食品非目标性检测技术
2016-03-06俞良莉陆维盈刘洁杜丽娟
俞良莉,陆维盈,刘洁,杜丽娟
(1.马里兰大学营养与食品科学系,美国马里兰 20742;
2.上海交通大学农业与生物学院,上海 200240; 3.北京工商大学人类营养与健康高精尖中心,北京 100048)
食品非目标性检测技术
俞良莉1,陆维盈2,刘洁3,杜丽娟2
(1.马里兰大学营养与食品科学系,美国马里兰 20742;
2.上海交通大学农业与生物学院,上海 200240; 3.北京工商大学人类营养与健康高精尖中心,北京 100048)
非目标性检测技术近年来在食品安全与质量分析领域得到了深入应用。非目标性检测技术创新性地通过仪器分析、指纹图谱及细胞分子生物学技术与化学计量学等手段的有机结合,综合分析食品的特征,是现代分析技术与食品质量控制的有机结合。非目标性检测技术的核心是检出非正常样品,而不是检测由何种物质造成样品的非正常。重点介绍了非目标性检测技术相关研究的最新进展,包括非目标性色谱、质谱、波谱,以及基于体外细胞的非目标性检测技术,广泛涉及了国内外的牛乳、枸杞、当归、牛至、蜂蜜等食品及食品原料。非目标性检测技术的研究工作能够在食品检验方面建立新的标准规范,推动食品工业健康快速发展,为保障群众的公共卫生安全水平以及生活质量的提高发挥重要作用。
非目标性检测技术;化学计量学;食品安全与质量;食品分析
近十年来,食品安全与质量问题对食品科学提出了新的任务与挑战。为了应对当前的食品安全与质量问题,实现食品安全与质量监控由“亡羊补牢”式的被动检查到主动防范的转变,食品安全与质量监控研究领域的科研工作者提出了一系列新技术、新方法,其中,非目标性检测技术(non-targeted detection technique)已成为近年来食品安全与质量分析领域的研究重点与热点之一。
1 非目标性检测技术及其特点
非目标性检测技术是以样品的整体化学或生物学信息为基础,通过基于分析化学、细胞和分子生物学、统计学、化学计量学等技术手段来综合分析食品特征的技术。非目标性检测的核心是检出非正常样品,而不是检出是什么造成非正常。传统的定向检测技术主要通过找出造成食品安全与质量问题的特定目标物质,并围绕此目标物质开展定性、定量检测工作。比如,找出特定的如三聚氰胺等受经济利益驱使的蓄意非法添加物质或掺假物质;检测中找出特定组分的农药、兽药残留等。然而,定向检测技术在现代的食品安全与质量检测中存在一定的局限性:对于食品中蓄意掺假或者伪造现象而言,由于掺假物质和手段的多样性,对于未知化学结构的添加物与异常的样品,无法通过对目标物的定向检测达到食品安全与质量监控的目的。而且,有限数量的目标物质的检测往往难以全面涵盖样品中重要的质量信息。相对地,非目标性检测技术采用指纹图谱技术结合化学计量学,包括主成分分析(principal component analysis,PCA),偏最小二乘法(partial least squares,PLS)等建模手段,解决了长期以来定向目标检测需要明确毒害物化学结构和标准品对照的缺陷。非目标性检测技术已经在掺假检测、食品及原料的溯源分析等诸多领域得到了深入的研究[1-2]。该技术已运用到各类食品检测领域,例如:色谱、质谱、波谱,以及基于体外细胞的非目标性检测分析技术等。
2 食品安全与质量检测中的非目标性检测技术
2.1 非目标性色谱检测技术
色谱技术是最常用的分析食品化学检测技术之一,主要是通过检测样品中特有的组成进行食品的检测。其中最具代表性的是高效液相色谱(high performance liquid chromatography,HPLC)及其相关技术。Lu等[3]采用超高效液相色谱(ultra performance liquid chromatography,UPLC)与微波辅助蛋白水解的技术,比较了牛乳蛋白与非乳蛋白的氨基酸相对比例的差异(图1[3])。

图1 牛乳与非乳蛋白全水解氨基酸指纹图谱主成分分析结果Fig.1PCA scores plot for all milk and non-milk proteins
Jablonski等[4]采用色谱法非定向地检测了脱脂奶粉中是否存在非乳外源蛋白。Zhao等[5]采集当归的样品,样品包括不同制造商的当归制成的保健品,进行HPLC指纹图谱的化学组成分析。Xie等[6]比较了不同基因型绞股蓝HPLC指纹图谱的不同区别。Blanch等[7]采用HPLC和HPLC-GC评价橄榄油和榛子油的真假。也有研究者用HPLC区分不同地区和不同品种的葡萄[8]。除了液相色谱和气相色谱外,其他色谱法也适用于非目标性检测的方法。如,反向薄层色谱法测定牛奶脂肪中混入的椰子油、大豆油等外源脂肪[9]。
2.2 非目标性质谱检测技术
近年来,一系列非目标性流动注射质谱(flow injection mass spectrometry,FIMS)指纹图谱技术在食品中得到了广泛应用。流动注射质谱具有分析速度快的优势。Chen等[10]首先提出了FIMS的概念,该研究分析了不同的因素,包括栽培年份、有机或者常规的种植方式、果实成熟度对于葡萄FIMS指纹图谱的影响。FIMS技术在最近的一系列区分有机与非有机种植模式的薄荷叶、鼠尾草等食品原料的工作中也得到了深入研究[11-15]。Gao等[14]在比较非有机与有机种植模式生产的牛至的工作中,运用了FIMS研究了牛至中化学组分的区别。结果表明,在非有机与有机种植的牛至中,百里香酚以及一些其他的活性分子的含量有着显著区别。此外,Zhao等[15]也运用了FIMS指纹图谱技术进行当归指纹图谱的分析,结果获得了与HPLC指纹图谱一致的结论。
Gao等[12]研究了有机与无机鼠尾草的FIMS指纹图谱(图2[12])。图2中,FIMS谱图的PCA分析表明了有机与无机种植模式下鼠尾草化学组分呈现出差异性。类似的不同种植模式的差异性也体现在薄荷叶[11]、罗勒叶[13]等植物原料中。Zhao等[15-16]也对二倍体与四倍体的绞股蓝与绞股蓝叶子和全草之间的区别进行分析。一系列的研究结果表明,FIMS技术与非目标性检测两者可以在实际的应用中得到很好地结合。
除了FIMS技术外,其他非目标性质谱检测技术也得到了很多报道。液质联用是将液相色谱法与质谱法相结合,其应用范围广泛。Lu等[17]建立了一种基于超高效液相色谱-质谱联用(UPLC-MS)与FIMS指纹图谱的方法,进行枸杞产地及其品种的非定向目标鉴别。他们将从宁夏采集的4种不同品种的枸杞干果与其他5个省份采集的枸杞干果用UPLC-MS和FIMS采集指纹图谱,并进行建模(图3[17]),为宁夏枸杞的优化育种提供了一定参考。
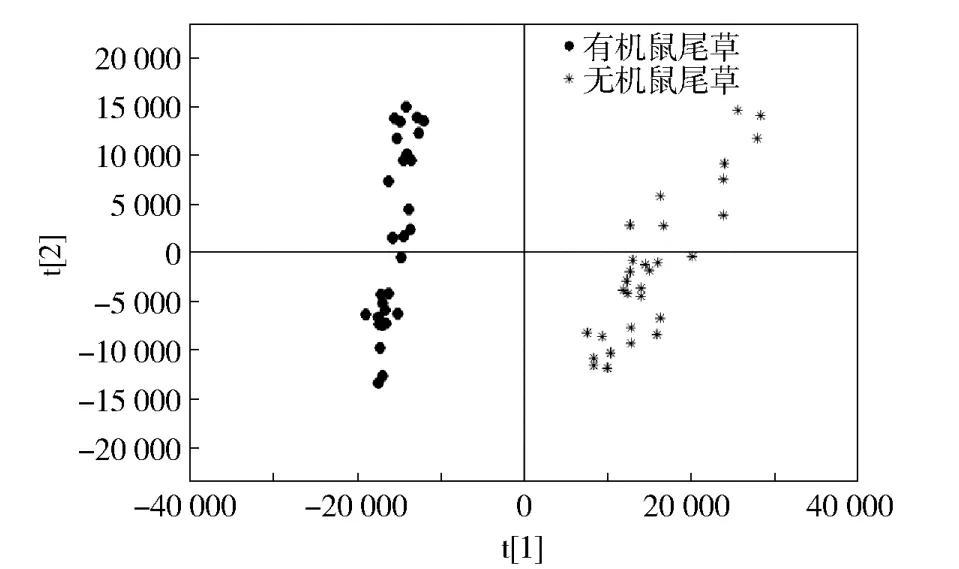
图2 使用FIMS指纹图谱的有机和无机鼠尾草的主成分分析结果Fig.2PCA scores plot for HPLC absolute peak areas of organic and conventional sage samples
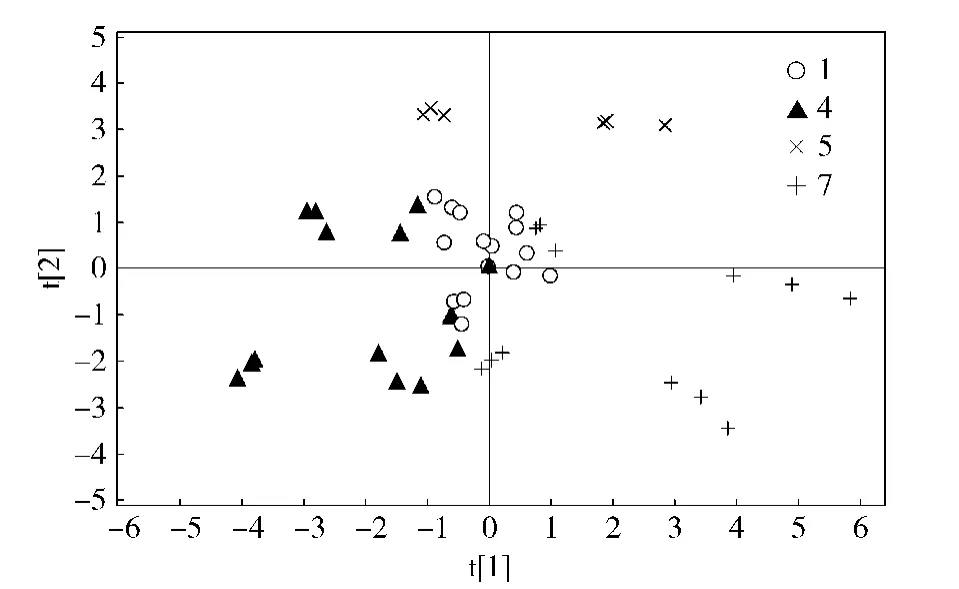
图3 不同品种宁夏枸杞提取物偏最小二乘法判别分析主成分图Fig.3PLS-DA scores plot differentiating NX cultivars using UPLC-MS peak areas
此外,Wang等[18]采用GC-MS采集传统种植和有机种植罗勒的指纹图谱对罗勒进行分类。Cordewener等[19]通过四级杆质谱研究了非目标性检测脱脂奶粉中潜在的非乳蛋白添加物的技术,该方法能够检测脱脂牛奶中加入的两种大豆分离蛋白和豌豆蛋白。Karlund等[20]采用LC-MS分析了常规与有机种植模式的3个不同品种草莓中的酚类、脂肪酸等代谢物的不同。此外,Fraser等[21]运用亲水性相互作用液相色谱-质谱联用,研究了茶叶水提物中的自由氨基酸和花青素的指纹图谱,为茶叶的品质等的鉴定提供参考。基质辅助激光解吸电离飞行时间质谱(matrix-assisted laser desorption/ionization mass spectrometry,MALDI-MS)被报道用于检测牛奶中掺假的成分如大豆油、棕榈油和动物脂肪[22],以及区别生鲜乳和加工的奶粉工作中[23]。
2.3 非目标性波谱检测技术
波谱检测技术是食品分析中的一类重要方法。波谱法主要包括红外光谱(infrared spectroscopy,IR)、NMR等。其中,近红外光谱技术(near infrared spectroscopy,NIR)是一种常用的波谱检测技术。NIR具有分析速度快、样品无需进行预处理、操作技术要求低等优点。非目标性红外光谱是一种有效的食品检测方法,其研究包括:采用NIR结合化学计量学模式识别测定牛奶中的乳清与尿素等掺假物[24],对生鲜牛奶和掺入增稠剂和伪蛋白的掺假样品进行鉴定[25],测定奶粉中的常用掺假物(淀粉、乳清和蔗糖)[26],通过全波长扫描IR检测螺旋藻粉的掺假等[27]。
拉曼光谱技术也被应用于非目标性检测。He等[28]将免疫磁珠和表面增强拉曼光谱结合检测混入牛奶中的卵白蛋白等外源蛋白,能够满足常规分析测定的需求。此外,NMR也被用来检测区别牛肉与马肉[29],确定酿酒的葡萄品种、酿酒的年份及地区[30],通过荧光光谱测定了来自不同喂养条件的羊奶[31]。
2.4 基于体外细胞的非目标性检测技术
肝脏是机体内负责代谢和解毒的重要器官,在外源性物质如药物、膳食补充剂、食品添加剂、食品污染物的代谢过程中发挥了重要作用。这也使得肝脏成为毒性物质作用的主要靶器官,导致急慢性肝毒、肝损伤或肝脏疾病。Liu等[32]将酚类化合物作用于人源肝癌HePG2/C3A细胞系及大鼠肝癌MH1C1细胞系,考察了他们对于细胞活力、氧化应激等指标的影响;综合各项指标按照毒性强弱分为4类,划分的情况与在体内的毒性基本一致(图4[32]);证明了利用易培养的肝细胞系,选取足够数量的受试化合物,通过检测细胞内活性氧的含量、线粒体膜电位、细胞内DNA含量、细胞内ATP含量和细胞LDH释放量、细胞线粒体CYP450酶活力等与细胞功能关系密切的指标,对相关物质的肝毒性进行非目标性评估的可行性。以体外肝细胞为载体、以多项肝毒性指标进行综合评价的肝毒性预警模型具有简便、快速、有效等优势。
2.5 其他检测技术
值得注意的是,非目标性检测作为一种通用的技术途径,还可在基因芯片等其他生物、化学检测手段中发挥巨大的应用潜力。如用凝胶电泳鉴定牛胶掺假[33],采用酶联免疫吸附技术鉴定驴奶中混入低价的牛奶等[34]。
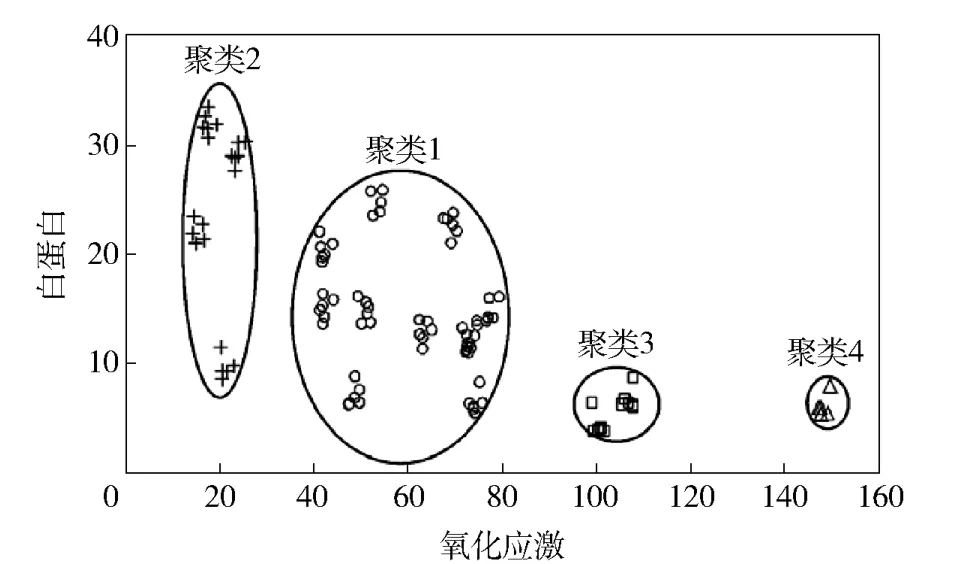
图416 种酚酸与5种植物提取物的8 MH1C1细胞活性评价指标聚类分析图Fig.4Cluster analysis for activity of 16 phenolics and 5 botanical extracts on 8 endpoint assays in MH1C1 cells
3 结论及展望
随着食品化学、仪器分析、代谢组学等相关科学研究的日趋成熟,非目标性检测技术将在食品检验领域发挥重大作用。为了推动此技术的发展,建立食品安全与质量相关的非目标性检测技术标准,开发集成非目标性检测技术相关模块的便携式检测设备等十分重要。总之,非目标性检测技术能够帮助推动食品工业健康、快速地发展,保障与提高广大群众的饮食健康以及生活质量。
[1]俞良莉,王硕,孙宝国.食品安全化学[M].上海:上海交通大学出版社,2014:283-311.
YU L L,WANG S,SUN B G.Food safety chemistry[M].Shanghai:Shanghai Jiao Tong University Press,2014:283-311.
[2]YU L L,WANG S,SUN B G.Food safety chemistry: toxicant occurence,analysis and mitigation[M].Boca Raton:CRC Press,2015:1-5.
[3]LU W,LV X,GAO B,et al.Differentiating milk and non-milk proteins by UPLC amino acid fingerprints combined with chemometric data analysis techniques[J].Journal of Agricultural and Food Chemistry,2015,63 (15):3996-4002.
[4]JABLONSKI J E,MOORE J C,HARNLY J M.Nontargeted detection of adulteration of skim milk powder with foreign proteins using UHPLC-UV[J].Journal of Agricultural and Food Chemistry,2014,62(22):5198-5206.
[5]ZHAO Y,SUN J,YU L L,et al.Chromatographic and mass spectrometric fingerprinting analyses of Angelica sinensis(Oliv.)diels-derived dietary supplements[J].Analytical and Bioanalytical Chemistry,2013,405(13): 4477-4485.
[6]XIE Z,ZHAO Y,CHEN P,et al.Chromatographic fingerprint analysis and rutin and quercetin compositions in the leaf and whole-plant samples of di-and tetraploid Gynostemma pentaphyllum[J].Journal of Agricultural and Food Chemistry,2011,59(7):3042-3049.
[7]BLANCH G P,CAJA M D M,RUIZ DEL CASTILLO M L,et al.Comparison of different methods for the evaluation of the authenticity of olive oil and hazelnut oil[J].Journal of Agricultural and Food Chemistry,1998,46 (8):3153-3157.
[8]FRAIGE K,PEREIRA-FILHO E R,CARRILHO E.Fingerprinting of anthocyanins from grapes produced in Brazil using HPLC-DAD-MS and exploratory analysis by principal component analysis[J].Food Chemistry,2014,145:395-403.
[9]RANI A,SHARMA V,ARORA S,et al.A rapid reversed-phase thin layer chromatographic protocol for detection of adulteration in ghee(clarified milk fat)with vegetable oils[J].Journal of Food Science and Technology,2015,52(4):2434-2439.
[10]CHEN P,HARNLY J M,LESTER G E.Flow injection mass spectral fingerprints demonstrate chemical differences in rio red grapefruit with respect to year,harvest time,and conventional versus organic farming[J].Journal of Agricultural and Food Chemistry,2010,58 (8):4545-4553.
[11]GAO B,LU Y,QIN F,et al.Differentiating organic from conventional peppermints using chromatographic and flow injection mass spectrometric(FIMS)fingerprints[J].Journal of Agricultural and Food Chemistry,2012,60(48):11987-11994.
[12]GAO B,LU Y,SHENG Y,et al.Differentiating organic and conventional sage by chromatographic and mass spectrometry flow injection fingerprints combined with principal component analysis[J].Journal of Agricultural and Food Chemistry,2013,61(12):2957-2963.
[13]LU Y,GAO B,CHEN P,et al.Characterisation of organic and conventional sweet basil leaves using chromatographic and flow-injection mass spectrometric(FIMS) fingerprints combined with principal component analysis[J].Food Chemistry,2014,154:262-268.
[14]GAO B,QIN F,DING T,et al.Differentiating organically and conventionally grown oregano using ultraperformance liquid chromatography mass spectrometry(UPLCMS),headspace gas chromatography with flame ionization detection(Headspace-GC-FID),and flow injection mass spectrum(FIMS)fingerprints combined with multivariate data analysis[J].Journal of Agricultural and Food Chemistry,2014,62(32):8075-8084.
[15]ZHAO Y,NIU Y,XIE Z,et al.Differentiating leaf and whole-plant samples of di-and tetraploid Gynostemma pentaphyllum(Thunb.)Makino using flow-injection mass spectrometric fingerprinting method[J].Journal of Functional Foods,2013,5(3):1288-1297.
[16]XIE Z,HUANG H,ZHAO Y,et al.Chemical composition and anti-proliferative and anti-inflammatory effects of the leaf and whole-plant samples of diploid and tetraploid Gynostemmapentaphyllum(Thunb.)Makino[J].Food Chemistry,2012,132(1):125-133.
[17]LU W,JIANG Q,SHI H,et al.Partial least-squaresdiscriminant analysis differentiating Chinese wolfberries by UPLC-MS and flow injection mass spectrometric (FIMS)fingerprints[J].Journal of Agricultural and Food Chemistry,2014,62(37):9073-9080.
[18]WANG Z,CHEN P,YU L,et al.Authentication of organically and conventionally grown basils by gas chromatography/mass spectrometry chemical profiles[J].Analytical Chemistry,2013,85(5):2945-2953.
[19]CORDEWENERJHG,LUYKXDMAM,FRANKHUIZEN R,et al.Untargeted LC-Q-TOF mass spectrometry method for the detection of adulterations in skimmed-milk powder[J].Journal of Separation Science,2009,32(8):1216-1223.
[20]KARLUND A,HANHINEVA K,LEHTONEN M,et al.Nontargeted metabolite profiles and sensory properties of strawberry cultivars grown both organically and conventionally[J].Journal of Agricultural and Food Chemistry,2015,63(3):1010-1019.
[21]FRASER K,HARRISON S J,LANE G A,et al.Nontargeted analysis of tea by hydrophilic interaction liquid chromatography and high resolution mass spectrometry[J].Food Chemistry,2012,134(3):1616-1623.
[22]GARCIA J S,SANVIDO G B,SARAIVA S A,et al.Bovine milk powder adulteration with vegetable oils or fats revealed by MALDI-QTOF MS[J].Food Chemistry,2012,131(2):722-726.
[23]COZZOLINO R,PASSALACQUA S,SALEMI S,et al.Identification of adulteration in milk by matrix‐assisted laser desorption/ionization time‐of‐flight mass spectrometry[J].Journal of Mass Spectrometry,2001,36(9):1031-1037.
[24]SANTOS P,PEREIRA-FILHO E,RODRIGUEZ-SAONA L.Rapid detection and quantification of milk adulteration using infrared microspectroscopy and chemometrics analysis[J].Food Chemistry,2013,138(1): 19-24.
[25]ZHANG L G,ZHANG X,NI L J,et al.Rapid identification of adulterated cow milk by non-linear pattern recognition methods based on near infrared spectroscopy[J].Food Chemistry,2014,145:342-348.
[26]BORIN A,FERRAO M F,MELLO C,et al.Leastsquares support vector machines and near infrared spectroscopy for quantification of common adulterants in powdered milk[J].Analytica Chimica Acta,2006,579 (1):25-32.
[27]WU D,NIE P,CUELLO J,et al.Application of visible and near infrared spectroscopy for rapid and non-invasive quantification of common adulterants in Spirulina powder[J].Journal of Food Engineering,2011,102(3): 278-286.
[28]HE L,HAYNES C L,DIEZ‐GONZALEZ F,et al.Rapid detection of a foreign protein in milk using IMSSERS[J].Journal of Raman Spectroscopy,2011,42 (6):1428-1434.
[29]JAKES W,GERDOVA A,DEFERNEZ M,et al.Authentication of beef versus horse meat using 60 MHz1H NMR spectroscopy[J].Food Chemistry,2015,175: 1-9.
[30]MONAKHOVA Y B,GODELMANN R,KUBALLA T,et al.Independent components analysis to increase efficiency of discriminant analysis methods(FDA and LDA):application to NMR fingerprinting of wine[J].Talanta,2015,141:60-65.
[31]HAMMAMI M,ROUISSI H,SALAH N,et al.Fluorescence spectroscopy coupled with factorial discriminant analysis technique to identify sheep milk from different feeding systems[J].Food Chemistry,2010,122(4): 1344-1350.
[32]LIU Y,FLYNN T J,FERGUSON M S,et al.Effects of dietary phenolics and botanical extracts on hepatotoxicity-related endpoints in human and rat hepatoma cells and statistical models for prediction of hepatotoxicity[J].Food and Chemical Toxicology,2011,49(8):1820-1827.
[33]AZIRA T N,MAN Y C,HAFIDZ R R M,et al.Use of principal component analysis for differentiation of gelatine sources based on polypeptide molecular weights[J].Food Chemistry,2014,151(20):286-292.
[34]PIZZANO R,SALIMEI E.Isoelectric focusing and ELISA for detecting adulteration of donkey milk with cow milk[J].Journal of Agricultural and Food Chemistry,2014,62(25):5853-5858.
Review on Non-targeted Detection Technique for Food Safety and Quality
Liangli(Lucy)Yu1,LU Weiying2,LIU Jie3,DU Lijuan2
(1.Department of Nutrition and Food Science,University of Maryland,College Park,MD 20742,USA; 2.School of Agriculture and Biology,Shanghai Jiao Tong University,Shanghai 200240,China; 3.Beijing Advanced Innovation Center for Food Nutrition and Human Health,Beijing Technology and Business University,Beijing 100048,China)
Non-targeted detection technique has been extensively applied in the food safety and quality in recent years.In general,a non-targeted detection combines analytical approaches,fingerprinting techniques and chemometrics to detect toxicants or foreign components in foods without knowing their chemical structures.The key purpose of non-targeted detection technique is to detect whether the sample is abnormal,without prior knowledge of what caused the abnormality.This manuscript introduces and reviews the current progress and the prospect non-targeted food detection techniques,including chromatographic,mass spectrometric,spectroscopic,cell-based non-targeted detection techniques.Foods and ingredients including milk,Chinese wolfberries,Chinese angelica,oregano,honey,etc.,were introduced.The nontargeted detection technique can help the healthy development of food industry and play an important role in protecting public welfare and human wellbeing.
non-targeted detection technique;chemometrics;food safety and quality assurance; food analysis
叶红波)
TS207.3
A
10.3969/j.issn.2095-6002.2016.06.001
2095-6002(2016)06-0001- 06
2016-11- 01
农业部公益性行业科研专项(201203069);国家高技术研究发展计划(863计划)项目(2013AA102202;2013AA102207);国家自然科学基金青年基金资助项目(31501553;31501479)。
俞良莉,女,教授,博士生导师,主要从事食品安全与营养方面的研究;陆维盈,男,副教授,博士生导师,主要从事食品安全与分析方面的研究。
俞良莉,陆维盈,刘洁,等.食品非目标性检测技术[J].食品科学技术学报,2016,34(6):1-6.
Liangli(Lucy)Yu,Lu Weiying,Liu Jie,et al.Review on non-targeted detection technique for food safety and quality[J].Journal of Food Science and Technology,2016,34(6):1-6.
专家论坛专栏
