-1224
Aksoy S Ç, Uzel A, Bedir E. 2016. Cytosine-type nucleosides from marine-derived Streptomyces rochei 06CM016. J Antibiot (Tokyo), 69(1): 51-56
Alvarez-Mico X, Jensen P R, Fenical W, et al. 2013. Chlorizidine, a cytotoxic 5H-pyrrolo[2,1-a]isoindol-5-one-containing alkaloid from a marine Streptomyces sp.. Org Lett, 15(5): 988-991
Amagata T, Xiao J, Chen Y P, et al. 2012. Creation of an HDAC-based yeast screening method for evaluation of marine-derived actinomycetes: discovery of streptosetin A. J Nat Prod, 75(12): 2193-2199
Aoyama T, Kojimal F, Imada C, et al. 1995. Pyrostatins A and B, new inhibitors of N-acetyl-β-D-glucosaminidase, produced by Streptomyces sp. SA-3501. J Enzyme Inhib, 8(4): 223-232
Arai M A, Koryudzu K, Ishibashi M. 2015. Inubosins A, B, and C are acridine alkaloids isolated from a culture of Streptomyces sp. IFM 11440 with Ngn2 promoter activity. J Nat Prod, 78(2): 311-314
Asolkar R N, Jensen P R, Kauffman C A, et al. 2006. Daryamides A-C, weakly cytotoxic polyketides from a marine-derived actinomycete of the genus Streptomyces Strain CNQ-085. J Nat Prod, 69(12): 1756-1759
Asolkar R N, Maskey R P, Helmke E, et al. 2002. Chalcomycin B, a new macrolide antibiotic from the marine isolate Streptomyces sp. B7064. J Antibiot (Tokyo), 55(10): 893-898
Bae M, Chung B, Oh K B, et al. 2015a. Hormaomycins B and C: new antibiotic cyclic depsipeptides from a marine mudflat-derived Streptomyces sp.. Mar Drugs, 13(8): 5187-5200
Bae M, Kim H, Moon K, et al. 2015b. Mohangamides A and B, new dilactone-tethered pseudo-dimeric peptides inhibiting Candida albicans isocitrate lyase. Org Lett, 17(3): 712-715
Bae M, Kim H, Shin Y, et al. 2013. Separacenes A-D, novel polyene polyols from the marine actinomycete, Streptomyces sp.. Mar Drugs, 11(8): 2882-2893
Bae M, Moon K, Kim J, et al. 2016. Mohangic Acids A-E, p-aminoacetophenonic acids from a marine mudflat-derived Streptomyces sp.. J Nat Prod, 79(2): 332-339
Bae M A, Yamada K, Ijuin Y, et al. 1996. Aburatubolactam A, a novel inhibitor of superoxide anion generation from a marine microorganism. Heterocycl Commun, 2(4): 315-318
Balk-Bindseil W, Helmke E, Weyland H, et al. 1995. Maremycin A and B, new diketopiperazines from a marine Streptomyces sp.. Liebigs Ann, (7): 1291-1294
Biabani M A F, Baake M, Lovisetto B, et al. 1998. Anthranilamides: new antimicroalgal active substances from a marine Streptomyces sp.. J Antibiot (Tokyo), 51(3): 333-340
Biabani M A F, Laatsh H, Helmke E, et al. 1997. δ -Indomycinone: a new member of pluramycin class of antibiotics isolated from marine Streptomyces sp.. J Antibiot (Tokyo), 50(10): 874-877
Boonlarppradab C, Kauffman C A, Jensen P R, et al. 2008. Marineosins A and B, cytotoxic spiroaminals from a marine-derived actinomycete. Org Lett, 10(24): 5505-5508
Bu Y Y, Yamazaki H, Ukai K, et al. 2014. Antimycobacterial nucleoside antibiotics from a marinederived Streptomyces sp. TPU1236A. Mar Drugs, 12(12): 6102-6112
Bugni T S, Woolery M, Kauffman C A, et al. 2006. Bohemamines from a marine-derived Streptomyces sp.. J Nat Prod, 69(11): 1626-1628
Capon R J, Skene C, Lacey E, et al. 2000. Lorneamides A and B: two new aromatic amides from a southern Australian marine actinomycete. J Nat Prod, 63(12): 1682-1683
Carlson J C, Li S Y, Burr D A, et al. 2009. Isolation and characterization of tirandamycins from a marinederived Streptomyces sp.. J Nat Prod, 72(11): 2076-2079
Che Q, Li J, Li D H, et al. 2016. Structure and absolute configuration of drimentine I, an alkaloid from Streptomyces sp. CHQ-64. J Antibiot (Tokyo), 69(6): 467-469
Chen C X, Wang J, Guo H, et al. 2013. Three antimycobacterial metabolites identified from amarine-derived Streptomyces sp. MS100061. Appl Microbiol Biotechnol, 97(9): 3885-3892
Chen G D, Gao H, Tang J S, et al. 2011. Benzamides and quinazolines from a mangrove actinomycetes Streptomyces sp. (No. 061316) and their inhibiting caspase-3 catalytic activity in vitro. Chem Pharm Bull, 59(4): 447-451
Chen Y L, Zhang W J, Zhu Y G, et al. 2014. Elucidating hydroxylation and methylation steps tailoring piericidin A1 biosynthesis. Org Lett, 16(3): 736-739
Chen Z B, Hao J J, Wang L P, et al. 2016. New α-glucosidase inhibitors from marine algae-derived Streptomyces sp. OUCMDZ-3434. Sci Rep, 6: 20004
Cheng C, Othman E M, Reimer A, et al. 2016. Ageloline A, new antioxidant and antichlamydial quinolone from the marine sponge-derived bacterium Streptomyces sp. SBT345. Tetrahedron Lett, 57(25): 2786-2789
Cheng Y B, Jensen P R, Fenical W. 2013. Cytotoxic and antimicrobial napyradiomycins from two marinederived, MAR 4 Streptomyces strains. Eur J Org Chem, (18): 3751-3757
Cho J Y, Kwon H C, Williams P G, et al. 2006a. Actinofuranones A and B, polyketides from a marine-derived bacterium related to the genus Streptomyces (Actinomycetales). J Nat Prod, 69(3): 425-428
Cho J Y, Kwon H C, Williams P G, et al. 2006b. Azamerone, a terpenoid phthalazinone from a marine-derived bacterium related to the genus Streptomyces (Actinomycetales). Org Lett, 8(12): 2471-2474
Cho K W, Lee H S, Rho J R, et al. 2001. New lactone-containing metabolites from a marinederived bacterium of the genus Streptomyces. J Nat Prod, 64(5): 664-667
Deng J J, Lu C H, Li S R, et al. 2014. p-terphenyl O-β-glucuronides, DNA topoisomerase inhibitors from Streptomyces sp. LZ35△gdmAI. Bioorg Med Chem Lett, 24(5): 1362-1365
Dickschat J S, Martens T, Brinkhoff T, et al. 2005. Volatiles released by a Streptomyces species isolated from the North Sea. Chem Biodivers, 2(7): 837-865
Ding L, Maier A, Fiebig H H, et al. 2011. Divergolides A-D from a mangrove endophyte reveal an unparalleled plasticity in ansa-macrolide biosynthesis. Angew Chem Int Ed, 50(7): 1630-1634
Ding L, Maier A, Fiebig H H, et al. 2012. Kandenols A-E, eudesmenes from an endophytic Streptomyces sp. of the mangrove tree Kandelia candel. J Nat Prod, 75(12): 2223-2227
Ding L, Münch J, Goerls H, et al. 2010. Xiamycin, a pentacyclic indolosesquiterpene with selective anti-HIV activity from a bacterial mangrove endophyte. Bioorg Med Chem Lett, 20(22): 6685-6687
Ding L, Pfoh R, Rühl S, et al. 2009. T-muurolol sesquiterpenes from the marine Streptomyces sp. M491 and revision of the configuration of previously reported amorphanes. J Nat Prod, 72(1): 99-101
Ding W J, Zhang S Q, Wang J H, et al. 2013. A new di-O-prenylated flavone from an actinomycete Streptomyces sp. MA-12. J Asian Nat Prod Res, 15(2): 209-214
El-Gendy M M A, Shaaban M, Shaaban K A, et al. 2008. Essramycin: a first triazolopyrimidine antibiotic isolated from nature. J Antibiot (Tokyo), 61(3): 149-157
Farnaes L, Coufal N G, Kauffman C A, et al. 2014. Napyradiomycin derivatives, produced by a marine-derived actinomycete, illustrate cytotoxicity by induction of apoptosis. J Nat Prod, 77(1): 15-21 Ford P W, Gadepalli M, Davidson B S. 1998. Halawanones A-D, new polycyclic quinones from a marine-derived streptomycete. J Nat Prod, 61(10): 1232-1236
Fremlin L, Farrugia M, Piggott A M, et al. 2011. Reveromycins revealed: new polyketide spiroketals from Australian marine-derived and terrestrial Streptomyces spp. A case of natural products vs. artifacts. Org Biomol Chem, 9(4): 1201-1211
Fu P, Johnson M, Chen H, et al. 2014. Carpatamides A-C, cytotoxic arylamine derivatives from a marine-derived Streptomyces sp.. J Nat Prod, 77(5): 1245-1248
Fu P, Kong F D, Wang Y F, et al. 2013. Antibiotic metabolites from the coral-associated actinomycete Streptomyces sp. OUCMDZ-1703. Chin J Chem, 31(1): 100-104
Fu P, La S, MacMillan J B. 2016. 1,3-Oxazin-6-one derivatives and bohemamine-type pyrrolizidine alkaloids from a marine-derived Streptomyces spinoverrucosus. J Nat Prod, 79(3): 455-462
Fu P, Yang C L, Wang Y, et al. 2012a. Streptocarbazoles A and B, two novel indolocarbazoles from the marine-derived actinomycete strain Streptomycessp. FMA. Org Lett, 14(9): 2422-2425
Fu P, Zhuang Y B, Wang Y, et al. 2012b. New indolocarbazoles from a mutant strain of the marine-derived actinomycete Streptomyces fradiae 007M135. Org Lett, 14(24): 6194-6197
Fukuda T, Miller E D, Clark B R, et al. 2011. Structures and biosynthesis of the pyridinopyrones, polyenepyrones from a marine-derived Streptomyces species. J Nat Prod, 74(8): 1773-1778
Han Z, Xu Y, McConnell O, et al. 2012. Two antimycin A analogues from marine-derived actinomycete Streptomyces lusitanus. Mar Drugs, 10(3): 668-676
Harinantenaina Rakotondraibe L, Rasolomampianina R, Park H Y, et al. 2015. Antiproliferative and antiplasmodial compounds from selected Streptomyces species. Bioorg Med Chem Lett, 25(23): 5646-5649
Harunari E, Imada C, Igarashi Y, et al. 2014. Hyaluromycin, a new hyaluronidase inhibitor of polyketide origin from marine Streptomyces sp.. Mar Drugs, 12(1): 491-507
Hassan H M, Boonlarppradab C, Fenical W. 2016. Actinoquinolines A and B, anti-inflammatory quinoline alkaloids from a marine-derived Streptomyces sp., strain CNP975. J Antibiot (Tokyo), 69(7): 511-514
Hawas U W, Shaaban M, Shaaban K A, et al. 2009. Mansouramycins A-D, cytotoxic isoquinolinequinones from a marine Streptomycete. J Nat Prod, 72(12): 2120-2124
Hayakawa Y, Shirasaki S, Shiba S, et al. 2007. Piericidins C7 and C8, new cytotoxic antibiotics produced by a marine Streptomyces sp.. J Antibiot, 60(3): 196-200
Hernandez I L C, Godinho M J L, Magalhães A, et al. 2000. N-acetyl-γ-hydroxyvaline lactone, an unusual amino acid derivative from a marine streptomycete. J Nat Prod, 63(5): 664-665
Hohmann C, Schneider K, Bruntner C, et al. 2009. Albidopyrone, a new α-pyrone-containing metabolite from marine-derived Streptomyces sp. NTK 227. J Antibiot (Tokyo), 62(2): 75-79
Hosoya T, Hirokawa T, Takagi M, et al. 2012. Trichostatin analogues JBIR-109, JBIR-110, and JBIR-111 from the marine sponge-derived Streptomyces sp. RM72. J Nat Prod, 75(2): 285-289
Hu Y C, MacMillan J B. 2012. A new peptide isolated from a marine derived Streptomyces bacillaris. Nat Prod Commun, 7(2): 211-214
Huang H, Cao Y, Tian L, et al. 2014. A new polyunsaturated acid from the marine-derived Streptomyces violans (No. HTTA-F04129). Chem Nat Compd, 50(3): 402-404
Huang H B, Yang T T, Ren X M, et al. 2012. Cytotoxic angucycline class glycosides from the deep sea actinomycete Streptomyces lusitanus SCSIO LR32. J Nat Prod, 75(2): 202-208
Huang X L, Gao Y, Xue D Q, et al. 2011. Streptomycindole, an indole alkaloid from a marine Streptomyces sp. DA22 associated with South China Sea sponge Craniella australiensis. Helv Chim Acta, 94(10): 1838-1842
Huang Y F, Tian L, Fu H W, et al. 2006a. One new anthraquinone from marine Streptomyces sp. FX-58. Nat Prod Res, 20(13): 1207-1210
Huang Y F, Tian L, Sun Y, et al. 2006b. Two new compounds from marine Streptomyces sp. FX-58. J Asian Nat Prod Res, 8(6): 495-498
Hughes C C, Kauffman C A, Jensen P R, et al. 2010. Structures, reactivities, and antibiotic properties of the marinopyrroles A-F. J Org Chem, 75(10): 3240-3250
Hughes C C, MacMillan J B, Gaudêncio S P, et al. 2009. The ammosamides: structures of cell cycle modulators from a marine-derived Streptomyces species. Angew Chem Int Ed, 48(4): 725-727
Hughes C C, Prieto-Davo A, Jensen P R, et al. 2008. The marinopyrroles, antibiotics of an unprecedented structure class from a marine Streptomyces sp.. Org Lett, 10(4): 629-631
Igarashi Y, Asano D, Furihata K, et al. 2012. Absolute configuration of pterocidin, a potent inhibitor of tumor cell invasion from a marine-derived Streptomyces. Tetrahedron Lett, 53(6): 654-656
Igarashi Y, Shimasaki R, Miyanaga S, et al. 2010. Rakicidin D, an inhibitor of tumor cell invasion from marine-derived Streptomyces sp.. J Antibiot (Tokyo), 63(9): 563-565
Igarashi Y, Zhou T, Sato S, et al. 2013. Akaeolide, a carbocyclic polyketide from marine-derived Streptomyces. Org Lett, 15(22): 5678-5681
Imamura N, Nishijima M, Adachi K, et al. 1993. Novel antimycin antibiotics, urauchimycins A and B, produced by marine actinomycete. J Antibiot (Tokyo), 46(2): 241-246
Itoh T, Kinoshita M, Aoki S, et al. 2003. Komodoquinone A, a novel neuritogenic anthracycline, from marine Streptomyces sp. KS3. J Nat Prod, 66(10): 1373-1377
Ivanova V, Oriol M, Montes M J, et al. 2001. Secondary metabolites from a Streptomyces strain isolated from Livingston Island, Antarctica. Z Naturforsch C, 56(1-2): 1-5
Iwata F, Sato S, Mukai T, et al. 2009. Lorneic acids, trialkyl-substituted aromatic acids from a marinederived actinomycete. J Nat Prod, 72(11): 2046-2048
Izumikawa M, Kawahara T, Hwang J H, et al. 2013. JBIR-107, a new metabolite from the marinesponge-derived actinomycete, Streptomyces tateyamensis NBRC 105047. Biosci Biotechnol Biochem, 77(3): 663-665
Izumikawa M, Khan S T, Komaki H, et al. 2010a. JBIR-31, a new teleocidin analog, produced by salt-requiring Streptomyces sp. NBRC 105896 isolated from a marine sponge. J Antibiot (Tokyo), 63(1): 33-36
Izumikawa M, Khan S T, Takagi M, et al. 2010b. Sponge-derived Streptomyces producing isoprenoids via the mevalonate pathway. J Nat Prod, 73(2): 208-212
Jang K H, Nam S J, Locke J B, et al. 2013. Anthracimycin, a potent anthrax antibiotic from a marine-derived actinomycete. Angew Chem Int Ed, 52(30): 7822-7824
Jeong S Y, Shin H J, Kim T S, et al. 2006. Streptokordin, a new cytotoxic compound of the methylpyridine class from a marine-derived Streptomyces sp. KORDI-3238. J Antibiot (Tokyo), 59(4): 234-240
Jiang Z D, Jensen P R, Fenical W. 1997. Actinoflavoside, a novel flavonoid-like glycoside produced by a marine bacterium of the genus Streptomyces. Tetrahedron Lett, 38(29): 5065-5068
Kaysser L, Bernhardt P, Nam S J, et al. 2012. Merochlorins A-D, cyclic meroterpenoid antibiotics biosynthesized in divergent pathways with vanadium-dependent chloroperoxidases. J Am Chem Soc, 134(29): 11988-11991
Kim D G, Moon K, Kim S H, et al. 2012. Bahamaolides A and B, antifungal polyene polyol macrolides from the marine actinomycete Streptomyces sp.. J Nat Prod, 75(5): 959-967
Kim H, Yang I, Patil R S, et al. 2014. Anithiactins A-C, modified 2-phenylthiazoles from a mudflat-derived Streptomyces sp.. J Nat Prod, 77(12): 2716-2719
Ko K, Lee S H, Kim S H, et al. 2014. Lajollamycins, nitro group-bearing spiro-β-lactone-γ-lactams obtained from a marine-derived Streptomyces sp.. J Nat Prod, 77(9): 2099-2104
Kock I, Maskey R P, Biabani M A F, et al. 2005. 1-Hydroxy-1-norresistomycin and resistoflavin methyl ether: new antibiotics from marine-derived streptomycetes. J Antibiot (Tokyo), 58(8): 530-534
Kondratyuk T P, Park E J, Yu R, et al. 2012. Novel marine phenazines as potential cancer chemopreventive and anti-inflammatory agents. Mar Drugs, 10(2): 451-464
Kunz A L, Labes A, Wiese J, et al. 2014. Nature's lab for derivatization: new and revised structures of a variety of streptophenazines produced by a sponge-derived Streptomyces strain. Mar Drugs, 12(4): 1699-1714
Kwon H C, Espindola A P D M, Park J S, et al. 2010. Nitropyrrolins A-E, cytotoxic farnesyl-α-nitropyrroles from a marine-derived bacterium within the actinomycete family Streptomycetaceae. J Nat Prod, 73(12): 2047-2052
Lane A L, Nam S J, Fukuda T, et al. 2013. Structures and comparative characterization of biosynthetic gene clusters for cyanosporasides, enediynederived natural products from marine actinomycetes. J Am Chem Soc, 135(11): 4171-4174
Le T C, Yang I, Yoon Y J, et al. 2016. Ansalactams B-D illustrate further biosynthetic plasticity within the ansamycin pathway. Org Lett, 18(9): 2256-2259
Lee H K, Lee D S, Lim J, et al. 1998. Topoisomerase I inhibitors from the Streptomyces sp. strain KM86-9B isolated from a marine sponge. Arch Pharm Res, 21(6): 729-733
Lee H S, Shin H J, Jang K H, et al. 2005. Cyclic peptides of the nocardamine class from a marinederived bacterium of the genus Streptomyces. J Nat Prod, 68(4): 623-625
Lee J, Kim H, Lee T G, et al. 2014. Anmindenols A and B, inducible nitric oxide synthase inhibitors from a marine-derived Streptomyces sp.. J Nat Prod, 77(6): 1528-1531
Li B, Chen G, Bai J, et al. 2011a. A bisamide and four diketopiperazines from a marine-derived Streptomyces sp.. J Asian Nat Prod Res, 13(12): 1146-1150
Li F C, Maskey R P, Qin S, et al. 2005. Chinikomycins A and B: isolation, structure elucidation, and biological activity of novel antibiotics from a marine Streptomyces sp. isolate M045#. J Nat Prod, 68(3): 349-353
Li J, Lu C H, Zhao B B, et al. 2008. Phaeochromycins F-H, three new polyketide metabolites fromStreptomyces sp. DSS-18. Beilstein J Org Chem, 4(1): 46
Li K, Li Q L, Ji N Y, et al. 2011b. Deoxyuridines from the marine sponge associated actinomycete Streptomyces microflavus. Mar Drugs, 9(5): 690- 695
Lian X Y, Zhang Z Z. 2013. Indanomycin-related antibiotics from marine Streptomyces antibioticus PTZ0016. Nat Prod Res, 27(23): 2161-2167
Liang Y, Chen L, Ye X W, et al. 2016a. New streptophenazines from marine Streptomyces sp. 182SMLY. Nat Prod Res: 1-7
Liang Y, Xie X, Chen L, et al. 2016b. Bioactive polycyclic quinones from marine Streptomyces sp. 182SMLY. Mar Drugs, 14(1): 1-11
Lin Z J, Antemano R R, Hughen R W, et al. 2010. Pulicatins A-E, neuroactive thiazoline metabolites from cone snail-associated bacteria. J Nat Prod, 73(11): 1922-1926
Lin Z J, Flores M, Forteza I, et al. 2012. Totopotensamides, polyketide-cyclic peptide hybrids from a mollusk-associated bacterium Streptomyces sp.. J Nat Prod, 75(4): 644-649
Lin Z J, Koch M, Pond C D, et al. 2014. Structure and activity of lobophorins from a turrid molluskassociated Streptomyces sp.. J Antibiot (Tokyo), 67(1): 121-126
Lin Z J, Reilly C A, Antemano R, et al. 2011. Nobilamides A-H, long-acting transient receptor potential vanilloid-1 (TRPV1) antagonists from mollusk-associated bacteria. J Med Chem, 54(11): 3746-3755
Liu D, Yang A G, Wu C M, et al. 2014. Lipid-lowering effects of farnesylquinone and related analogues from the marine-derived Streptomyces nitrosporeus. Bioorg Med Chem Lett, 24(22): 5288-5293
Liu N, Song F Y, Shang F, et al. 2015. Mycemycins A-E, new dibenzoxazepinones isolated from two different Streptomycetes. Mar Drugs, 13(10): 6247-6258
Lorente A, Pla D, Cañedo L M, et al. 2010. Isolation, structural assignment, and total synthesis of barmumycin. J Org Chem, 75(24): 8508-8515
Luo M H, Tang G L, Ju J H, et al. 2016. A new diketopiperazine derivative from a deep seaderived Streptomyces sp. SCSIO 04496. Nat Prod Res, 30(2): 138-143
Macherla V R, Liu J, Bellows C, et al. 2005. Glaciapyrroles A, B, and C, pyrrolosesquiterpenes from a Streptomyces sp. isolated from an Alaskan marine sediment. J Nat Prod, 68(5): 780-783
Mahyudin N A, Blunt J W, Cole A L J, et al. 2012. The isolation of a new S-methyl benzothioate compound from a marine-derived Streptomyces sp.. J Biomed Biotechnol, 2012: 894708
Manam R R, Teisan S, White D J, et al. 2005. Lajollamycin, a nitro-tetraene spiro-β-lactone-γlactam antibiotic from the marine actinomycete Streptomyces nodosus. J Nat Prod, 68(2): 240-243
Martin G D A, Tan L T, Jensen P R, et al. 2007. Marmycins A and B, cytotoxic pentacyclic C-glycosides from a marine sediment-derived actinomycete related to the genus Streptomyces. J Nat Prod, 70(9): 1406-1409
Maskey R P, Helmke E, Fiebig H H, et al. 2002. Parimycin: isolation and structure elucidation of a novel cytotoxic 2, 3-dihydroquinizarin analogue of γ-indomycinone from a marine Streptomycete isolate. J Antibiot (Tokyo), 55(12): 1031-1035
Maskey R P, Sevvana M, Usón I, et al. 2004. Gutingimycin: a highly complex metabolite from a marine streptomycete. Angew Chem Int Ed, 43(10): 1281-1283
Matsuo Y, Kanoh K, Jang J H, et al. 2011. Streptobactin, a tricatechol-type siderophore from marine-derived Streptomyces sp. YM5-799. J Nat Prod, 74(11): 2371-2376
Miller E D, Kauffman C A, Jensen P R, et al. 2007. Piperazimycins: cytotoxic hexadepsipeptides from a marine-derived bacterium of the genus Streptomyces. J Org Chem, 72(2): 323-330
Mitchell S S, Nicholson B, Teisan S, et al. 2004. Aureoverticillactam, a novel 22-atom macrocyclic lactam from the marine actinomycete Streptomyces aureoverticillatus. J Nat Prod, 67(8): 1400-1402
Mitova M I, Lang G, Wiese J, et al. 2008. Subinhibitory concentrations of antibiotics induce phenazine production in a marine Streptomyces sp.. J Nat Prod, 71(5): 824-827
Moon K, Ahn C H, Shin Y, et al. 2014. New benzoxazine secondary metabolites from an arctic actinomycete. Mar Drugs, 12(5): 2526-2538
Motohashi K, Inaba K, Fuse S, et al. 2011. JBIR-56 and JBIR-57, 2 (1H)-pyrazinones from a marine sponge-derived Streptomyces sp. SpD081030SC-03. J Nat Prod, 74(7): 1630-1635
Motohashi K, Irie K, Toda T, et al. 2008. 5-Dimethylallylindole-3-carboxylic acid and A80915G-8-acid produced by marine-derivedStreptomyces sp. MS239. J Antibiot, 61(2): 75-80
Motohashi K, Takagi M, Shin-Ya K. 2010a. Tetracenoquinocin and 5-iminoaranciamycin from a sponge-derived Streptomyces sp. Sp080513GE-26. J Nat Prod, 73(4): 755-758
Motohashi K, Takagi M, Shin-Ya K. 2010b. Tetrapeptides possessing a unique skeleton, JBIR-34 and JBIR-35, isolated from a sponge-derived actinomycete, Streptomyces sp. Sp080513GE-23. J Nat Prod, 73(2): 226-228
Motohashi K, Toda T, Sue M, et al. 2010c. Isolation and structure elucidation of tumescenamides A and B, two peptides produced by Streptomyces tumescens YM23-260. J Antibiot (Tokyo), 63(9): 549-552
Mukku V J R V, Speitling M, Laatsch H, et al. 2000. New butenolides from two marine streptomycetes. J Nat Prod, 63(11): 1570-1572
Mullowney M W, Ó hAinmhire E, Shaikh A, et al. 2014. Diazaquinomycins E-G, novel diaza-anthracene analogs from a marine-derived Streptomyces sp.. Mar Drugs, 12(6): 3574-3586
Na M, Meujo D A F, Kevin D, et al. 2008. A new antimalarial polyether from a marine Streptomyces sp. H668. Tetrahedron Lett, 49(44): 6282-6285
Nachtigall J, Schneider K, Bruntner C, et al. 2011. Benzoxacystol, a benzoxazine-type enzyme inhibitor from the deep-sea strain Streptomyces sp. NTK 935. J Antibiot (Tokyo), 64(6): 453-457
Nakamura H, Iitaka Y, Kitahara T, et al. 1977. Structure of Aplasmomycin. J Antibiot (Tokyo), 30(9): 714-719
Nam S J, Kauffman C A, Jensen P R, et al. 2011. Isolation and characterization of actinoramides A—C, highly modified peptides from a marine Streptomyces sp.. Tetrahedron, 67(35): 6707-6712
Nam S J, Kauffman C A, Paul L A, et al. 2013. Actinoranone, a cytotoxic meroterpenoid of unprecedented structure from a marine adapted Streptomyces sp.. Org Lett, 15(21): 5400-5403
Nong X H, Zhang X Y, Xu X Y, et al. 2016. Nahuoic acids B−E, polyhydroxy polyketides from the marine-derived Streptomyces sp. SCSGAA. J Nat Prod, 79(1): 141-148
Ohlendorf B, Schulz D, Erhard A, et al. 2012. Geranylphenazinediol, an acetylcholinesterase inhibitor produced by a Streptomyces species. J Nat Prod, 75(7): 1400-1404
Okami Y, Hotta K, Yoshida M, et al. 1979. New aminoglycoside antibiotics, istamycins A and B. J Antibiot (Tokyo), 32(9): 964-966
Okami Y, Okazaki T, Kitahara T, et al. 1976. Studies on marine microorganisms. V. A new antibiotic, aplasmomycin, produced by a streptomycete isolated from shallow sea mud. J Antibiot (Tokyo), 29(10): 1019-1025
Pan E D, Jamison M, Yousufuddin M, et al. 2012. Ammosamide D, an oxidatively ring opened ammosamide analog from a marine-derived Streptomyces variabilis. Org Lett, 14(9): 2390-2393
Pan H Q, Zhang S Y, Wang N, et al. 2013. New spirotetronate antibiotics, lobophorins H and I, from a South China Sea-derived Streptomyces sp. 12A35. Mar Drugs, 11(10): 3891-3901
Pathirana C, Jensen P R, Dwight R, et al. 1992. Rare phenazine L-quinovose esters from a marine actinomycete. J Org Chem, 57(2): 740-742
Pérez M, Crespo C, Schleissner C, et al. 2009. Tartrolon D, a cytotoxic macrodiolide from the marinederived actinomycete Streptomyces sp. MDG-04-17-069. J Nat Prod, 72(12): 2192-2194
Pérez M, Schleissner C, Fernández R, et al. 2016. PM100117 and PM100118, new antitumor macrolides produced by a marine Streptomyces caniferus GUA-06-05-006A. J Antibiot (Tokyo), 69(5): 388-394
Phipps R K, Blunt J W, Cole A L J, et al. 2004. Anthracycline derivatives from a marine-derived New Zealand Streptomycete. Arkivoc, 2004(10): 94-100
Pimentel-Elardo S M, Buback V, Gulder T A M, et al. 2011. New tetromycin derivatives with antitrypanosomal and protease inhibitory activities. Mar Drugs, 9(10): 1682-1697
Poumale H M P, Ngadjui B T, Helmke E, et al. 2006. New anthraquinones from a marine Streptomyces sp. -isolation, structure determination and biological activities. Z Naturforsch B, 61(11): 1450-1454
Pusecker K, Laatsch H, Helmke E, et al. 1997. Dihydrophencomycin methyl ester, a new phenazine derivative from a marine Streptomycete. J Antibiot (Tokyo), 50(6): 479-483
Quitschau M, Schuhmann T, Piel J, et al. 2008. The new metabolite (S)-cinnamoylphosphoramide from Streptomyces sp. and its total synthesis. Eur J Org Chem, (30): 5117-5124
Raju R, Khalil Z G, Piggott A M, et al. 2014. Mollemycin A: an antimalarial and antibacterial glyco-hexadepsipeptide-polyketide from an Australianmarine-derived Streptomyces sp. (CMB-M0244). Org Lett, 16(6): 1716-1719
Raju R, Piggott A M, Barrientos Diaz L X, et al. 2010a. Heronapyrroles A-C: farnesylated 2-nitropyrroles from an Australian marine-derived Streptomyces sp.. Org Lett, 12(22): 5158-5161
Raju R, Piggott A M, Conte M M, et al. 2010b. Heronamides A-C, new polyketide macrolactams from an Australian marine-derived Streptomyces sp. A biosynthetic case for synchronized tandem electrocyclization. Org Biomol Chem, 8(20): 4682-4689
Raju R, Piggott A M, Conte M M, et al. 2009. Naseseazines A and B: a new dimeric diketopiperazine framework from a marine-derived actinomycete, Streptomyces sp.. Org Lett, 11(17): 3862-3865
Raju R, Piggott A M, Khalil Z, et al. 2012. Heronamycin A: a new benzothiazine ansamycin from an Australian marine-derived Streptomyces sp.. Tetrahedron Lett, 53(9): 1063-1065
Salem S M, Kancharla P, Florova G , et al. 2014. Elucidation of final steps of the marineosins biosynthetic pathway through identification and characterization of the corresponding gene cluster. J Am Chem Soc, 136(12): 4565-4574
Jose S L, Marta M I, Julia P B, et al. 2003. New cytotoxic indolic metabolites from a marine Streptomyces. J Nat Prod, 66(6): 863-864
Sato S, Iwata F, Mukai T, et al. 2009. Indoxamycins A-F. Cytotoxic tricycklic polypropionates from a marine-derived actinomycete. J Org Chem, 74(15): 5502-5509
Sato S, Iwata F, Yamada S, et al. 2011. Usabamycins A-C: new anthramycin-type analogues from a marine-derived actinomycete. Bioorg Med Chem Lett, 21(23): 7099-7101
Schleissner C, Pérez M, Losada A, et al. 2011. Antitumor actinopyranones produced by Streptomyces albus POR-04-15-053 isolated from a marine sediment. J Nat Prod, 74(7): 1590-1596
Schneemann I, Kajahn I, Ohlendorf B, et al. 2010. Mayamycin, a cytotoxic polyketide from a Streptomyces strain isolated from the marine sponge Halichondria panicea. J Nat Prod, 73(7): 1309-1312
Schumacher R W, Talmage S C, Miller S A, et al. 2003. Isolation and structure determination of an antimicrobial ester from a marine sediment-derived bacterium. J Nat Prod, 66(9): 1291-1293
Shaaban K A, Helmke E, Kelter G, et al. 2011. Glucopiericidin C: a cytotoxic piericidin glucoside antibiotic produced by a marine Streptomyces isolate. J Antibiot (Tokyo), 64(2): 205-209
Shaaban K A, Shaaban M, Facey P, et al. 2008. Electrospray ionization mass spectra of piperazimycins A and B and gamma-butyrolactones from a marine-derived Streptomyces sp.. J Antibiot (Tokyo), 61(12): 736-746
Shin B, Ahn S, Noh M, et al. 2015. Suncheonosides A−D, benzothioate glycosides from a marinederived Streptomyces sp.. J Nat Prod, 78(6): 1390-1396
Shin H J, Lee H S, Lee J S, et al. 2014. Violapyrones H and I, new cytotoxic compounds isolated from Streptomyces sp. associated with the marine starfish Acanthaster planci. Mar Drugs, 12(6): 3283-3291
Sitachitta N, Gadepalli M, Davidson B S. 1996. New α-pyrone-containing metabolites from a marinederived actinomycete. Tetrahedron, 52(24): 8073-8080
Socha A M, Garcia D, Sheffer R, et al. 2006. Antibiotic bisanthraquinones produced by a Streptomycete isolated from a Cyanobacterium. J Nat Prod, 69(7): 1070-1073
Song Y X, Huang H B, Chen Y C, et al. 2013. Cytotoxic and antibacterial marfuraquinocins from the deep south china sea-derived Streptomyces niveus SCSIO 3406. J Nat Prod, 76(12): 2263-2268
Song Y X, Li Q L, Liu X, et al. 2014. Cyclic hexapeptides from the deep south china sea-derived Streptomyces scopuliridis SCSIO ZJ46 active against pathogenic gram-positive bacteria. J Nat Prod, 77(8): 1937-1941
Song Y X, Liu G F, Li J, et al. 2015. Cytotoxic and antibacterial angucycline- and prodigiosinanalogues from the deep-sea derived Streptomyces sp. SCSIO 11594. Mar Drugs, 13(3): 1304-1316
Strangman W K, Kwon H C, Broide D, et al. 2009. Potent inhibitors of pro-inflammatory cytokine production produced by a marine-derived bacterium. J Med Chem, 52(8): 2317-2327
Stritzke K, Schulz S, Laatsch H, et al. 2004. Novel caprolactones from a marine streptomycete. J Nat Prod, 67(3): 395-401
Sugiyama R, Nishimura S, Matsumori N, et al. 2014. Structure and biological activity of 8-deoxyheronamide C from a marine-derived Streptomyces sp.: heronamides target saturated hydrocarbon chains inlipid membranes. J Am Chem Soc, 136(14): 5209-5212
Sun D D, Sun W, Yu Y X, et al. 2014a. A new glutarimide derivative from marine sponge-derived Streptomyces anulatus S71. Nat Prod Res, 28(19): 1602-1606
Sun P, Maloney K N, Nam S J, et al. 2011. Fijimycins A-C, three antibacterial etamycin-class depsipeptides from a marine-derived Streptomyces sp.. Bioorg Med Chem, 19(22): 6557-6562
Sun Y, Takada K, Nogi Y, et al. 2014b. Lower homologues of ahpatinin, aspartic protease inhibitors, from a marine Streptomyces sp.. J Nat Prod, 77(7): 1749-1752
Supong K, Thawai C, Suwanborirux K, et al. 2012. Antimalarial and antitubercular C-glycosylated benz[α]anthraquinones from the marine-derived Streptomyces sp. BCC45596. Phytochem Lett, 5(3): 651-656
Takada K, Ninomiya A, Naruse M, et al. 2013. Surugamides A-E, cyclic octapeptides with four D-amino acid residues, from a marine Streptomyces sp. LC−MS-aided inspection of partial hydrolysates for the distinction of D- and L-amino acid residues in the sequence. J Org Chem, 78(13): 6746-6750
Takahashi C, Takada T, Yamada T, et al. 1994. Halichomycin, a new class of potent cytotoxic macrolide produced by an actinomycete from a marine fish. Tetrahedron Lett, 35(28): 5013-5014
Tapiolas D M, Roman M, Fenical W, et al. 1991. Octalactins A and B: cytotoxic eight-membered-ring lactones from a marine bacterium, Streptomyces sp.. J Am Chem Soc, 113(12): 4682-4683
Trischman J A, Tapiolas D M, Jensen P R, et al. 1994. Salinamides A and B: anti-inflammatory depsipeptides from a marine Streptomycete. J Am Chem Soc, 116(2): 757-758
Ueda J Y, Khan S T, Takagi M, et al. 2010. JBIR-58, a new salicylamide derivative, isolated from a marine sponge-derived Streptomyces sp. SpD081030ME-02. J Antibiot (Tokyo), 63(5): 267-269
Um S, Choi T J, Kim H, et al. 2013a. Ohmyungsamycins A and B: cytotoxic and antimicrobial cyclic peptides produced by Streptomyces sp. from a volcanic island. J Org Chem, 78(24): 12321-12329
Um S, Kim Y J, Kwon H, et al. 2013b. Sungsanpin, a lasso peptide from a deep-sea streptomycete. J Nat Prod, 76(5): 873-879
Vicente J, Stewart A K, Wagoner R M, et al. 2015. Monacyclinones, new angucyclinone metabolites isolated from Streptomyces sp. m7_15 associated with the puertorican sponge Scopalina ruetzleri. Mar Drugs, 13(8): 4682-4700
Wang F F, Xu M J, Li Q S, et al. 2010. p-Aminoacetophenonic acids produced by a mangrove endophyte Streptomyces sp. (strain HK10552). Molecules, 15(4): 2782-2790
Wang P, Xi L J, Liu P P, et al. 2013. Diketopiperazine derivatives from the marine-derived actinomycete Streptomyces sp. FXJ7.328. Mar Drugs, 11(4): 1035-1049
Wei R B, Xi T, Li J, et al. 2011. Lobophorin C and D, new kijanimicin derivatives from a marine sponge-associated actinomycetal strain AZS17. Mar Drugs, 9(3): 359-368
Williams D E, Dalisay D S, Li F L, et al. 2013. Nahuoic acid A produced by a Streptomyces sp. isolated from a marine sediment is a selective SAM-competitive inhibitor of the histone methyltransferase SETD8. Org Lett, 15(2): 414-417
Williams D E, Dalisay D S, Patrick B O, et al. 2011. Padanamides A and B, highly modified linear tetrapeptides produced in culture by a Streptomyces sp. isolated from a marine sediment. Org Lett, 13(15): 3936-3939
Williams D E, Izard F, Arnould S, et al. 2016. Structures of nahuoic acids B−E produced in culture by a Streptomyces sp. isolated from a marine sediment and evidence for the inhibition of the histone methyl transferase SETD8 in human cancer cells by nahuoic acid A. J Org Chem, 81(4): 1324-1332
Wilson M C, Nam S J, Gulder T A M, et al. 2011. Structure and biosynthesis of the marine streptomycete ansamycin ansalactam A and its distinctive branched chain polyketide extender unit. J Am Chem Soc, 133(6): 1971-1977
Wu C Y, Tan Y, Gan M L, et al. 2013a. Identification of elaiophylin derivatives from the marine-derived actinomycete Streptomyces sp. 7-145 using pcr-based screening. J Nat Prod, 76(11): 2153-2157
Wu S J, Fotso S, Li F C, et al. 2006. N-carboxamidostaurosporine and selina-4(14), 7(11)-diene-8, 9-diol, new metabolites from a marine Streptomyces sp.. J Antibiot (Tokyo), 59(6): 331-337
Wu S J, Fotso S, Li F C, et al. 2007. Amorphane sesquiterpenes from a marine Streptomyces sp.. J Nat Prod, 70(2): 304-306
Wu Z C, Li S M, Li J, et al. 2013b. Antibacterial andcytotoxic new napyradiomycins from the marinederived Streptomyces sp. SCSIO 10428. Mar Drugs, 11(6): 2113-2125
Xie X C, Mei W L, Zhao Y X, et al. 2006. A new degraded sesquiterpene from marine actinomycete streptomyces. 0616208. Chinese Chem Lett, 17(11): 1463-1465
Xie Z P, Liu B, Wang H P, et al. 2012. Kiamycin, a unique cytotoxic angucyclinone derivative from a marine Streptomyces sp.. Mar Drugs, 10(3): 551-558
Xu L Y, Quan X S, Wang C, et al. 2011. Antimycins A19and A20, two new antimycins produced by marine actinomycete Streptomyces antibioticus H74-18. J Antibiot (Tokyo), 64(10): 661-665
Xu M J, Liu X J, Zhao Y L, et al. 2012. Identification and characterization of an anti-fibrotic benzopyran compound isolated from mangrove-derived Streptomyces xiamenensis. Mar Drugs, 10(3): 639-654
Xu X L, Yin L Y, Wang S Y, et al. 2013. Cycloheximide acid A, a new cycloheximide derivative from marine derived Streptomyces sp. from East China Sea. Rec Nat Prod, 7(4): 292-295
Xu Z L, Baunach M, Ding L, et al. 2014. Biosynthetic code for divergolide assembly in a bacterial mangrove endophyte. Chembiochem, 15(9): 1274-1279
Yang A G, Si L L, Shi Z P, et al. 2013a. Nitrosporeusines A and B, unprecedented thioester-bearing alkaloids from the Arctic Streptomyces nitrosporeus. Org Lett, 15(20): 5366-5369
Yang X W, Peng K, Liu Z, et al. 2013b. Strepsesquitriol, a rearranged zizaane-type sesquiterpenoid from the deep-sea-derived actinomycete Streptomyces sp. SCSIO 10355. J Nat Prod, 76(12): 2360-2363
Yao C B F, Schiebel M, Helmke E, et al. 2006. Prefluostatin and new urauchimycin derivatives produced by Streptomycete isolates. Z Naturforsch B, 61(3): 320-325
Yixizhuoma, Tsukahara K, Toume K, et al. 2015. Novel cytotoxic isobenzofuran derivatives from Streptomyces sp. IFM 11490. Tetrahedron Lett, 56(46): 6345-6347
Yuan G J, Hong K, Lin H P, et al. 2013. New azalomycin F analogs from mangrove Streptomyces sp. 211726 with activity against microbes and cancer cells. Mar Drugs, 11(3): 817-829
Yuan G J, Lin H P, Wang C, et al. 2011.1H and13C assignments of two new macrocyclic lactones isolated from Streptomyces sp. 211726 and revised assignments of Azalomycins F3a, F4aand F5a. Magn Reson Chem, 49(1): 30-37
Zafrir Ilan E, Torres M R, Prudhomme J, et al. 2013. Farnesides A and B, sesquiterpenoid nucleoside ethers from a marine-derived Streptomyces sp., strain CNT-372 from Fiji. J Nat Prod, 76(9): 1815-1818
Zhang H Y, Wang H P, Cui H L, et al. 2011. A new anthracene derivative from marine Streptomyces sp. W007 exhibiting highly and selectively cytotoxic activities. Mar Drugs, 9(9): 1502-1509
Zhang J L, Qian Z Y, Wu X K, et al. 2014a. Juanlimycins A and B, ansamycin macrodilactams from Streptomyces sp.. Org Lett, 16(10): 2752-2755 Zhang Q B, Mándi A, Li S M, et al. 2012a. N-N-Coupled indolo-sesquiterpene atropo-diastereomers from a marine-derived actinomycete. Eur J Org Chem, (27): 5256-5262
Zhang W J, Ma L, Li S M, et al. 2014b. Indimicins A-E, bisindole alkaloids from the deep-sea-derived Streptomyces sp. SCSIO 03032. J Nat Prod, 77(8): 1887-1892
Zhang W J, Li S M, Zhu Y G, et al. 2014c. Heronamides D−F, polyketide macrolactams from the deep-seaderived Streptomyces sp. SCSIO 03032. J Nat Prod, 77(2): 388-391
Zhang W J, Liu Z, Li S M, et al. 2012b. Spiroindimicins A-D: new bisindole alkaloids from a deep-seaderived actinomycete. Org Lett, 14(13): 3364-3367
Zhang Y, Zhou X, Huang H B, et al. 2013. 03219A, a new Δ8,9-pregnene isolated from Streptomyces sp. SCSIO 03219 obtained from a South China Sea sediment. J Antibiot (Tokyo), 66(6): 327-331
Zhang Y M, Li H Y, Hu C, et al. 2016. Ergosterols from the culture broth of marine Streptomyces anandii H41-59. Mar Drugs, 14(5)
Zhen X, Gong T, Liu F, et al. 2015. A new analogue of echinomycin and a new cyclic dipeptide from a marine-derived Streptomyces sp. LS298. Mar Drugs, 13(11): 6947-6961
Zhou X, Huang H B, Li J, et al. 2014. New antiinfective cycloheptadepsipeptide congeners and absolute stereochemistry from the deep sea-derived Streptomyces drozdowiczii SCSIO 10141. Tetrahedron, 70(42): 7795-7801
New Natural Products From the Marine-Derived Streptomyces Actinobacteria
WANG Cong, MEI Xian-Gui, ZHU Wei-Ming*
(Key Laboratory of Marine Drugs, Ministry of Education of China, School of Medicine and Pharmacy, Ocean University of China, Qingdao 266003, China) *Corresponding author, E-mail: weimingzhu@ouc.edu.cn
Marine-derived Streptomyces were the chief source of the bioactive marine natural products (MNPs) from marine microbial origin owing to their unique physiological and metabolic functions. According to statistic results for MNPs from 2010 to 2013, marine-derived Streptomyces attributed to 60% MNPs sourced from marine-derived actinobacteria. This paper reviews the sources, structures and bioactivities of the 547 new MNPs of Streptomyces origin in 40 years from 1976 to June 2016. These MNPs have diverse structures including alkaloids, polyketides, sterols and terpenoids, and so on. Nitrogen compounds are the major structural types making up 61% MNPs from Streptomyces. And 67% MNPs sourced from Streptomyces showed bioactivities such as cytotoxicity, antimicrobial activity, antimalaric and insecticidal activity.
marine-derived actinobacteria; Streptomyces sp.; natural products; sources; chemical structures; bioactivities
R282.77
10.12036/hykxjk20160711002
* 资助项目: 国家自然科学基金项目(81561148012, U1501221, U1406402-1, 41376148)。王聪, 男, 博士研究生, E-mail: wangcong123206@163.com
① 通讯作者: 朱伟明, 男, 教授, 从事海洋微生物药物化学和天然药物的优化等研究工作, E-mail: weimingzhu@ouc.edu.cn
2016-07-11, 收修改稿日期: 2016-08-02
