Effect of clopidogrel on development of chemically induced colitis-associated cancer in mice
2016-02-15YANGXiaowenWANGShiqiSONGJueminLUXuechunLIUXiaoyanZHANGShuzhuoYANHaitaoMAXiaoyunZHENGJianquanWEIXiaoli
YANG Xiao-wen,WANG Shi-qi,SONG Jue-min,LU Xue-chun,LIU Xiao-yan,ZHANG Shu-zhuo,YAN Hai-tao,MA Xiao-yun,ZHENG Jian-quan,WEI Xiao-li
(1.Institute of Pharmacology and Toxicology,Academy of Military Medical Sciences,Beijing 100850,China;2.Tumor Hospital of Shanxi Medical University,Taiyuan 030001,China;3.Chinese PLA General Hospital,Beijing 100853,China)
·ORIGINAL ARTICLE·
Effect of clopidogrel on development of chemically induced colitis-associated cancer in mice
YANG Xiao-wen1,WANG Shi-qi1,SONG Jue-min2,LU Xue-chun3,LIU Xiao-yan1,ZHANG Shu-zhuo1,YAN Hai-tao1,MA Xiao-yun1,ZHENG Jian-quan1,WEI Xiao-li1
(1.Institute of Pharmacology and Toxicology,Academy of Military Medical Sciences,Beijing 100850,China;2.Tumor Hospital of Shanxi Medical University,Taiyuan 030001,China;3.Chinese PLA General Hospital,Beijing 100853,China)
OBJECTIVE To investigate the effect of clopidogrel(Clog),a platelet aggregation inhibitor,on the development of colitis-associated colon cancer(CAC)and its possible mechanism. METHODS To establish a CAC model,male BALB/c mice were treated with single azoxymethane(AOM)10 mg·kg-1by ip.One week later,the mice drank 2.5%dextran sulfate sodium(DSS)for one week and water for two weeks,which lasted three cycles.From the first day mice received 2.5%DSS water,Clog 12.5,25.0 and 50.0 mg·kg-1was ig administered once a day.Body mass,clinical symptoms,the number of colon tumor and tumor size in colon tissue were recorded.Hyperplasia of tumors was analyzed by HE staining.In the early inflammatory phase of the CAC model,the length of colons was measured,histological structure and epithelium cell proliferation of colon tissues were evaluated by HE staining and Ki67 staining,respectively.In the tumorigenesis and progression phase of the CAC model,epithe⁃lium cell proliferation of colon tissues was evaluated by Ki67 staining.The mRNA expression of tumor necrosis factor-α(TNF-α)was detected by real-time quantitative PCR.The expression of chemokine(C-X-C motif)ligand 2(CXCL2)and its receptor 2(CXCR2)in colon tissues was detected by PCR and immu⁃nohistochemistry.RESULTS Compared with model group,clinical symptoms of mice in Clog 12.5 mg·kg-1group were alleviated,the size of colon tumors was decreased(P<0.05),and hyperplasia of tumors was reduced(P<0.05).During the inflammatory phase,the clinical symptoms of mice in Clog 12.5 mg·kg-1group were significantly alleviated(P<0.05),the decrease of body mass was reduced(P<0.01),the colon shrinkage was ameliorated(P<0.01),the inflammatory injury and epithelium cell proliferation in colon tissues were reduced(P<0.05).During the tumorigenesis and progression phase,epithelium cell prolif⁃eration in colon tissues in Clog 12.5 mg·kg-1group was reduced(P<0.01),and the mRNA and protein expression of TNF-α,CXCL2 and CXCR2 of colon tissues was decreased(P<0.05).CONCLUSION Clog can alleviate inflammation during the CAC early inflammatory phase and inhibit the formation of CAC.The antitumor effect of Clog may be related to the decrease in expression of CXCL2 and CXCR2.
clopidogrel;colitis;colon cancer;inflammatory bowel diseases;platelets
Inflammatoryboweldiseases(IBD) are chronic inflammatory disorders of the intestinaltract,which are associated with an immunological imbalance in the intestinal mucosa.Ulcerative colitis(UC)and Crohn disease(CD)are the most common types.About 15%of the IBD patients develop into colorectal cancer,known as colitis-associated colon cancer(CAC)[1].The risk of CAC is elevated significantly with theseverity,extent of injury and duration of the colitis[2]. Increasing evidence has confirmed the close causal relationship between inflammation and tumori⁃genesis and progression of CAC.Therefore,inhibiting inflammatory progression of CAC has been considered a novel strategy that may decrease the risk and severity of CAC.
Thromboembolismisoneoffourleading causes of death in IBD patients[3].Accumulated evidence indicates that platelets may play a crucial regulatory role in the progression of IBD. Platelets are main effector cells that respond to hemostasis and thrombosis in the body.Recent studies highlight that platelets also have func⁃tions of potent pro-inflammatory cells,and are involved in occurrence and progression of multiple inflammations and cancers[4-5].In IBD patients,platelets are highly activated and have a unique spontaneously aggregated feature. Platelets present many changes including morphological al⁃terations,over-secretion of granular content,and release of microparticles[6].Therefore,inhibition of platelets activation and aggregation may pre⁃vent the inflammatory progression of CAC.
Platelets could help malignant tumors with tumorigenesis[7],angiogenesis[8],invasion and metastasis[9].Recent clinical studies showed that long term and low dose administration of aspirin could reduce the incidence and mortality of many inflammation-induced cancers[10].Aspirin could protect the body from malignant cancers by inhibiting the platelet production of thromboxane A2and preventing platelets activation,aggregation,and degranulation[8].Varieties of leukocytes infil⁃trate into the tumor microenvironment,including neutrophils or tumor-associated neutrophils(TANs). TANs are either pro-tumor or anti-tumor,while all the existing clinical studies manifest that TANs are correlated with poorclinicalout⁃comes[11-12].Therefore,TANs are often associated with a pro-tumor role.Studies also suggest that platelets have interactions with neutrophils and are closely related to neutrophils motility[13-14]. Promoting TANs infiltration to facilitate tumor cells survival may be another mechanism of platelet pro-tumor role.
According to the studies mentioned above,an increased level of platelet activation and aggre⁃gation is involved in the development of inflam⁃mation and tumorigenesis.Chronic and relapsing inflammation occurring in IBD has been classically associated with an increased risk of colorectal cancer.Therefore,we hypothesize that anti-platelet aggregation drugs may play a therapeutic role by delaying the development of inflammatory pro⁃gression of CAC,thereby affecting the occur⁃rence and development of CAC.
In the present study,a CAC mouse model was chemically induced by administration of dex⁃tran sulfate sodium(DSS)in the drinking water combined with the injection of azoxymethane(AOM).DSS ingestion causes damage to the epithelial cell barrier and recapitulates the inflam⁃matory condition of human UC[15].A colonic tumor was well formed within a short time by AOM injection[15-16].An anti-platelet aggregation agent clopidogrel(Clog)was applied to model mice to observe the anti-tumor effect of Clog and explore the possible mechanism.
1 MATERIALS AND METHODS
1.1 Reagents and instruments
Clog(HubeiTuochukangyuanPharm& Chem,China),AOM and agarose(Sigma-Aldrich,America),DSS(MW:36 000-50 000,MP Bio⁃medicals,America),Trizol(Invitrogen,America),the reverse transcriptase kit and SYBR Green reagent kit(TaKaRa,Japan),2×Taq PCR Mas⁃terMix(Biomed,China),Primers(AuGCT DNASYN Biotechnology,China,Tab.1),goat antimouse chemokine(C-X-C motif)ligand 2(CXCL2)antibody(R&D,America), rabbit anti-mouse chemokine(C-X-C motif) receptor 2(CXCR2)antibody(Abcam, Britain), HRP-conjugated secondary antibody and DAB kit(ZSGB-BIO,China).EC-900-1001EcoTM Real-Time PCR Sys⁃tem(Illumina,America),BS214 Delectronic scales(Sartorius,Germany),BX51 Fluorescence microscope(Olympus,Japan),2020D GEL-Transilluminator(Kelin Hengda,China),Veriti 96 Thermal cycler(ABI,America).

Tab.1 Primer sequences for PCR
1.2 Mouse model of CAC and treatment
Male BALB/c mice(20-24 g),8-10 weeks old,were purchased and kept in the Animal Center of Academy of Military Medical Sciences.The animal experiments were performed with approval of the Institutional Animal Care and Use Committee of Beijing Institute of Pharmacology and Toxicology.
After one week of adaption,the mice were weighed and randomly divided to 5 groups(15 mice per group):normal control group(ig a single dose of saline),model group,and Clog 12.5,25.0 and 50.0 mg·kg-1groups, Clog was suspended in 1%CMC-Na.The CAC mouse model was developed as Fig.1,ip a single dose of AOM 10 mg·kg-1.One week later,the mice drank 2.5%dextran sulfate sodium(DSS)for one week and water for two weeks,which lasted three cycles.The 1stday DSS water was adminis⁃treted was defined as the experiment 1stday.All groups were administrated every day until the mice were sacrificed on the 35thday(5 mice of per group)and 56thday(all remained mice). The body mass,stool consistency,and hemato⁃chezia of the mice were recorded every 2-3 d. The symptoms of stool consistency and hematoche⁃zia served as the clinical score according to a previ⁃ously admitted standard[17].When the mice were sacrificed,whole colons were collected for further studies,and the full length of the intestine was measured from the cecum to the end of the rectum.
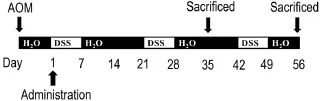
Fig.1 Scheme formouse colitis-associated colon cancer(CAC)model.To establish a mouse CAC model,the mice were ip administered with carcinogen azoxymethane(AOM)10 mg·kg-1,a week later,followed by three cycles of oral 2.5% dextran sulfate sodium(DSS)administration.Clog 12.5,25.0 and 50.0 mg·kg-1groups were ig administered every day until mice were sacrificed on the 35thday(5 mice per group)and 56thday(all the remaining mice)from the first day of DSS administration.
1.3 Mouse model of CAC inflammatory phase and treatment
Male BALB/c mice(20-24 g),8-10 weeks old,were divided into three groups(16 mice per group):normal control group(1%CMC-Na),mod⁃el group(a single ip administration of AOM 10 mg·kg-1,and a week later,treated with 2.5%DSS in drinking water for 8 d)(Fig.2),and Clog 12.5 mg·kg-1group(ig administration of Clog 12.5 every day from the first day of DSS administration).On the 8thday after DSS admin⁃istration,the mice were sacrificed,and the whole colons were collected for further studies. The full length of the intestine was measured from the cecum to the end of the rectum.

Fig.2Scheme for mouse CAC inflammatory phase model.The mice were ip administered with AOM 10 mg·kg-1,one week later,administered with 2.5%DSS in drinking water for 8 d.
1.4 Histopathological analysis of mouse colon tissue by HE staining
Colon sections were embedded in paraffin for HE staining.The intestine was longitudinally sheared and washed with normal saline before being rolled as rings by the Swiss-roll method and fixed in 10% oxymethylene.Histological alter⁃nations were observed under a microscope.Thedegree of inflammation,ulceration,hyperplasia and extent of lesions in colon tissues were scored according to previously admitted standards[18-19].
1.5 Expression of Ki67,CXCL2,and CXCR2 protein in mouse colon tissue detected by immunohistochemistry analysis
Paraffin-embedded colon sections were deparaffinized and rehydrated.Heat-induced antigen retrieval was performed.Colon sections were incubated with 3%hydrogen peroxide for 10 min. After blocking with the appropriate antisera for 1 h,sections were incubated with anti-Ki67(1∶200),anti-CXCR2(1∶100),and anti-CXCL2(1∶100)antibodies,respectively,at 4℃ overnight.After incubation with HRP-conjugated secondary anti⁃body,positive signals were visualized by DAB kit and counter-stained with hematoxylin.Four fields from each section were examined and analyzed using IPP 6.0.The positive content was calculat⁃ed:average optical density=integrating optical den⁃sity/calculated area.
1.6 TNF- αmRNA expression in mouse colon tis⁃sue detected by real-time quantitative PCR(qPCR)
Total RNA of colon tissue was isolated and cDNA prepared using the reverse transcriptase kit according to the manufacturer′s protocol.
qPCR analysis was conducted for 40 cycles(95℃for 5 s and 60℃for 31 s).The procedures were conducted in strict accordance with to the instructions.β-Actin was used as an internal con⁃ trol.Each sample was performed with three dupli⁃cates.TNF-αmRNA expression was analyzed with 2-△△Ctmethod.
1.7 CXCL2andCXCR2mRNA expression in mouse colon tissue detected by semi-quanti⁃tative reverse transcriptase-PCR(RT-PCR)
Total RNA of colon tissue was isolated and cDNA prepared according to the instructions. RT-PCR analysis was conducted for 35 cycles(94℃ for 30 s,56℃ for 30 s,and 72℃ for 1 min)in 50 μL reaction mixture.PCR products were fractionated on a 1.2%agarose gel and visualized by ethidium bromide staining under an ultraviolet transilluminator.The band intensities were measured using Image J analysis software,and mRNA expression ofCXCL2andCXCR2was calculated with the ratios toGAPDH.
1.8 Statistic analysis
Data were presented asx±s.GraphPad Prism 6 software was used for statistical analysis. One-way ANOVA analysis and Studentttest were used as appropriate.P<0.05 was consid⁃ered statistically significant.
2 RESULTS
2.1 Effect of Clog on tumorigenesis of CAC model mice
2.1.1 Clinical score

Fig.3 Effect of clopidogrel(Clog)on clinical score of CAC model mice.See Fig.1 for the mouse treatment.x±s,n=15(the 1st-35thday),n=7-10(the 35th-56thday).**P<0.01,compared with normal control group;#P<0.05,##P<0.01,compared with model group.
As shown in Fig.3,compared with normalcontrolgroup,body mass was significantly decreased(P<0.01),while bloody stool and diar⁃rhea were increased when DSS administration in model group.Those symptoms were relieved with the withdrawal of DSS.Compared with model group,Clog 12.5,25.0 and 50.0 mg·kg-1treat⁃ment had little effect on relieving the decrease of body mass(data not shown).Each dosage of Clog could reduce the clinical score at multiple time points,mostly in the early period of CAC development(P<0.05,P<0.01).
2.1.2 Tumor size
As shown in Tab.2,on the 56thday,there was no tumor formed in normal control group,and the colonic neoplasm formation rate was 100%in model group.Compared with model group,tumorsize was decreased in Clog 12.5 mg·kg-1group(P<0.05),but tumor number and tumor load did not change significantly in Clog 12.5 mg·kg-1group.
2.1.3 Degree of hyperplasia of tumors
As shown in Fig.4,compared with model group,the degree of hyperplasia was decreased in Clog 12.5 mg·kg-1group.Tumors from 4/7 mice of model group were classified as severegrade dysplasia,compared with tumors from 1/8 mice of Clog 12.5 mg·kg-1group. Hyperplasia score was decreased from 2.9±0.9 to 2.0±0.7 in Clog 12.5 mg·kg-1group(P<0.05).But there was no statistically significant difference in Clog 25.0 and 50.0 mg·kg-1groups.
2.2 Effect of Clog during inflammatory phase of CAC
2.2.1 Body mass,clinical score and colon length
As shown in Fig.5,compared with normal control group,body mass and colon lengths de⁃clined,and clinical score was elevated(P<0.01)in model group.On the 8thday,compared with model group,body mass and clinical score were decreased(P<0.01)in Clog 12.5 mg·kg-1group,and the contraction of colon was relieved from(6.2±0.5)cm to(7.3±0.7)cm(P<0.01)in Clog 12.5 mg·kg-1group.

Tab.2 Effect of Clog on tumor size,tumor number and tumor load of CAC model mice

Fig.4 Effect of Clog on tumor hyperplasia of CAC model mice detected by HE staining.See Fig.1 for the mouse treat⁃ment.Dashed lines mark the borders between adenomas and normal epithelium.B was the semi-quantitative result of A.x±s,n=7-8. *P<0.05,compared with model group.

Fig.5 Effect of Clog on body mass(A),clinical score(B)and colon length(C)of mice during inflammatory phase of CAC.See Fig.2 for the mouse treatment.Body mass change(%)=(final body mass-initial body mass)/initial body mass× 100%.x±s,n=16.**P<0.01,compared with normal control group;#P<0.05,##P<0.01,compared with model group.
2.2.2 Histological structure of mouse colon tissues
As shown in Fig.6,compared with normal control group,infiltration of inflammatory cells,extent of ulceration and lesions,and degree of hyperplasia were elevated in colon tissues of model group.Compared with model group,the inflammation score,ulceration score,hyperplasia score and extent of lesions score were decreased in Clog 12.5 mg·kg-1group(P<0.05,P<0.01).
2.2.3 Epithelium cell proliferation
As shown in Fig.7,compared with normal control group,the range of Ki67 staining of mouse colon tissues was significantly increased in model group(P<0.01).After Clog 12.5 mg·kg-1treatment,the range was decreased(P<0.05). Compared with model group,the proliferation of epithelium cells in colon tissues was decreased in Clog 12.5 mg·kg-1group(P<0.05).
2.3 Effect of Clog on mouse colon during tumorigenesis and progression phase of CAC
2.3.1 TNF-αmRNA expression

Fig.6 Effect of Clog on histological structure of mouse colon tissues during inflammatory phase of CAC detected by HE staining.See Fig.2 for the mouse treatment.Double sided arrows indicate borders of ulcers;single sided arrows indicate infiltration of inflammatory cells.B was semi-quantitative results of A.x±s,n=8.**P<0.01,compared with normal control group;#P<0.05,##P<0.01,compared with model group.

Fig.7 Effect of Clog on epithelium cell proliferation of mouse colon tissues during inflammatory phase of CAC detected by Ki67 staining.See Fig.2 for the mouse treatment.Brown color indicated Ki67 staining.Positive content was calculated:average absorbance(AA)=integrated absorbance(IA)/calculated area.B was semi-quantitative results of A.x±s,n=8.**P<0.01,compared with normal control group;#P<0.05,compared with model group.
As shown in Fig.8,compared with normal control group,mRNA expression ofTNF-αin colon tissues was increased(P<0.01)in model group during tumorigenesis and progression phase of CAC.On the 35thday of CAC develop⁃ment,compared with model group,TNF-αmRNA expression in mouse colon tissues was decreased(P<0.01)in Clog 12.5 mg·kg-1group,while on the 56thday of CAC development,TNF-αmRNA expression did not significantly decrease in Clog 12.5 mg·kg-1group.

Fig.8 Effect of Clog on tumor necrosis factor- α(TNF- α)expression in mouse colon tissues during tumorigen⁃esis and progression phase of CAC detected by qPCR. See Fig.1 for the mouse treatment.x±s,n=5(on the 35thday);n=7-8(on the 56thday).**P<0.01 compared with normal con⁃trol group;##P<0.01,compared with model group.
2.3.2 Epithelium cell proliferation
As shown in Fig.9,compared with normal control group,the range of Ki67 staining in colon tissues was increased during the tumorigenesis and progression phase of CAC(P<0.01)in model group.Compared with model group,the range of Ki67 staining in Clog12.5 mg·kg-1group was significantly decreased on the 35thday of CAC development(P<0.01),while on the 56thday,the range of Ki67 staining had no significant decrease in Clog 12.5 mg·kg-1group.

Fig.9 Effect of Clog on epithelium cell proliferation in mouse colon tissues during tumorigenesis and pro⁃gression phase of CAC detected by Ki67 staining.See Fig.1 for the mouse treatment.Brown color indicated Ki67 staining. B was the semi-quantitative results of A.x±s,n=5(on the 35thday);n=7-8(on the 56thday).*P<0.05,**P<0.01 compared with normal control group;##P<0.01,compared with model group.
2.3.3 CXCL2andCXCR2mRNA expression
As shown in Fig.10,on both the 35thday and 56thday,compared with normal control group,bend intensities ofCXCL2andCXCR2mRNA were increased in model group.Compared with model group,mRNA expression ofCXCL2andCXCR2was decreased in Clog 12.5 mg·kg-1group(P<0.05,P<0.01).
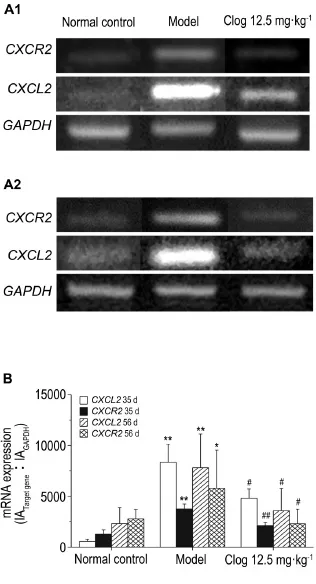
Fig.10 Effect of Clog on mRNA expression of chemo⁃kine(C-X-C motif)ligand 2(CXCL2)and its receptor 2(CXCR2)in mouse colon tissues during tumorigen⁃esis and progression phase of CAC detected by realtime RT-PCR.See Fig.1 for the mouse treatment.A1:on the 35thday;A2:on the 56thday;B was the semi-quantitative results of A1 and A2,respectively.x±s,n=5(on the 35thday),n=7-8(on the 56thday).*P<0.05,**P<0.01,compared with normal control group;#P<0.05,##P<0.01,compared with model group.
2.3.4 Protein expression of CXCL2 and CXCR2
As shown in Fig.11,on both the 35thday and the 56thday,compared with normla control group,the color depth and range of CXCL2 and CXCR2 immunohistochemical staining became darker and larger in colon tissues in model group.Compared with model group,protein expression ofCXCL2 and CXCR2 in mouse colon tissues of Clog 12.5 mg·kg-1group was reduced(P<0.01).

Fig.11 Effect of Clog on protein expression of CXCL2 and CXCR2 in mouse colon tissues during tumorigen⁃esis and progression phase of CAC detected by immunohistochemical staining.See Fig.1 for the mouse treatment.Brown color indicated CXCL2 staining or CXCR2 staining.A1:on the 35thday;A2:on the 56thday;B was semiquantitative results of A1 and A2,respectively.x±s,n=5(on the 35thday),n=7-8(on the 56thday).**P<0.01,compared with normal control group;##P<0.01,compared with model group.
3 DISCUSSION
In this study,we developed a mouse model of CAC and observed the effect of an anti-platelet aggregation drug Clog on CAC tumorigenesis and progression.The colonic neoplasm forma⁃tion rate was 100%after AOM/DSS treatment,which is consistent with the previous study[20].In the third DSS cycle,some mice died of repeated stimulation of DSS water,which resulted in the varied number of mice in different groups.In addition,the tolerance of inflammatory stimulation in each mouse was different,leading to a high value of standard deviation.
Our results showed that Clog was able to alleviate the clinical symptoms,delay the forma⁃tion of colonic tumor,and reduce the hyperpla⁃sia level of tumors in mice.Clog 12.5 mg·kg-1was the most effective dose,whereas a higher dose of Clog(25-50 mg·kg-1)did not produce a bettereffectas expected.Therefore, Clog 12.5 mg·kg-1was chosen for the following studies. As a platelet aggregation inhibitor,Clog is mainly used to prevent heart attack and stroke in clinic. This was the first study to reveal that Clog pre⁃sented an antineoplastic effect at a relatively low dosage.However,we could not explain why a higher dosage of Clog loses its effect.Obviously,further explorations are needed.
In the early stage of the CAC model,inflam⁃mation is the dominant pathologic process.It was found that Clog 12.5 mg·kg-1significantly al⁃leviated the symptoms of decrease of body mass,bloody diarrhea,and shrinkage of colon length induced by inflammation in the inflamma⁃tory phase.Histological analysis also showed diminished injury and decreased cell proliferation in colon tissues from Clog treated group.During the later stage of the CAC model,colorectal cancers occurred.In the tumorigenesis and progression phase,Clog 12.5 mg·kg-1significantly decreased the range of Ki67 staining and TNF-α expression in colon tissues on the 35thday,and the effect of Clog was eliminated on the 56thday. Ki67 is a proliferation-related antigen.The larger range of Ki67 staining indicates a higher level of cell proliferation in the tissue.TNF-α played a key role in the transition from colitis to colorectal cancer. Blockade of TNF-α diminished tissue injury and resulted in a smaller tumor number and smaller tumor size[21].Therefore,a drug that can decrease the level of TNF-α may prevent tumorigenesis of CAC.Our results indicated that Clog delayed the inflammatory progression and ameliorated tumori⁃genesis in the early stage.However,the effect of Clog was limited in the later stage of CAC.
Shanget al[22]found that infiltration of TANs was closely related to the tumor growth of CAC,and the recruitment of TANs depended on CX⁃CL2 and CXCR2 interaction.Our study found that Clog reduced the mRNA and protein level of CXCL2 and CXCR2 in both samples from the 35thday and 56thday compared with model group,indicating thatClog mayreduce the recruitment of neutrophils to the colon tissues in CAC mice.Studies have confirmed that platelets could promote the movement of neutrophils,and blockade of platelets activation could significantly inhibit the motility and activity of neutrophils[13,23]. Therefore,Clog may exert anti-tumor effect by inhibiting platelet activation and then reducing the infiltration of TANs.In addition,CXCL2 is also a potent pro-angiogenesis agent that combines with its receptor CXCR2 and promotes angiogenesis and neovascularization in tumor[24].This may be another mechanism of anti-tumor effect of Clog.
In summary,our results revealed that Clog could delay tumorigenesis of AOM/DSS induced CAC.It decreased the size and hyperplasia level of the tumor.The potential mechanisms may be asso⁃ciated with reducing the severity of inflammatory lesions,inhibiting colon epithelium cell proliferation,and decreasing the recruitment of neutrophils at the tumor site.Our results suggested that anti-platelet aggregation agent Clog may be used as an adju⁃vant therapy for CAC in clinical application.
ACKNOWLEDGMENTS:We thank WANG He-mei and QU Wen-sheng for guidance and manufacturing of histo⁃logical analysis.
REFERENCES:
[1]Dyson JK,Rutter MD.Colorectal cancer in inflam⁃matory bowel disease:what is the real magnitude of the risk?[J].World J Gastroenterol,2012,18(29):3839-3848.
[2]Herszényi L,Barabás L,Miheller P,Tulassay Z. Colorectal cancer in patients with inflammatory bowel disease:the true impact of the risk[J].Dig Dis,2015,33(1):52-57.
[3]JacksonLM,O′Gorman PJ, O′ConnellJ,Cronin CC,Cotter KP,Shanahan F.Thrombosis in inflammatory bowel disease:clinical setting,procoagulant profile and factorⅤLeiden[J].QJM,1997,90(3):183-188.
[4]Yoshida H, Granger DN.Inflammatory bowel disease:a paradigm for the link between coagulation and inflammation[J].Inflamm Bowel Dis,2009,15(8):1245-1255.
[5]Voudoukis E,Karmiris K,Koutroubakis IE.Multi⁃potent role of platelets in inflammatory bowel dis⁃eases:a clinical approach[J].World J Gastroen⁃terol,2014,20(12):3180-3190.
[6]Collins CE,Cahill MR,Newland AC,Rampton DS. Platelets circulate in an activated state in inflamma⁃tory bowel disease[J].Gastroenterology,1994,106(4):840-845.
[7]Sharma D,Brummel-Ziedins KE,Bouchard BA,Holmes CE.Platelets in tumor progression:a host factor that offers multiple potential targets in the treatment of cancer[J].J Cell Physiol,2014,229(8):1005-1015.
[8]Menter DG,Tucker SC,Kopetz S,Sood AK,Crissman JD,Honn KV.Platelets and cancer:a casual or causal relationship:revisited[J].Cancer Metastasis Rev,2014,33(1):231-269.
[9]Gay LJ,Felding-Habermann B.Contribution of platelets to tumour metastasis[J].Nat Rev Cancer,2011,11(2):123-134.
[10]Chan AT,Zauber AG, Hsu M, Breazna A,Hunter DJ,Rosenstein RB,et al.Cytochrome P450 2C9 variants influence response to celecoxib for prevention of colorectal adenoma[J].Gastroen⁃terology,2009,136(7):2127-2136.e1.
[11]Houghton AM.The paradox of tumor-associated neutrophils:fueling tumor growth with cytotoxic substances[J].Cell Cycle,2010,9(9):1732-1737.
[12]Sionov RV,Fridlender ZG,Granot Z.The multi⁃faceted roles of neutrophils play in the tumor micro⁃environment[J].Cancer Microenviron,2015,8(3):125-158.
[13]Lam FW,Vijayan KV,Rumbaut RE.Platelets and their interactions with other immune cells[J].Compr Physiol,2015,5(3):1265-1280.
[14]Sreeramkumar V, Adrover JM, Ballesteros I,Cuartero MI,Rossaint J,Bilbao I,et al.Neutro⁃phils scan for activated platelets to initiate inflam⁃mation[J].Science,2014,346(6214):1234-1238.
[15]Thaker AI,Shaker A,Rao MS,Ciorba MA.Modeling colitis-associated cancer with azoxymethane(AOM)and dextran sulfate sodium(DSS)[J].J Vis Exp,2012,2012(67):e4100-e4100.
[16]Kanneganti M,Mino-Kenudson M,Mizoguchi E. Animal models of colitis-associated carcinogenesis[J].J Biomed Biotechnol,2011,2011:342637.
[17]Wirtz S,Neufert C,Weigmann B,Neurath MF. Chemically induced mouse models of intestinal inflammation[J].Nat Protoc,2007,2(3):541-546.
[18]Maeda S,Hsu LC,Liu H,Bankston LA,Iimura M,Kagnoff MF,et al.Nod2 mutation in Crohn′s disease potentiates NF-kappaB activity and IL-1beta processing[J].Science,2005,307(5710):734-738.
[19]Zaki MH,Vogel P,Malireddi R,Anand PK,Bertin J,Green DR,et al.The NOD-like receptor NLRP12 attenuates colon inflammation and tumorigenesis[J].Cancer Cell,2011,20(5):649-660.
[20]Suzuki R,Kohno H,Sugie S,Nakagama H,Tanaka T.Strain differences in the susceptibility to azoxymethane and dextran sodium sulfate-induced colon carcinogenesis in mice[J].Carcinogenesis,2006,27(1):162-169.
[21]Popivanova BK,Kitamura K,Wu Y,Kondo T,Kagaya T,Kaneko S,et al.Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis[J].J Clin Invest,2008,118(2):560-570.
[22]Shang K,Bai YP,Wang C,Wang Z,Gu HY,Du X,et al.Crucial involvement of tumor-associated neutrophils in the regulation of chronic colitis-asso⁃ciated carcinogenesis in mice[J].PLoS One,2012,7(12):e51848.
[23]Vieira-de-Abreu A,Campbell RA,Weyrich AS,Zimmerman GA.Platelets:versatile effector cells in hemostasis,inflammation,and the immune continuum[J].Semin Immunopathol,2012,34(1):5-30.
[24]Keeley EC,Mehrad B,Strieter RM.Chemokines as mediators of tumor angiogenesis and neovascu⁃larization[J].Exp Cell Res,2011,317(5,SI):685-690.
氯吡格雷在化学诱导小鼠结肠炎相关肿瘤发生中的作用
杨晓雯1,王士奇1,宋觉敏2,卢学春3,刘晓燕1,张树卓1,闫海涛1,马晓芸1,郑建全1,魏晓莉1
(1.军事医学科学院毒物药物研究所,北京 100850;2.山西医科大学肿瘤医院,山西太原 030001;3.中国人民解放军总医院,北京 100853)
目的 探究血小板聚集抑制剂氯吡格雷(Clog)对结肠炎相关结肠癌(CAC)形成过程的影响及其作用机制。方法 雄性BALB/c小鼠分为5组,正常对照组,模型组,Clog 12.5,25.0和50.0 mg·kg-1组。CAC模型组首先1次ip给予氧化偶氮甲烷(AOM)10 mg·kg-1,1周后,每天饮用〔2.5%葡聚糖硫酸钠(DSS)1周+生理盐水2周〕3个周期建立CAC模型。自给予2.5%DSS饮用水起,Clog 12.5,25.0和50.0 mg·kg-1每天ig给药1次至模型建立结束。记录小鼠的体质量,临床症状,小鼠结肠肿瘤的数目和大小,HE染色评价肿瘤的异型性。在CAC小鼠早期炎症阶段,测量小鼠的结肠长度,HE染色和Ki67染色分别评价结肠组织病理变化和结肠组织上皮细胞的增殖水平。在肿瘤形成与发展阶段,Ki67染色评价结肠组织上皮细胞增殖水平,实时荧光定量PCR法检测肿瘤坏死因子α(TNF-α)mRNA的表达,PCR和免疫组化法检测趋化因子(C-X-C结构域)配体2(CXCL2)及其受体CXCR2的表达。结果 与模型组相比,Clog 12.5 mg·kg-1可缓解小鼠的临床症状,减小结肠肿瘤平均直径(P<0.05),降低肿瘤异型性(P<0.05)。在CAC早期炎症阶段,Clog 12.5 mg·kg-1可缓解小鼠临床症状(P<0.05)和体质量下降(P<0.01),增加结肠长度(P<0.01),减轻结肠组织炎症损伤(P<0.05),降低上皮细胞增殖水平(P<0.05);在CAC肿瘤形成与发展阶段,Clog 12.5 mg·kg-1可降低结肠组织上皮细胞增殖水平(P<0.05),减少结肠组织TNF-αmRNA水平、CXCL2和CXCR2 mRNA及蛋白表达水平(P<0.05)。结论 Clog可缓解CAC早期炎症阶段的炎症发展,抑制结肠肿瘤的形成。其抗肿瘤作用可能与减少CXCL2与CXCR2的表达有关。
氯吡格雷;结肠炎;结肠癌;炎性肠病;血小板
国家科技重大专项(2012-ZX09301003)
魏晓莉,E-mail:weixl@bmi.ac.cn;郑建全,E-mail:jqzheng@bmi.ac.cn
2016-01-19接受日期:2016-09-05)
R979.1
A
1000-3002-(2016)09-0910-11
10.3867/j.issn.1000-3002.2016.09.002
(本文编辑:贺云霞)
Foundation item:The project supported by National Science and Technology Major Project of China(2012-ZX09301003)
Biography:YANG Xiao-wen,major in molecular cancer.
s:WEI Xiao-li,E-mail:weixl@bmi.ac.cn;ZHENG Jian-quan,E-mail:jqzheng@bmi.ac.cn
