Effects of calcium channel blockers on growth cone and their clinical implications
2016-02-16JisunKIMVivianSZETOZhongpingFENG
Ji-sun KIM,Vivian Y SZETO,Zhong-ping FENG
(Department of Physiology,University of Toronto,1 King′s College Circle, Toronto,Ontario,Canada M5S 1A8)
·REVIEW·
Effects of calcium channel blockers on growth cone and their clinical implications
Ji-sun KIM*,Vivian Y SZETO*,Zhong-ping FENG
(Department of Physiology,University of Toronto,1 King′s College Circle, Toronto,Ontario,Canada M5S 1A8)
Calcium signaling is a critical component of neuronal development,and is mediated by numerous calcium channels,exchangers and calcium-binding proteins.Growth cones use calcium gradient as a tool to pathfind correctly,ultimately directing the appropriate growth of axons.Conse⁃quently,dysfunctions of calcium channels could lead to incorrect pathfinding,thus resulting in develop⁃mental disorders.It is important to understand the pharmacological modulators of the calcium channels present in the growth cone-some already widely used in other tissue pathologies,like cardiovascular disease.Further,L-type channel blockers such as dihydropyridines have been instrumental in under⁃standing the properties of L-type channels as well as their heterogeneous nature.Investigation of channel property often uses pharmacological inhibition to reveal their functionality.Recent studies show the channel modulators as promising drug targets in the treatment of neurodevelopmental disorders.The goal of this article is to discuss the potential effects of pharmacological regulators of growth cone calcium channels on outgrowth activity.
calcium;growth cone;L-type calcium channels;ryanodine receptor calcium release channel;inositol(1,4,5)-trisphosphate receptors
Calcium ion is a key secondary messenger required in a variety of signaling pathways[1], therefore intracellular calcium levels are tightly regulated by numerous factors to ensure neuronal development,cell survival and proper cellular functions[2].In a growth cone,calcium signaling dictates axon steering and extension/retraction in response to guidance molecules,in a process that is essential to neuronal development and maturation[3-4].
The physicalproperties ofcalcium ions determine its role as an important signaling factor. As a divalent cation,calcium can generate stable complexes with other compounds,in contrast to the monovalent ions,such as Na+,K+,or Cl-[5].Its relatively large radius along with the complex electron shell configuration allow Ca2+to form bonds with sites with irregular geometry,often found in biological macromolecules[5].Further, calcium ion can form high-affinity interactions with proteins via an EF-hand binding motif or C2 binding domains[5].
Neurons have established a mechanism to maintain the cytosolic calcium concentrations low at 10-100 nmol·L-1range,about four orders of magnitude below the ambient extracellular concentration[5-6].Plasma membrane calcium ATPases and sodium-calcium exchangerscontribute to create this steep concentration gradient across the cell membrane,providing a driving force for calcium entry into the cytosol through various types of calcium channels[5]. Intracellularly,endoplasmic reticulum(ER)serves as a potent calcium source at mmol·L-1levels,and is believed to extend beyond soma into growth cone[6].In addition,mitochondria as well as calcium binding proteins act as buffers to ensure localized calcium signaling and maintain calcium homeo⁃stasis in developing neurons[7].
An axonal growth cone is the highly dynamic fan-shaped distal tip of an axon.It can take on many shapes and sizes by extending and retracting theirmembrane protrusions,to probe their environment constantly.These protrusions are comprised of filopodia,tapered finger-like projections, and lamelliopodia,flatsheet-like protrusions. Growth cone protrusion and motility are driven by polymerization and depolymerization of actin filaments and microtubules,thus cytoskeleton dynamics is essential in axonal outgrowth.During axon pathfinding,a growth cone constantly undergoes dynamic changes in its structure, allowing it to lay down new regions of axon along its path to a target[8].A growth cone decodes guidance cues and steers the neurite navigation using intracellular calcium as a tool[9].Axon and growth cone behaviours include both outgrowth and retraction as a part of the normal develop⁃mental process[10-11].Furthermore,following axotomy in an adult,elongating mode must be reinitiated to form new growth cones and regenerate the severed neurite[9].
Intracellular calcium dynamics can contribute to different transcription and cytoskeletal modulations. Therefore,it is critical for developing neurons to precisely regulate the intracellular calcium levels in a well-controlled manner.When this intricate interplay among different channels,exchangers and buffers is disrupted,it can result in developmental disorders due to inappropriate connections formed between different brain regions[12-13].
Intracellular calcium level has been linked to the development of autism spectrum disorder (ASD)[12,14-15].ASD is a multi-faceted,polygenic psychiatric disorder,but one of the defining features is the abnormal development of brain connectivity[16].Several studies have suggested thatthe potentialcause forsuch abnormal connectivity is altered calcium homeostasis[12,15].
Therefore,it is imperative to understand the factors involved in calcium regulation at the growth cone level of a developing neuron.As mentioned above,the key players of calcium regulation in this region is voltage-gated calcium channels(VGCCs)(L-type)on plasma membrane and ryanodine receptors(RyRs)and inositol (1,4,5)-trisphosphate receptors(IP3Rs)on the membrane of ER.Pharmacological agents that mediate the channel activity are essential in understanding the physiologicalfunctions of these channels and their impact on growth cone motility. Furthermore, understanding their mechanisms of action provides us an insight to theirpotentialuse astherapeuticagents in managing developmental abnormalities.Here we summarize the potential utility of known pharma⁃cological modulators in better understanding the functionality and ultimately treatmentmodality in normal growth cone maturation.
1 VOLTAGE-GATED CALCIUM CHANNELS
L-Type channels are the first calcium channels to be functionally expressed during neuronal development,indicating theirsignificance in early stages of neurite outgrowth.Interestingly, their involvement decreases over time,and is replaced by N-type channels that are present along axonal plasma membrane and in growth cone[17].In most cases,multiple calcium channel types coexist in the same neuron and it has been speculated that they contribute to different neuronal functions, possibly depending on their localization[18-19]. In more established neurons, L-type channels are mainly presentin the somatodendritic compartment where they allow postsynaptic integration during basal synaptic transmission and alter synaptic plasticity whereasthe localization of N-and P/Q-type calcium channels on the release face of nerve terminals is preferred for their involvement in exocytosis[19-20].
During neuronal development,extracellular guidance cues act through calcium transients to alter the trajectory of the neurite(chemoattraction or chemorepulsion).Typically,guidance cues or adhesion molecules interact with receptors on the growth cone.At the growth cone,calcium influx through VGCC is a crucial response to a guidance molecule or adhesion molecule[21].
Aside from regulating growth cone turning, L-type VGCCs are responsible for spontaneous calcium transients in developing neurons.The frequency and amplitude of these transients are inversely correlated with the motility ofthe growth cone[22].The larger and more inactive growth cones display high frequency spontaneous calcium transients while smaller rapidly advancing growth cones show calcium transients that are smaller and less frequent.To determine if it is a causal relationship we can utilize L-type VGCC blockers.
Chemotrophic factors like netrins,semaphorins, slits,neurotrophins,ephrins and cell adhesion proteins can mediate a negative voltage change, change in levels of cAMP or cGMP resulting in the activation of L-type VGCCs[23].Calcium influx via VGCC,especially L-type,is significant at the growth cone because this further induces calcium release from the internal store ER[21](Fig.1).There are two mechanisms,termed IP3 induced calcium release(IICR)and calcium-induced calcium release (CICR).The initial calcium influx also binds directly to and activates ER resident calcium channel, RyR,commencing CICR.Calcium influx can activate phospholipase C that then hydrolyzes PIP2into IP3.With the modulation protein kinase and scaffolding by Homer 1,IP3initiates calcium transients via IP3Rs.This release is termed IICR.

Fig.1 Calcium conducting channels presentat growth cone.
Calcium concentration gradient establishment is critical in growth cone steering as well as growth cone extension/retraction.This is facilitated by numerous types of calcium-conducting channels in this micro-environment,but in Fig.1,we have depicted L-type calcium channels on plasma membrane,and RyR and IP3R on ER.Subtypes of L-type calcium channels require varying levels of activation thresholds-both high and low. Activation of these can further release calcium from internal stores such as the ER via RyR and IP3R.
1.1Structure and biophysical properties of voltage-gated calcium channels
The VGCC family contains at least ten members that are distinguished by their structure,subunit composition,location,biophysical properties and pharmacology.The central pore-forming α1subunit expresses the major biophysical and functional properties of the channel.This subunit is associated with a number of auxiliary subunits,α2δ,β,and γ that control channel expression,membrane incor⁃poration,and the drug binding and gating charac⁃teristics of the central unit[24].
L-Type channels have a unitary conductance ranging from 20 and 27 ps using 110 mmol·L-1barium(Ba2+)as the charge carrier.L-Type channels require large departures from resting potential to become activated and typically begin to open at potentials positive to-10 mV,although they can activate at significantly more negative potentials in chromaffin cells,sensory neurons,and cardiac cells[7,25].Using Ca2+as a charge carrier,L-type currents are smaller and inactive rapidly during depolarization[26].The Ca2+-dependentinactivation has a number of characteristic properties and the inactivation attributable to Ca2+influx is the greatest at depolarization at which Ca2+entry through the channel is maximal[27].
L-Type calcium channels have four subtypes:Cav1.1,Cav1.2,Cav1.3 and Cav1.4.Recent studies have revealed that there is a significant functional diversity among L-type calcium channels expressed in neurons.For example,Cav1.3 and Cav1.4 have low activation thresholds,and also are significantly less sensitive to dihydropyridines(DHPs)compared to Cav1.2[28].
1.2 Pharmacological properties of voltage-gated calcium channels
The L-type channels have been well charac⁃terized by small molecule ligands/blockers,as they are a major therapeutic class of cardiovascular drugs,and have been extensively characterized[29]. There are three main classes of organic L-type channel blockers:phenylalkylamines(verapamil), benzodiazapines(diltiazem),and 1,4-DHP(eg, nitrendipine,nifedipine,and nimodipine)[7].The DHP antagonists bind preferentially to the pore and activation gate with the highest affinity in the active conformation,a state favoured by depolarization,thus providing more potent inhibition at depolarized potentials[30-31].(-)-Bay-K 8644 which increases both the open time and the single channel conductance,as one of the most used DHP agonists.L-Type channels are also blocked by certain native peptide toxins such as ω-agatoxinⅢA(ω-AgaⅢA)[9,32].ω-AgaⅢA reduces the current amplitude without affecting the time course,and unlike DHPs,its action is voltageindependent by blocking L-type channels at all potentials[32].
Blocking of L-type VGCC reduces the growth cone response to guidance molecules such as netrin-1.However,due to the lack of drugs for Cav1.2 and Cav1.3,the major neuronal subtypes, it is hard to distinguish the role of each subtype. Both channels are equally inhibited by isradipine, reducing channel current.Recently,N,N-disubsti⁃tuted pyrimodinetrione was proposed to be Cav1.3 specific[33]although efficacy is not yet confirmed in animal models.
1.3 Research and therapeutic applications of voltage-gated calcium channels
Attention has been paid to the α1subunit as the major pore forming and voltage-sensing component of channel.With the recognition that the auxiliary subunits have important roles in modulating both the trafficking and biophysical properties of the complex there is increasing interest in the role of these subunits as drug targets. There are additional drug binding sites present on both the major α1subunit and also on the α2δ subunit with which different structural classes of drugs interact[34].
There are reports of the anti-epileptic actions of the Ca2+channel blockers[29,35].In one study L-type channel activator,Bay K 8644 was epilep⁃togenic,and the 1,4-DHP antagonists were effective in reducing numberofexperimentalseizure states[35].Considering T-type channels are a significant target for a number of existing antiepileptic agents,it is unclear whether the antiepileptic properties of 1,4-DHP is due to a primary action at the L-type channel[29].
A number of L-type VGCC blockers have been extensively studied and approved for clinical use for decades.Therefore employing these blockers in the treatment of other L-type VGCC disorders,like neuronal developmental diseases could be achieved.Commonly used L-type VGCC blockers,their effective doses and clinical appli⁃cations are highlighted in Tab.1.Effective doses in cell culture research range from 0.1 μmol·L-1to over 100 mmol·L-1.It means that there are numerous channel blockers at various concentrations could be used.The range of doses usedin vivois even larger,owing to the large differences in absorption,mass and possibly biotransformation of drugs between species.L-Type calcium channels have many clinical applications as shown in Tab.1 suggesting that if additional clinical uses for the drugs are demonstrated,approval for their use in humans could be expedited.
1.4 Involvement in neurodevelopmental issues,potential use of voltage-gated calcium channel blockers
Timothy syndrome(TS)is an arrhythmia disorder that is associated with dysfunction in multiple organ systems,including congenital heart disease,syndactyly,immune deficiency,and autism.This disorder results from a recurrent,de novomissense mutation of Cav1.2 L-type calcium channel gene[15].The Cav1.2 gene is expressed in multiple tissues.Functional expression in heterologous system has demonstrated that the disease-associated mutation causes abnormal Ca2+current.The gain-of-function mechanism is mediated through failed channelinactivation, suggesting that calcium channel blockers could be useful in treating TS[15].

Tab.1 Commonly used L-type voltage-gated calcium channel blockers under research and clinical settings
Fetal alcohol spectrum disorders are caused by peri-pregnancy consumption of alcohol and symptomatically characterized by persistent and debilitating learning and memory deficits in the child.Zucca,et al[51]show that even low concen⁃trations of ethanol can disrupt long-term potentiation of the frequency of γ-aminobutyric acid(GABA) A receptor mediated spontaneous post-synaptic currents through inhibiting L-type calcium channels resulting in abnormal CA3 pyramidal neuronal synapse maturation.Mah,et al[50]demonstrated that acute ethanol attenuates both spontaneous and KCl-induced calcium transients by L-type VGCC in the growth cone of stage 3 neurons. However,chronic exposure to alcohol causes increased L-type VGCC calcium amplitudes owing to an increase in channelexpression,not functionality.Clearly,growth cone calcium currents play an important role in the pathobiology of fetal alcohol spectrum disorders and can be targeted in treatment.
Congenitalstationary nightblindness ischaracterized by difficulty seeing low light conditions and often accompanied by loss of visual acuity and severe myopia.Nachman and colleagues[54]have shown the critical role L-type calcium channels play in the outgrowth and structural remodeling of retinal cells.Nicardipine blockage resulted in inhibited axon remodeling,inhibited axon retraction, inhibited lamellipodium formation, reduced number of total processes,reduced total neurite outgrowth and the production of irregularly long and thin processes.Congenital stationary night blindness type 2 has been linked to the lack of functionalCav1.4 causing inefficientsynaptic transmission in the adult;however,the implica⁃tions of dysregulated Cav1.4 during development have recently been a proposed mechanism[48]. Liu and others[48]found that mice lacking functional Cav1.4 showed irregular development at the ribbonsynapsebetweenphotoreceptorsand second-order neurons.Compared to wild-type ribbon synapse formation,mice with genetically enhanced or reduced Cav1.4 activation showed retention of immature synapse morphological state.This suggests that depending on the mutation and its resulting effect on the functionality of the channel,Cav1.4 on growth cones maturing into synapses may be a therapeutic target for this type of night blindness.Interestingly,in mutated mice,synaptic ribbon formation remains largely normal until postnatal day 13,therefore fetuses with Cav.1.4 mutationsmayhave a decent window of opportunity for treatment.Additionally, photoreceptors show high levels of axonal and synaptic plasticity.
2ENDOPLASMICRETICULUM AND INTRACELLULAR CALCIUM STORE
Clearly,calcium entry into cytosol from the extracellular environment is an essential event in modulating the intracellular calcium levels,as demonstrated by numerous types of channels that modulate this process[55-56].Additionally,there are intracellular organelles that serve as calcium stores[5-6].These include ER,mitochondria,and nucleus.Recentstudies have revealed that some of these sources like ER and mitochondria, are found in the growth cone,contributing to local calcium dynamics[57].
ER is a single and continuous membranebound organelle,and is known to dictate lipid and protein biosynthesis;its membrane is the production site of all transmembrane proteins and lipids forvarious organelles.Moreover, recently synthesized proteins to be secreted to cell exterior are initially delivered to the ER lumen.In addition to these functions,ER lumen also holds a high level of calcium ions,at mmol·L-1levels[6].ER can take on heterogeneous shapes, depending on the cell types and developmental stages;there are three main domains[58]-nuclear envelope,smooth ER,and ribosome-bound rough ER.It is present throughout the cytoplasm, consisting of cisternae,flattened sheets,and tubules that form irregular polygons[59].In a neuron, smooth ER is more prevalent compared to other types,but rough ER is still present throughout dendrites and especially at branch points and close to synapses[60].ER connects the soma with the entire dendritic arbor and axon[61].ER is an integral part of the secretory pathway in neuronal growth and synaptic transmission.
RyRs and IP3Rs regulate the release of Ca2+from ER.These two types of channels are sensitive to Ca2+and their activation contributes to the rapid rise of Ca2+transients during the CICR and IICR.Both classes of receptors have three isoforms and some isoforms are more prevalent in certain tissues[57,62].
ER displays a heterogenous distribution of IP3Rs and RyRs,but how this affects local Ca2+signaling and trafficking has not been throughly investigated[61].
3 RYANODINE RECEPTORS
RyR is a Ca2+channel with fast gating and high conductance.It is regulated by Ca2+,Mg2+, ATP,calmodulin and caffeine among others[62].In cardiac myocytes,Ca2+that enters through theL-type channel activates RyR isoform 2 to create a localcalcium spark,inducing excitationcontraction coupling.Recovery occurs as Ca2+is pumped back into ER/SR via sarco(endo)plasmic Ca2+-ATPase(SERCA)[56].RyRs and IP3Rs are large tetrameric channel proteins that share a four-leaf clover like structure.They are also asso⁃ciated with various proteins that modulate their opening[57].The three RyR isoforms,classified as″skeletal muscle″,″heart″,and″brain″types are currently known as RyR1-RyR3.RyRs are shown to be localized with VGCCs[63].
3.1Structure and biophysical properties of ryanodine receptors
The RyR channels are modulated by a number of factors including Ca2+,ATP,calmodulin,as well as cellular processes such as phosphorylation and oxidation[64].They can also be manipulated with pharmacologicalagents like ryanodine, caffeine and ruthenium red.Steady-state single RyR channel activity is bell-shaped and a function of cytosolic Ca2+concentration.The channels are activated by Ca2+concentrations near 1-10 μmol·L-1but inhibited at higher concentrations(1-10 mmol·L-1). The RyRs proteins share significant homology in sequences that form the channel′s pore.The distinguishing functional attribute of each RyR channels likely underlies the spatiotemporal complexity of intracellular Ca2+signaling in cells[65]. Furthermore,RyRs have a high calcium conductance of 100-150 ps(more than L-type VGCCs and IP3Rs)despite the structural similarity of the conduction pores.RyRs are distributed across the entire nervous system,with differential distribution in different neuronal types and neuronal compartment[6].
The three isoforms of RyRs are differentially localized in dendrites and spines of central nervous system neurons.Studies of RyR property have reported inconsistent effects of agonists and antagonists on spine motility,unlike the effects onN-methyl-D-aspartate(NMDA)or AMPA receptors. A recent study showed that RyR2 and RyR3 isoforms mediate the action of brain-derived neurotrophic factor(BDNF)on dendritic spines and on cognitive tasks associated with the hippo⁃campus[66-67].In other words,BDNF-induced remodeling ofhippocampaldendritic spines required functional RyRs in rats.RyR2 and RyR3 are shown to be involved in long-term potentiation (LTP)as theirexpression levels increase following spatial memory training while the selective knockdown of the isoforms impairs memory formation[68].
RyR activation can also lead to the production of IP3,which in turn activates IP3Rs.IP3Rs are predominantlyfound in soma and proximal dendrites while RyRs are also found in distal processes and spines.There is growing evidence that the regulation of calcium movement in and out of ER can interfere with physiological growth, morphology and plasticity of neurons.Furthermore, the disturbed regulation of internal calcium sources can initiate neuronalinjuries orneurological diseases such as Alzheimer,Parkinson,and Huntington disease[69].
3.2Pharmacological modulators of ryanodine receptors
RyRs can be studied by pharmacological manipulation with blockers such as ruthenium red,high ryanodine concentrations,dantrolene and activators like caffeine,4-chloro-m-cresol and ryanodine at low concentration[70].Ryanodine locks the channel at open-state at low concentrations (Tab.2)while locking in closed-state at high concentrations[71]. The pharmacodynamics of dantrolene action on RyRs is still largely unknown. Animal studies suggest the binding sequence is at residues 590-609 of RyR1 at the N-terminal region[72].Dantrolene displays competitive binding in the presence of ryanodine.Ryanodine is believed to bind at residues 500-1300.Both binding sites are trypsin-cleavage sensitive[73].Caffeine stimulates spontaneous RyR calcium release by reducing the ER luminal calcium threshold for RyR opening.
3.3 Applications of ryanodine receptor channel blockers in research and clinic
Tab.2 summarized the RyR blockers and their application in animals studies as well as in clinics.All of the calcium-conducting channelspresent on ER are critical in calcium regulation during development.For example,RyR1 and RyR2 knock-out mice die early during the embryonic development[74].There seems to be a high degree of redundancy in the Ca2+release channel expression,but the function of this redundancy is not clearly understood.Recent studies have suggested that the localization of ER in the growth cones is important in developing neurons[75-76]. RyR activation was required in normal dendrite arborization[75].In regenerating axons an elevated RyR2 increased the axonal dieback rate,and inhibiting the RyR2 receptors reduced the rate of axonal degeneration[77].Tab.2 contains the agonists and antagonists to RyRs.Although originally named after plant alkaloid ryanodine,many more pharmacologicalagentshave been used to manipulate RyRs activity in research.As noted in Tab.2,in vitrostudies show ryanodine,like other physiological modulators of RyRs,have opposite effects below/beyond a threshold concentration. In contrast,the remaining drugs discussed in Tab.2 do not reverse the effects,depending on varying concentrations,although notalways confirmed by research.Like L-type channel antagonists,RyR-affecting drugs have a wide range of approved uses in humans.
Furthermore,intracellular calcium homeostasis has been shown to relate to the development of autism spectrum disorder(ASD).ASD isa multi-faceted,polygenic psychiatric disorder,and one of the defining features is the abnormal developmentofbrain connectivity[16].Several studies have suggested that the potential causefor such abnormal connectivity is altered calcium homeostasis[12,15,75,88].In particular,a genome-wide association study by Lu,et al[88]revealed a single nucleotide polymorphism(SNP)in the gene coding RyR2.It appears that calcium release from ER, especially via RyRs is important in creating ap⁃propriate pathfinding patterns.These studies col⁃lectively suggestthe RyRs involvementin development,however,understanding the effects of RyR blockers and agonists on growth cone behaviors and neuronal development are still at the infancy.
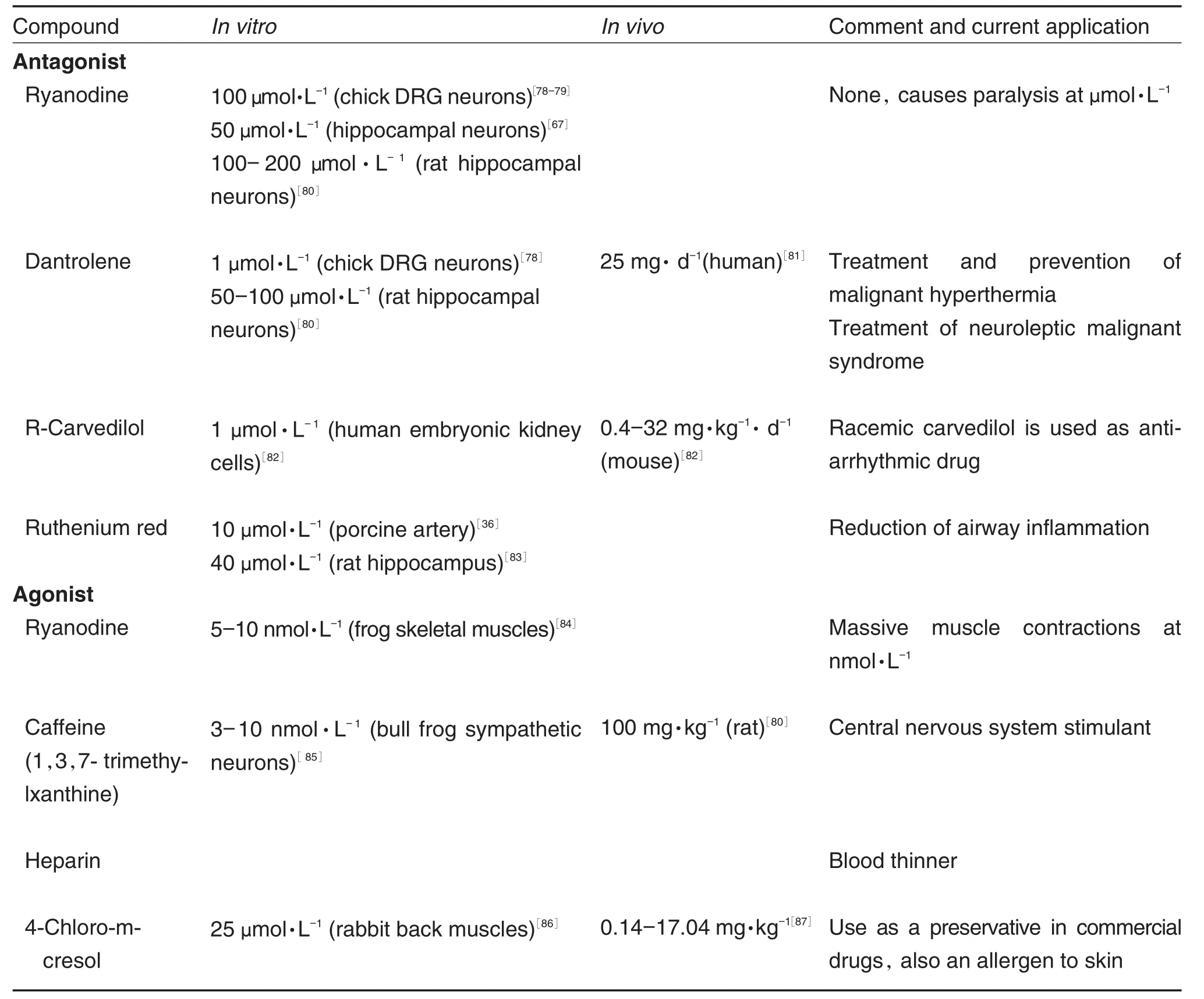
Tab.2 Commonly used ryanodine receptor blockers and activators under research and clinical settings
4 INOSITOL(1,4,5)-TRISPHOSPHATE RECEPTORS
There are three isoforms of IP3Rs,named IP3R1-IP3R3,with IP3R1 being the predominant neu⁃ronal type[6].These channels are large oligomeric structures formed by association of four subunits. Most cells express this group of channels,but they are enriched in cerebellar Purkinje cells and hippocampalCA1pyramidalcells[89].In hippocampus, IP3Rs are present at high concentrations in dendritic shafts and cell bodies,whereas RyRs are present primarily in dendritic spines and axons[89]. GABAergic neurons in cerebellum are highly saturated with IP3R,especially in dendritic spines[6].IP3Rs are believed to mediate the action of acetylcholine and other neurotransmitters, by releasing calcium and subsequently changing AMPA and NMDA receptor functions.Specifically, LTP2 is induced by release of Ca2+via IP3Rs located in the dendrites,which is initiated by NMDA-R activation[90]. IP3signals are both necessary and sufficient for inducing growth cone turning.Photolytic release of caged IP3on one side of growth cone induced IICR and caused the growth cone to turn towards that side.Additionally,calcium and IP3imaging showed higher concentrations on the attracted side of the growth cone,in response to neuronal growth factors[91].
In developing PC12 cells,microtubule dynamics at the growth cone are mediated by α7 nicotinic receptor activation,thus calcium release through IP3Rs[92].Furthermore,α7 nicotinic acetylcholine receptors(α7nAChRs)have also been implicated with calcium release from ER through RyRs in more established neurons to regulate synaptic signaling and neurotransmitter release[93].
Studies have established the connection between transient receptor potential channels (TRPCs)and the chemoattractor,BDNF.In cultured rat cerebellar granule cells,elevation of[Ca2+]iin growth cone was abolished when TRPC3 and TRPC6 were inhibited[94].TRPC family members bind to different regions of IP3R at their C terminus,indirectly regulating the channel activity.It was further demonstrated that an IP3R dependent Ca2+influx via TRPC channels contributes to elevation of[Ca2+]iin cultured cerebellar granule cells,highlighting the involvement of IP3Rs in calcium regulation in axon pathfinding at growth cone regions[95].
4.1Structure and biophysical properties of IP3Rs
IP3Rs absolutely require IP3for activation, along with low levels of Ca2+[96].Calcium has a biphasic effect on the IP3Rs,and this regulation is mediated by the direct action of Ca2+on the receptor and indirectly through calmodulin,which could be inhibitory or activating[56].Like RyRs, some isoforms of IP3Rs are more prevalent on certain cell types than others[63].
Calcium release from IP3R can also be extracellular calcium independent.In neurons a membrane voltage sensorcauses G-protein activation,phospholipase C activation,and then ultimatelycalcium release from IP3R.These calcium-independent,depolarization-triggered IP3R intracellularcalcium-release events are likely important to neuronalmaturation[97]. During neuronal development,there are spontaneous excitatory postsynaptic current(EPSC)events that can activate this process,triggering IP3R to drive neuronal outgrowth.Studies using IP3R blockers,under cadmium-blocked VGCC conditions, could explore the effects on neuronal outgrowth, providing insight into how this mode of calciumsignaling can influence neuronal development.
4.2 Pharmacological modulators of IP3Rs
α-Methyl-2-benzofuranethanamine(2-APB) and xestospongin C are the most commonly used IP3R blockers,despite xestospongin′s debated effectiveness[98-101].2-APB is membrane permeable and blocks store-operated calcium entry.Interestingly,whether its binding domain interacts with that of IP3Rs is poorly understood[98-99]. Both xestospongin C and 2-APB lack specificity to IP3R and only seem to be effective in isoform IP3R1 Heparin,not only lacks specificity,but also is membrane impermeable due to being polyanionic[102-103].However heparin is the strongest inhibitor of IP3R,effective on all three isoforms. Due to its polyanionic structure that mimics IP3, heparin displays competitive binding on the N-terminal core[98].
4.3Research and clinical applications of IP3Rs drugs
Tab.3 summarized the IP3R receptor blockers and their applications in laboratories as well as in clinics.Of the channels reviewed in this paper, IP3R have fewereffective antagonists and agonists.Notably,2meSADP does not directly regulate IP3R,but it acts through stimulating the production of IP3(a cellular agonist to IP3R). Currently there are few applications of IP3R blockers in humans,specifically none regarding the brain,perhaps owing to non-specific effects and membrane impermeability.The possibility of clinical uses for IP3R drugs is in its infancy. Further development of specific and effective IP3R-manipulating drugs is advised.
Both the depletion of ER stores and inhibition of IP3R blockers induce neurite growth cone retraction,growth arrest,and interfered with dorso-ventral axis formation[105].Further,IP3R is required to modulate ER stress and prevent brain damage.This was demonstrated in neuro⁃degenerative disease like Huntington′s in mouse models[106].
Pfister,et al[107]observed that mice deficient in cystic fibrosis transmembrane conductance regulator(CFTR)also differed in the levels of other ion channels.Specifically,in the olfactory epithelium, IP3R were more intensely stained compared to the controlgroup.CFTR-mutation showed progressive neuronal loss and defects in olfactory cell function.This was associated with hypersen⁃sitive immune responses to chemicals and reduced regenerative ability following mathimazoleinduced neurodegeneration[109].IP3R is involved in the neuropeptide Y signal transduction pathway. This suggests that aside from CFTR mutation, the olfactory and taste sensitivities of childrenwith CF may be mediated by IP3R.Dysregulation of IP3Rs may play a significant role in many severe diseases.
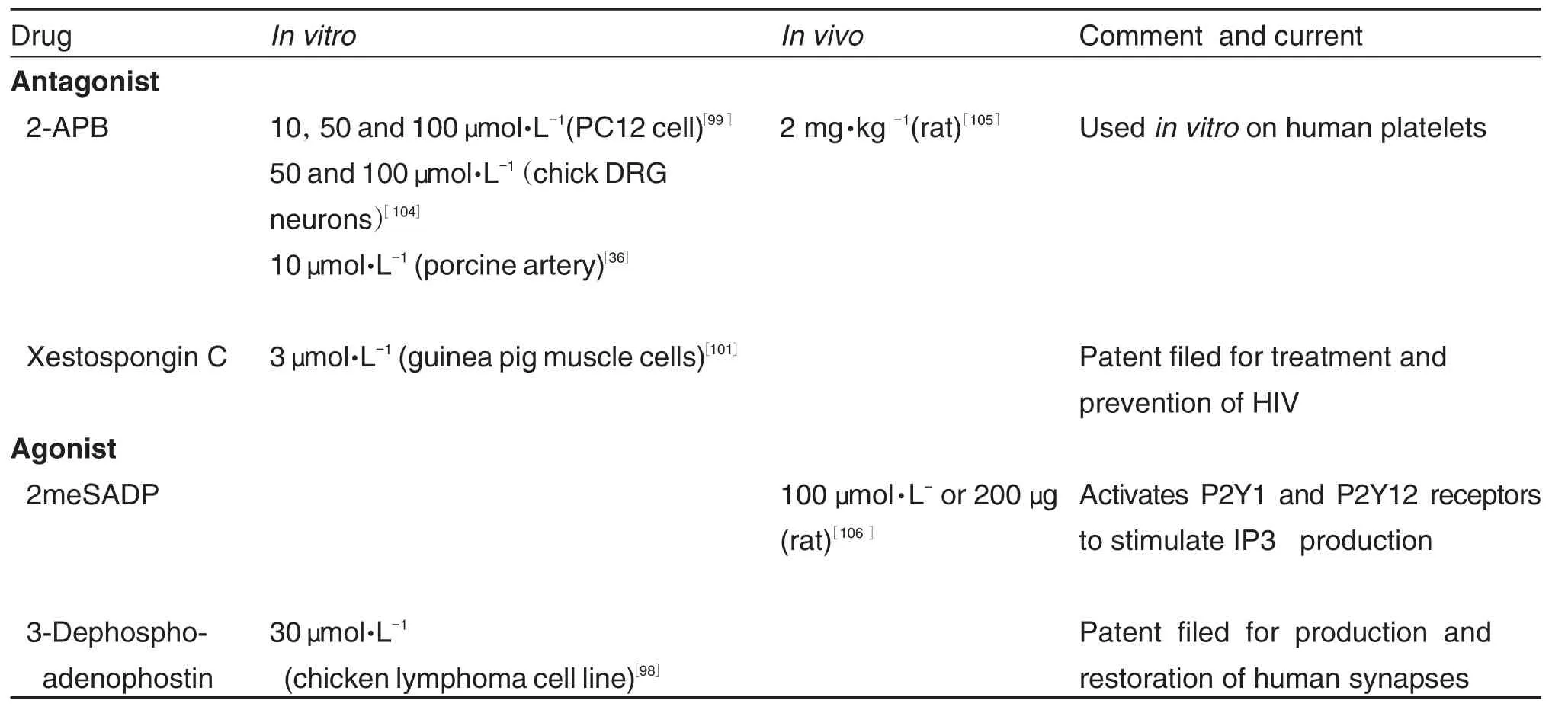
Tab.3 Commonly used IP3receptor blockers under research and clinical settings
5 CONCLUSION
The use of channel blockers and activators has afforded us a better understanding of calcium regulation in a developing neuron.This process is critical in potentially utilizing these pharmaco⁃logical agents in managing calcium homeostasisrelated neurological disorders.Same concept could be applied to treating neurodegenerative diseases where calcium homeostasis is disrupted, and also to cases where regeneration of new neurons is required following injury.Nonetheless, it would also be equally critical to assess the possibilities of off-target effects and negative side-effects while ensuring the specificity of the drugs.Development of more effective modulators is only possible through this learning process.
REFERENCES:
[1] Clapham DE.Calcium signaling[J].Cell,2007,131(6):1047-1058.
[2] Rosenberg SS,Spitzer NC.Calcium signaling in neuronal development[J].Cold Spring Harb Perspect Biol,2011,3(10):a004259.
[3] Nicol X,Hong KP,Spitzer NC.Spatial and temporal second messenger codes for growth cone turning[J].Proc Natl Acad Sci USA,2011,108(33):13776-13781.
[4] Yamada RX,Sasaki T,Ichikawa J,Koyama R,Matsuki N,Ikegaya Y.Long-range axonal calcium sweep induces axon retraction[J].J Neurosci,2008,28(18):4613-4618.
[5] Bading H.Nuclear calcium signalling in the regu⁃lation of brain function[J].Nat Rev Neurosci,2013,14(9):593-608.
[6] Segal M,Korkotian E.Endoplasmic reticulum calcium stores in dendritic spines[J].Front Neuroanat,2014,8:64.
[7] Bean BP.Classes of Calcium channels in verte⁃brate cells[J].Annu Rev Physiol,1989,51(1989):367-384.
[8] Dent EW,Gupton SL,Gertler FB.The growth cone cytoskeleton in axon outgrowth and guidance[J].Cold Spring Harb Perspect Biol,2011,3(3):a001800.
[9] Rossi F,Gianola S,Corvetti L.Regulation of intrinsic neuronal properties for axon growth and regeneration[J].Prog Neurobiol,2007,81(1):1-28.
[10] Dickson BJ.Molecular mechanisms of axon guid⁃ance[J].Science,2002,298(5600):1959-1964.
[11] Luo L,O′Leary DD.Axon retraction and degen⁃eration in development and disease[J].Annu Rev Neurosci,2005,28:127-156.
[12] Palmieri L,Papaleo V,Porcelli V,Scarcia P,Gaita L,Sacco R,et al.Altered Calcium homeo⁃stasis in autism-spectrum disorders:evidence from biochemical and genetic studies of the mito⁃chondrial aspartate/glutamate carrier AGC1[J]. Mol Psychiatry,2010,15(1):38-52.
[13] Liao P,Soong TW.CaV1.2 channelopathies:from arrhythmias to autism,bipolar disorder,and immunodeficiency[J].Pflugers Arch,2010,460(2):353-359.
[14] Napolioni V,Persico AM,Porcelli VA.The mito⁃chondrial aspartate/glutamate carrier AGC1 and Calcium homeostasis:physiological links and abnormalities in autism[J].Mol Neurobiol,2011,44(1):83-92.
[15] Splawski I,Timothy KW,Sharpe LM,Decher N,Kumar P,Bloise R,et al.CaV1.2 Calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism[J].Cell,2004,119(1):19-31.
[16] Belmonte MK,Allen G,Beckel-Mitchener A,Boulanger LM,Carper RA,Webb SJ.Autism and abnormal development of brain connectivity[J].J Neurosci,2004,24(42):9228-9231.
[17] Pravettoni E,Bacci A,Coco S,Forbicini P,Matteoli M,Verderio C.Different localizations and functions of L-type and N-type calcium channels during development of hippocampal neurons[J]. Dev Biol,2000,227(2):581-594.
[18] Doughty JM,Miller AL,Langton PD.Non-speci⁃ficity of chloride channel blockers in rat cerebral arteries:block of the L-type calcium channel[J]. J Physiol,1998,507(Pt2):433-439.
[19] Wu LG,Westenbroek RE,Borst JG,Catterall WA,Sakmann B.Calcium channel types with distinct presynaptic localization couple differen⁃tially to transmitter release in single calyx-type synapses[J].J Neurosci,1999,19(2):726-736.
[20] Stanley EF,Mirotznik RR.Cleavage of syntaxin prevents G-protein regulation of presynaptic calcium channels[J].Nature,1997,385(6614):340-343.
[21] Hong K,Nishiyama M,Henley J,Tessier-Lavigne M,Poo MM.Calcium signalling in the guidance of nerve growth by netrin-1[J].Nature,2000,403(6765):93-98.
[22] Tang F,Dent EW,Kalil K.Spontaneous calcium transients in developing cortical neurons regulate axon outgrowth[J].J Neurosci,2003,23(3):927-936.
[23] Nishiyama M,Von Schimmelmann MJ,Togashi K,Findley WM,Hong K.Membrane potential shifts caused by diffusible guidance signals direct growth-cone turning[J].Nat Neurosci,2008,11(7):762-771.
[24] Triggle DJ.L-type calcium channels[J].Curr Pharm Des,2006,12(4):443-457.
[25] Scott RH,Pearson HA,Dolphin AC.Aspects of vertebrate neuronal voltage-activated calcium currents and their regulation[J].Prog Neurobiol,1991,36(6):485-520.
[26] Tsien RW,Lipscombe D,Madison DV,Bley KR,Fox AP.Multiple types of neuronal calcium channels and theirselective modulation[J]. Trends Neurosci,1988,11(10):431-438.
[27] Imredy JP,Yue DT.Mechanism of Ca2+-sensitive inactivation of L-type Ca2+channels[J].Neuron,1994,12(6):1301-1318.
[28] Lipscombe D,Helton TD,Xu WF.L-Type calcium channels:the low down[J].J Neurophysiol,2004,92(5):2633-2641.
[29] Triggle DJ.Drug targets in the voltage-gated calcium channel family:why some are and some are not[J].Assay Drug Dev Technol,2003,1(5):719-733.
[30] Tikhonov DB,Zhorov BS.Structural model for dihydropyridine binding to L-type calcium channels[J].J Biol Chem,2009,284(28):19006-19017.
[31] Tanabe T,Takeshima H,Mikami A,Flockerzi V,Takahashi H,Kangawa K,et al.Primary structure of the receptor for calcium channel blockers from skeletalmuscle[J].Nature,1987,328(6128):313-318.
[32] Mintz IM,Venema VJ,Adams ME,Bean BP. Inhibition of N-and L-type Ca2+channels by the spider venom toxin omega-Aga-IIIA[J].Proc Natl Acad Sci USA,1991,88(15):6628-6631.
[33] Kang S,Cooper G,Dunne SF,Luan CH, Surmeier DJ,Silverman RB.Structure-activity relationship of N,N′-disubstituted pyrimidinetriones as CaV1.3 calcium channel-selective antagonists for Parkinson′s disease[J].J Med Chem,2013,56(11):4786-4797.
[34] Rampe D,Triggle DJ.New synthetic ligands for L-type voltage-gated calcium channels[J].Prog Drug Res,1993,40:191-238.
[35] Cosford ND,Meinke PT,Stauderman KA,Hess SD. Recent advances in the modulation of voltage-gated ion channels for the treatment of epilepsy[J]. Curr Drug Targets CNS Neurol Disord,2002,1(1):81-104.
[36] Nguyen HT,Nguyen HT,Islam MZ,Obi T,Pothinuch P,Zar PP,et al.Pharmacological characteristics of Artemisia vulgaris L.in isolated porcine basilar artery[J].J Ethnopharmacol,2016,182:16-26.
[37] Putrenko I,Ghavanini AA,Meyer Schöniger KS,Schwarz SK.Central nervous system-toxic lidocaine concentrations unmask L-type Ca2+currentmediated action potentials in rat thalamocortical neurons: an in vitro mechanism of action study[J].Anesth Analg,2016,122(5):1360-1369.
[38] Miao XN,Siu KL,Cai H.Nifedipine attenuation of abdominal aortic aneurysm in hypertensive and non-hypertensive mice:Mechanisms and implications[J].J Mol Cell Cardiol,2015,87(2015):152-159.
[39] Regueiro J,Olguin N,Simal-Gandara J,Sunol C. Toxicity evaluation of new agricultural fungicides in primary cultured cortical neurons[J].Environ Res,2015,140(2015):37-44.
[40] Hunter AL,Unosson J,Bosson JA,Langrish JP,Pourazar J,Raftis JB,et al.Effect of wood smoke exposure on vascular function and thrombus formation in healthy fire fighters[J].Part Fibre Toxicol,2014,11(2014):62.
[41] Vedunova M,Sakharnova T,Mitroshina EA,Pimashkin A,Zakharov Y,Dityatev A,et al.Sei⁃zure-like activity in hyaluronidase-treated dissociated hippocampal cultures[J].Front Cell Neurosci,2013,7:149.
[42] Yang Y,Gao M,Guo Y,Qiao J.Calcium antag⁃onists,diltiazem and nifedipine,protect broilers againstlow temperature-induced pulmonary hypertension and pulmonary vascular remodeling[J].Anim Sci J,2010,81(4):494-500.
[43] Sirivanasandha B,Sakaew A,Sutthivaiyakit K,Raksamani K,Waitayawinyu P,Rushatamukay⁃anunt P,et al.An equivalence trial comparing labetalol and diltiazem in controlling emergence hypertension after supratentorial tumor surgery[J].J Med Assoc Thai,2015,98(11):1104-1111.
[44] Martella G, Costa C, Pisani A, Cupini L,CalabresiP.Antiepileptic drugs on calcium currents recorded from corticaland PAG neurons:therapeutic implications for migraine[J].Cephalalgia,2008,28(12):1315-1326.
[45] Werhahn KJ,Trinka E,Dobesberger J,Unter⁃berger I,Baum P,Deckert-Schmitz M,et al.A randomized,double-blind comparison of antiepi⁃leptic drug treatment in the elderly with newonset focal epilepsy[J].Epilepsia,2015,56(3):450-459.
[46] Töllner K,Twele F,Löscher W.Evaluation of the pentylenetetrazole seizure threshold test in epileptic mice as surrogate model for drug testing against pharmacoresistant seizures[J].Epilepsy Behav,2016,57(Pt A):95-104.
[47] Mao XY,Cao YG,Ji Z,Zhou HH,Liu ZQ,Sun HL.Topiramate protects against glutamate excitotoxicity via activating BDNF/TrkB-dependent ERK pathway in rodent hippocampal neurons[J].Prog Neuropsychopharmacol Biol Psychiatry,2015,60(2015):11-17.
[48] Liu XN,Kerov V,Haeseleer F,Majumder A,Artemyev N,Baker SA,et al.Dysregulation of Cav1.4 channels disrupts the maturation of photo⁃receptor synaptic ribbons in congenital stationary night blindness type 2[J].Channels,2013,7(6):514-523.
[49] Kitayama M,Hirota K,Kudo M,Kudo T,Ishihara HA.Inhibitory effects of intravenous anaesthetic agents on K+-evoked glutamate release from rat cerebrocortical slices.Involvement of voltage-sensitive Ca2+channels and GABA(A)receptors[J].Naunyn Schmiedebergs Arch Pharmacol,2002,366(3):246-253.
[50] Mah SJ,Fleck MW,Lindsley TA.Ethanol alters calcium signaling in axonal growth cones[J]. Neuroscience,2011,189:384-396.
[51] Zucca S,Valenzuela CF.Low concentrations of alcoholinhibitBDNF-dependentGABAergic plasticity via L-type Ca2+channel inhibition in developing CA3 hippocampal pyramidal neurons[J].J Neurosci,2010,30(19):6776-6781.
[52] Thomas G,Chung M,Cohen CJ.A dihydropyridine(Bay K 8644)that enhances calcium currents in guinea pig and calf myocardial cells.A new type of positive inotropic agent[J].Circ Res,1985,56(1):87-96.
[53] Bechem M,Hoffmann H.The molecular mode of action of the Ca agonist(-)BAY K 8644 on the cardiac Ca channel[J].Pflugers Arch,1993,424(3-4):343-353.
[54] Nachman-Clewner M,St Jules R, Townes-Anderson E.L-type calcium channels in the photoreceptor ribbon synapse:localization and role in plasticity[J].J Comp Neurol,1999,415(1):1-16.
[55] Zheng JQ,Poo MM.Calcium signaling in neuronal motility[J].Annu Rev Cell Dev Biol,2007,23(2007):375-404.
[56] Berridge MJ,Bootman MD,Roderick HL.Calcium signalling:dynamics,homeostasis and remodelling[J].Nat Rev Mol Cell Biol,2003,4(7):517-529.
[57] Verkhratsky A.Physiology and pathophysiology of the calcium store in the endoplasmic reticulum of neurons[J].Physiol Rev,2005,85(1):201-279.
[58] Cui-Wang T,Hanus C,Cui T,Helton TA,Watson D,Harris KM,et al.Local zones of endoplasmic reticulum complexity confine cargo in neuronal dendrites[J].Cell,2012,148(1-2):309-321.
[59] Torre ER,Steward O.Protein synthesis within dendrites:glycosylation of newly synthesized proteins in dendrites of hippocampal neurons in culture[J].J Neurosci,1996,16(19):5967-5978.
[60] Hanus C,Ehlers MD.Specialization of biosyn⁃thetic membrane trafficking for neuronal form and function[J].Curr Opin Neurobiol,2016,39(2016):8-16.
[61] Ramirez OA,Couve A.The endoplasmic reticulum and protein trafficking in dendrites and axons[J].Trends Cell Biol,2011,21(4):219-227.
[62] Lanner JT,Georgiou DK,Joshi AD,Hamilton SL. Ryanodine receptors: structure, expression,molecular details,and function in calcium release[J].Cold Spring Harb Perspect Biol,2010,2(11):a003996.
[63] Mak DO,Foskett JK.Inositol 1,4,5-trisphos⁃phate receptors in the endoplasmic reticulum:A single-channel point of view[J].Cell Calcium,2015,58(1):67-78.
[64] Matsuo N,Tanda K,Nakanishi K,Yamasaki N,Toyama K,Takao K,et al.Comprehensive behavioral phenotyping of ryanodine receptor type 3(RyR3)knockout mice:decreased social contact duration in two social interaction tests[J].Front Behav Neurosci,2009,3:3.
[65] Copello JA,Barg S,Sonnleitner A,Porta MA,Fill M,Schindler H,et al.Differential activation by Ca2+,ATP and caffeine of cardiac and skeletal muscle ryanodine receptors after block by Mg2+[J].J Membr Biol,2002,187(1):51-64.
[66] Adasme T,Haeger P,Paula-Lima AC,Mer⁃cedes Casas-Alarcon M,Angelica Carrasco M,Hidalgo C.Involvement of ryanodine receptors in neurotrophin-induced hippocampal synaptic plasticity and spatial memory formation[J].Proc NatlAcadSciUSA,2011,108(7):3029-3034.
[67] Adasme T,Paula-Lima A,Hidalgo C.Inhibitory ryanodine prevents ryanodine receptor-mediated Ca2+release without affecting endoplasmic reticulum Ca2+content in primary hippocampal neurons[J]. Biochem Biophys Res Commun,2015,458(1):57-62.
[68] Martín ED,Buño W.Caffeine-mediated presyn⁃aptic long-term potentiation in hippocampal CA1 pyramidal neurons[J].J Neurophysiol,2003,89(6):3029-3038.
[69] Jaskova K, Pavlovicova M, Jurkovicova D. Calcium transporters and their role in the devel⁃opment of neuronal disease and neuronal damage[J].Gen Physiol Biophys,2012,31(4):375-382.
[70] Zhao F,Li P,Chen SR,Louis CF,Fruen BR. Dantrolene inhibition of ryanodine receptor Ca2+release channels.Molecular mechanism and isoform selectivity[J].J Biol Chem,2001,276(17):13810-13816.
[71] Meissner G.Ryanodine activation and inhibition of the Ca2+release channel of sarcoplasmic retic⁃ulum[J].J Biol Chem,1986,261(14):6300-6306.
[72] Paul-Pletzer K,Yamamoto T,Bhat MB,Ma J,Ikemoto N,Jimenez LS,et al.Identification of a dantrolene-binding sequence on the skeletal muscle ryanodine receptor[J].J Biol Chem,2002,277(38):34918-34923.
[73] Wu Y,Aghdasi B,Dou SJ,Zhang JZ,Liu SQ,Hamilton SL.Functional interactions between cytoplasmic domains of the skeletal muscle Ca2+release channel[J].J Biol Chem,1997,272(40):25051-25061.
[74] Takeshima H,Komazaki S,Hirose K,Nishi M,Noda T,Iino M.Embryonic lethality and abnormal cardiacmyocytesin mice lackingryanodine receptor type 2[J].EMBO J,1998,17(12):3309-3316.
[75] Wayman GA,Bose DD,Yang D,Lesiak A,Bruun D,Impey S,et al.PCB-95 modulates the calcium-dependent signaling pathway responsible for activity-dependent dendritic growth[J].Environ Health Perspect,2012,120(7):1003-1009.
[76] Dailey ME,Bridgman PC.Dynamics of the endo⁃plasmic reticulum and other membranous organ⁃elles in growth cones of cultured neurons[J].J Neurosci,1989,9(6):1897-1909.
[77] Stirling DP,Cummins K,Chen S,Stys P. Axoplasmic reticulum Ca2+release causes secondary degeneration of spinal axons[J].Ann Neurol,2014,75(2):220-229.
[78] Yamane M,Yamashita N,Yamamoto H,Iizuka A,Shouji M,Usui H,et al.Semaphorin3a facilitates axonal transport through a local calcium signaling and tetrodotoxin-sensitive voltage-gated sodium channels[J].Biochem Biophys Res Commun,2012,422(2):333-338.
[79] Iketani M,Imaizumi C,Nakamura F,Jeromin A,Mikoshiba K,Goshima Y,et al.Regulation of neurite outgrowth mediated by neuronal calcium sensor-1 and inositol 1,4,5-trisphosphate receptor in nerve growth cones[J].Neuroscience,2009,161(3):743-752.
[80] Nakamura-Maruyama E,Miyamoto O,Okabe N,Himi N,Feng L,Narita K,et al.Ryanodine receptors contribute to the induction of ischemic tolerance[J].Brain Res Bull,2016,122:45-53.
[81] TimminsMA,RosenbergH,LarachMG,Sterling C,Kraeva N,Riazi S.Malignant hyper⁃thermia testing in probands without adverse anesthetic reaction[J].Anesthesiology,2015,123(3):548-556.
[82] Zhang J,Zhou Q,Smith CD,Chen H,Tan Z,Chen B, etal. Non-β-blocking R-carvedilol enantiomer suppresses Ca2+waves and stressinduced ventricular tachyarrhythmia without lowering heart rate or blood pressure[J].Biochem J,2015,470(2):233-242.
[83] Xu Y,Tanaka M,Chen L,Sokabe M.DHEAS induces short-term potentiation via the activation of a metabotropic glutamate receptor in the rathippocampus[J].Hippocampus,2012,22(4):707-722.
[84] Bull R,Marengo JJ,Suárez-Isla BA,Donoso P,Sutko JL,Hidalgo C. Activation ofcalcium channels in sarcoplasmic reticulum from frog muscle by nanomolar concentrations of ryanodine[J].Biophys J,1989,56(4):749-756.
[85] Nohmi M,Hua SY,Kuba K.Basal Ca2+and the oscillation of Ca2+in caffeine-treated bullfrog sympathetic neurones[J].J Physiol,1992,450(1992):513-528.
[86] Herrmann-FrankA,RichterM,Sarközi S,Mohr U,Lehmann-Horn F.4-Chloro-m-cresol,a potent and specific activator of the skeletal muscle ryanodine receptor[J].Biochim Biophys Acta,1996,1289(1):31-40.
[87] Iaizzo PA,Johnson BA,Nagao K,Gallagher WJ.4-Chloro-m-cresol triggers malignant hyper⁃thermia in susceptible swine at doses greatly exceeding those found in drug preparations[J]. Anesthesiology,1999,90(6):1723-1732.
[88] Lu A,Cantor RM.Allowing for sex differences increases power in a GWAS of multiplex Autism families[J].Mol Psychiatry,2012,17(2):215-222.
[89] Sharp AH,Mcpherson PS,Dawson TM,Aoki C,Campbell KP,Snyder SH.Differential immu⁃nohistochemical localization of inositol 1,4,5-trisphosphate-and ryanodine-sensitive Ca2+release channels in rat brain[J].J Neurosci,1993,13(7):3051-3063.
[90] Raymond CR,Redman SJ.Spatial segregation of neuronal calcium signals encodes different forms of LTP in rat hippocampus[J].J Physiol,2006,570(1):97-111.
[91] Akiyama H,Kamiguchi H.Second messenger networks for accurate growth cone guidance[J]. Dev Neurobiol,2015,75(4):411-422.
[92] Nordman JC,Kabbani N.Microtubule dynamics at the growth cone are mediated by α7 nicotinic receptor activation of a Gαq and IP3 receptor pathway[J].FASEB J,2014,28(7):2995-3006.
[93] Jones CK,Byun N,Bubser M.Muscarinic and nicotinic acetylcholine receptoragonists and allosteric modulators for the treatment of schizo⁃phrenia[J].Neuropsychopharmacology,2012,37(1):16-42.
[94] Tai YL,Feng SJ,Du WL,Wang YZ.Functional roles of TRPC channels in the developing brain[J].Pflugers Arch,2009,458(2):283-289.
[95] Jia Y,Zhou J,Tai Y,Wang Y.TRPC channels promote cerebellar granule neuron survival[J]. Nat Neurosci,2007,10(5):559-567.
[96] Taylor CW,Traynor D.Calcium and inositol trisphosphate receptors[J].J MembrBiol,1995,145(2):109-118.
[97] Ryglewski S,Pflueger HJ,Duch C.Expanding the neuron′s calcium signaling repertoire:intra⁃cellular calcium release via voltage-induced PLC and IP3R activation.[J].PLoS Biol,2007,5(4):e66.
[98] Saleem H,Tovey SC,Riley AM,Potter BV,Taylor CW.Stimulation of inositol 1,4,5-trispho⁃sphate(IP3)receptor subtypes by adenophostin A and its analogues[J].PLoS One,2013,8(2):e58027.
[99] Ansari N,Hadi-Alijanvand H,Sabbaghian MA,Khodagholi F.Interaction of 2-APB,dantrolene,and TDMT with IP3R and RyR modulates ER stress-induced programmed cell deathⅠandⅡin neuron-like PC12 cells:an experimental and computational investigation[J].J Biomol Struct Dyn,2014,32(8):1211-1230.
[100] Maruyama T,Kanaji T,Nakade S,Kanno T,Mikoshiba K. 2APB, 2-aminoethoxydiphenyl borate,a membrane-penetrable modulator of Ins(1,4,5)P3-induced Ca2+release[J].J Biochem,1997,122(3):498-505.
[101] Gafni J,Munsch JA,Lam TH,Catlin MC,Costa LG,Molinski TF,et al.Xestospongins:potent membrane permeable blockers of the inositol 1,4,5-trisphosphate receptor[J].Neuron,1997,19(3):723-733.
[102] Nilsson T,Zwiller J,Boynton AL,Berggren PO. Heparin inhibits IP3-induced Ca2+release in permeabilized pancreatic β-cells[J].FEBS Lett,1988,229(1):211-214.
[103] Salminen S,Luomanmaki K.The binding of sodium and potassium ions by heparin[J].Biochim Biophys Acta,1963,69(1963):533-537.
[104] Saleem H,Tovey SC,Molinski TF,Taylor CW. Interactions of antagonists with subtypes of inositol 1,4,5-trisphosphate(IP3)receptor[J].Br J Pharmacol,2014,171(13):3298-3312.
[105] Nicoud IB,Knox CD,Jones CM,Anderson CD,Pierce JM,Belous AE,et al.2-APB Protects against liver ischemia-reperfusion injury by reducing cellular and mitochondrial calcium uptake[J]. Am J Physiol Gastrointest Liver Physiol,2007,293(3):G623-G630.
[106] Higo T,Hamada K,Hisatsune C,Nukina N,Hashikawa T,Hattori M,et al.Mechanism of ER stress-induced brain damage by IP3 receptor[J].Neuron,2010,68(5):865-878.
[107] Pfister S,Weber T,Haertig W,Schwerdel C, Elsaesser R,Knuesel I,et al.Novel role of cystic fibrosis transmembrane conductance regulator in maintaining adult mouse olfactory neuronalhomeostasis[J].JCompNeurol,2015,523(3):406-430.
钙离子通道调节剂在神经元的生长锥的作用及临床意义
Ji-Sun KIM*,Vivian Y SZETO*,冯中平
(Department of Physiology,University of Toronto,1 King's College Circle,Toronto,Ontario,Canada M5S 1A8)
钙是神经发育过程中重要的信号分子,其生物学功能通过众多的钙离子通道、交换蛋白及钙结合蛋白来实现。在神经发育过程中,神经元的生长锥利用钙离子的浓度差来进行正确爬行,并最终引导轴突向正确的方向生长。然而,如果钙离子通道出现功能障碍,将会引起生长锥错误地爬行,从而导致神经发育相关疾病的发生。了解特异性表达于生长锥的钙通道,并获得能调控这些钙通道的调节剂对于治疗神经发育相关疾病至关重要。这些调节剂可能已广泛用于其他组织病变,如心血管疾病的治疗。但它们对生长锥上钙通道的调控仍有待进一步研究。最近的研究表明,离子通道调节剂可以作为治疗神经发育相关疾病的药物靶标。本文讨论钙通道调节剂在生长锥引导轴突生长过程中的潜在作用。
钙;生长锥;L型钙离子通道;兰尼碱受体钙释放通道;肌醇(1,4,5)-三磷酸受体
冯中平,E-mail:zp.feng@utoronto.ca,Tel:+1(416)946-0671
2016-03-20 接受日期:2016-06-13)
R966
:A
:1000-3002-(2016)06-0640-16
10.3867/j.issn.1000-3002.2016.06.004
Biography:Ji-sun KIM,female,BSc&Graduate Student, main research field is neuroscience,E-mail:jsk.kim@mail. utoronto.ca;Vivian Y SZETO,female,HBSc&Project Student,main research field is neuroscience,E-mail:v.szeto@ mail.utoronto.ca
Zhong-ping FENG,Tel:+1(416) 946-0671,E-mail:zp.feng@utoronto.ca
*Co-first author.
*共同第一作者。
(本文编辑:乔 虹)
猜你喜欢
杂志排行
中国药理学与毒理学杂志的其它文章
- Incretin hormone glucagon-like peptide 1 and gut microbiota
- Drug reward memory:implication from drug-induced conditioned place preference model
- Pathological role of transient receptor potential melastatin member 2 channel in neurodegenerative diseases and Alzheimer disease
- Reversal effects of desipramine on resistance of U251/TR cells to temozolomide
- Role of calcium-activated potassium channels in neuronal pacemaker activity
- Developing high quality chemical probes targeting ubiquitin-specific proteases
