Apolipoprotein E polymorphisms increase the risk of post-stroke depression
2016-02-09XuebinLiJieWangAndingXuJianminHuangLanqingMengRuiyaHuangJunliWang1StrokeCenterNeurologyDivisiontheFirstAffiliatedHospitalofJinanUniversityGuangzhouGuangdongProvinceChinaDepartmentofNeurologytheAffiliatedHospitalof
Xue-bin Li, Jie Wang, An-ding Xu Jian-min Huang Lan-qing Meng Rui-ya Huang Jun-li Wang1 Stroke Center & Neurology Division, the First Affiliated Hospital of Jinan University, Guangzhou, Guangdong Province, China Department of Neurology, the Affiliated Hospital of Youjiang Medical University for Nationalities, Baise, Guangxi Zhuang Autonomous Region, China3 Department of Nephrology, the Affiliated Hospital of Youjiang Medical University for Nationalities, Baise, Guangxi Zhuang Autonomous Region, China Department of Laboratory Medicines, the Affiliated Hospital of Youjiang Medical University for Nationalities, Baise, Guangxi Zhuang Autonomous Region, China
Apolipoprotein E polymorphisms increase the risk of post-stroke depression
Xue-bin Li1,2,*,#, Jie Wang3,#, An-ding Xu1,*, Jian-min Huang2, Lan-qing Meng2, Rui-ya Huang2, Jun-li Wang4
1 Stroke Center & Neurology Division, the First Affiliated Hospital of Jinan University, Guangzhou, Guangdong Province, China
2 Department of Neurology, the Affiliated Hospital of Youjiang Medical University for Nationalities, Baise, Guangxi Zhuang Autonomous Region, China
3 Department of Nephrology, the Affiliated Hospital of Youjiang Medical University for Nationalities, Baise, Guangxi Zhuang Autonomous Region, China
4 Department of Laboratory Medicines, the Affiliated Hospital of Youjiang Medical University for Nationalities, Baise, Guangxi Zhuang Autonomous Region, China
How to cite this article:Li XB, Wang J, Xu AD, Huang JM, Meng LQ, Huang RY, Wang JL (2016) Apolipoprotein E polymorphisms increase the risk of post-stroke depression. Neural Regen Res 11(11):1790-1796.
Open access statement:This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Funding:This study was supported in part by the National Natural Science Foundation of China, No. 81160146.
Graphical Abstract
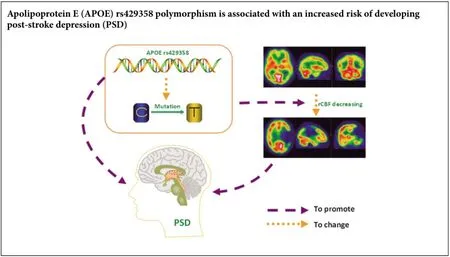
Recent reports have shown that apolipoprotein E (APOE) polymorphisms are involved in neurodegenerative disease. However, it is unclear whetherAPOEaffects post-stroke depression. Accordingly, we hypothesized thatAPOEpolymorphisms modify the risk of post-stroke depression. Here, we performed a hospital-based case-control study (including 76 cerebral infarction cases with post-stroke depression, 88 cerebral infarction cases without post-stroke depression, and 109 controls without any evidence of post-stroke depression or cerebral infarction) to determine possible association betweenAPOErs429358 and rs7412 polymorphisms and risk of post-stroke depression. Our findings show no difference among the groups with regards genotype distribution of the rs7412 polymorphism. In contrast,APOEgenotypes with rs429358-C alleles increased the risk of post-stroke depression. Further, the rs429358 polymorphism was associated with significantly decreased regional cerebral blood flow values in the left temporal lobe of post-stroke depression cases. Additionally, the rs429358 polymorphism was not only associated with depression severity, but with increasing serum levels of total cholesterol. These results suggest that theAPOErs429358 polymorphism is associated with increased risk of developing post-stroke depression, and thatAPOErs429358-C allele genotypes may be detrimental to recovery of nerve function after stoke. Indeed, these findings provide clinical data for future post-stroke depression gene interventions.
nerve regeneration; apolipoprotein E; genetic polymorphism; post-stroke depression; risk; regional resting-state cerebral blood flow; rs429358; rs7412; cerebral infarction; neural regeneration
Introduction
As the third leading cause of death worldwide, and a major health issue in the elderly population, stroke not only results in physical impairments such as disability, but also leads to social nonparticipation and psychological disease (Robinson and Jorge, 2015; Weaver and Liu, 2015). Of these, post-stroke depression (PSD) is the most common neuropsychiatric impairment, and often accompanied by apathy, fatigue, feelings of worthlessness, sleep changes, and anhedonia. Indeed, PSD is the most significant factor in causing low quality of life in stroke patients (Dwyer Hollender, 2014; Eum and Yim, 2015; Robinson and Jorge, 2015). However, to date, PSD pathogenesis remains unclear. Studies show that PSD may be related to certain behavioral, neurobiological, and social factors (Robinson and Jorge, 2015). Additionally, molecular epidemiological studies suggest that genetic factors play an important role in PSD pathogenesis (Tang et al., 2013; Zhou et al., 2015; Zhao et al., 2016). Yet despite considerable efforts in past decades, PSD-related genes have not been identified. Apolipoprotein E (APOE), a major chylomicron apoprotein, plays an important physiological role in regulation of overall lipid and lipoprotein homeostasis (Zhao et al., 2014). Further,APOEplays an important role in neuronal repair (Hatters et al., 2006; Zhang et al., 2013). Recently, studies have shown that two polymorphisms of theAPOEgene (namely, rs429358 T > C and rs7412 C > T) may be associated withAPOEdysfunction and increased risk of several neurological and cardiovascular diseases, including Alzheimer’s disease (Cosentino et al., 2008; Jofre-Monseny et al., 2008; McGuinness et al., 2010; Seripa et al., 2011; Yi et al., 2014; Lagos et al., 2015; Yuan et al., 2015). However, association between these polymorphisms and PSD has not yet been investigated. In addition, their correlation with regional cerebral blood flow (rCBF), a valuable marker for brain function including neural regeneration and activity (Venkat et al., 2016), has not previously been performed. Thus, in this study, we hypothesized thatAPOErs429358 and rs7412 polymorphisms modify PSD risk by disrupting recovery of brain function after stoke. Accordingly, we performed a hospital-based case-control study to determine the influence of these two polymorphisms on PSD risk and brain function.
Subjects and Methods
Subjects
This study was a hospital-based retrospective case-control study (Figure 1). The procedures were performed in accordance with theGuidelines for Medical Research Projects Involving Human Samples, and the protocol was approved by the Medical Ethics Committee of the Affiliated Hospital of Youjiang Medical University for Nationalities, China (approved No. 2011016). Every patient with a newly diagnosed cerebral infarction was recruited at the Affiliated Hospital of Youjiang Medical University for Nationalities by Youjiang Cancer Institution staff members using a standard interviewer-administered questionnaire. The inclusion criteria for cases were: (1) cerebral infarction confirmed by magnetic resonance imaging (MRI) and/or computed tomography (CT) scanning, according to diagnostic criteria from the 4thCerebral-Vascular Diseases Conference of China (Wu, 1997); (2) the study objective was understood and informed consent provided; (3) ability to complete the necessary investigations and questionnaires; (4) cases without a history of receiving blood cholesterol-lowering agents or antidepressants; (5) cases undergoing rCBF perfusion imaging examination; and (6) 6-month follow-up completed, and available blood samples and clinical data. The exclusion criteria were: (1) cases with depression but without cerebral infarction; (2) cases with a history of substance abuse, psychotic disorder, bipolar disorder, psychiatric illness, severe aphasia or dysarthria, intracranial hemorrhage, unconsciousness, severe infection, chronic inflammatory disease, tumors, hematologic disease, autoimmune disease, or severe heart, liver, or kidney damage; and (3) cases that died, dropped out, or had absent information during follow-up. Based on these inclusion and exclusion criteria, 164 patients with first-ever acute cerebral infarction were consecutively recruited within 7 days of stroke incidence from April 2013 to July 2014. All cases received subsequent stroke assessments by a single trained neurologist and psychiatrist, at both acute and chronic stages, specifically, approximately 2 weeks and 1, 3, and 6 months after stroke onset. Patients were screened and assessed for PSD using the 24-item Hamilton Depression Rating Scale (HAMD) questionnaire (Ning, 1986) and World Health Organisation Composite International Diagnostic Interview (Kessler and Üstün, 2004). Patients with cerebral infarction were included in the PSD group (n= 76) if they had both HAMD scores ≥ 8 and were diagnosed with depression. Alternatively, they were included in the non-PSD (NPSD) group (n= 88). During the same period, controls (n= 109) without any evidence of psychiatric disorder, mental illness, heart vessel disease, or other brain disorders, were randomly selected from a pool of healthy volunteers who had visited a general health check-up center at the same hospital for a routinely scheduled physical examination. Controls were frequently matched to cases with respect to age (± 5 years) and gender. Of those asked, 100% agreed to participate in this investigative study.
After providing written consent, 4 mL peripheral blood samples were obtained from all participants at the Affiliated Hospital of Youjiang Medical University for Nationalities forAPOEgenotype analysis. Demographic information and clinical data were also collected at the same hospital using a standard interviewer-administered questionnaire and/or medical records.
rCBF perfusion imaging assay
All subjects underwent rCBF perfusion imaging to examine brain function one month after stoke. rCBF perfusion imaging analysis was performed using a Siemens Symbia T2 single-photon emission computed tomography (SPECT)/CT Dual Head System (Siemens, Munich, Germany) between 8:00 a.m. and 9:00 a.m. Subjects were orally treated with potassium perchlorate (400 mg), and with their heads in a head holder, were instructed to keep their eyes closed for 30minutes. Subjects were then injected with 740 MBq (20 mCi)99mTc- ethyl cysteinate dimer (99mTc-ECD, HTA Co., Ltd., Beijing, China). Fifteen minutes after injection, SPECT/CT data were acquired using a 128 × 128 matrix with a zoom of 1.23 and 15% symmetric energy window at 140 keV. Images were acquired at a rate of 35 s/frame by rotating the camera a total of 360° at 6° intervals. In total, 36 frames were collected. Data were analyzed using CBF perfusion imaging software (Brain Tomo PROC; Siemens) by two experienced nuclear medicine physicians that were blinded to the patients’ clinical data. Relative rCBF (rrCBF) values in regions of interest were calculated by: average rCBF value in region of interest/average rCBF value in ipsilateral cerebellar cortex.

Figure 1 Study design.

Table 1 Participants demographic and clinical characteristics

Table 2 APOE rs429358 and rs7412 polymorphisms and PSD risk
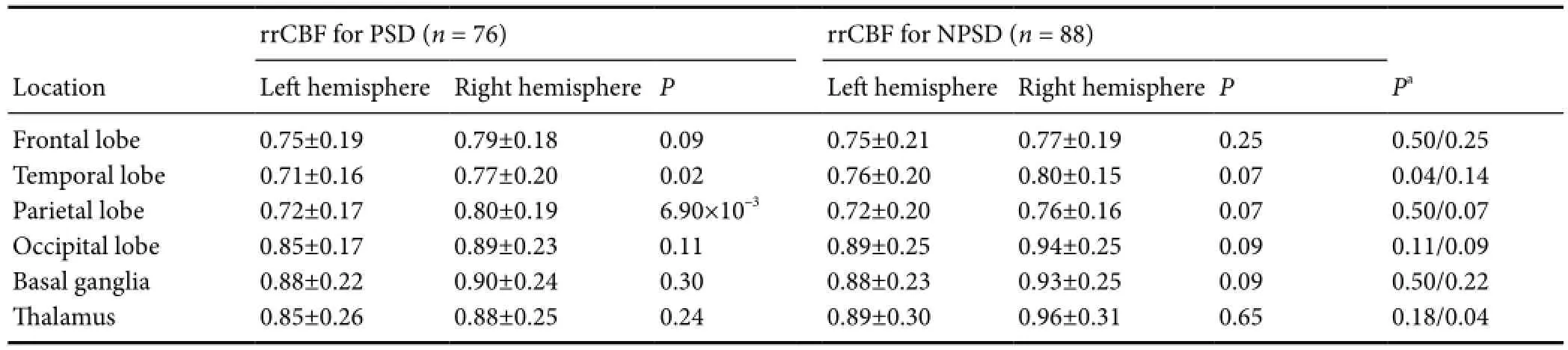
Table 4 rrCBF in PSD and NPSD cases

Table 5 Effect of APOE rs429358 polymorphism on rrCBF in PSD cases
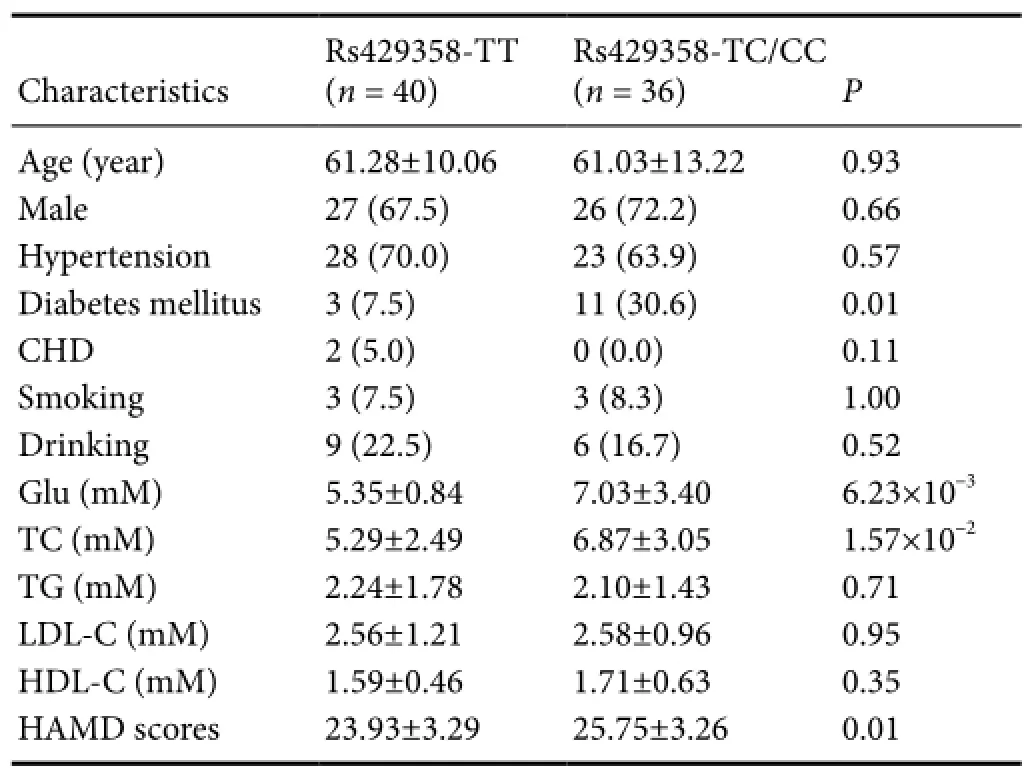
Table 3 Association between APOE rs429358 polymorphism and clinical characteristics of PSD cases
APOEgenotyping
Genomic DNA was extracted from peripheral blood leukocytes.APOErs429358 and rs7412 genotypes were determined by TaqMan polymerase chain reaction (TaqMan-PCR) using an iCycler iQ real-time PCR detection system (iQ5; Bio-Rad, Hercules, CA, USA). Each PCR was performed in 25 μL total volumes consisting of 1 × TaqMAN®Universal Master Mix II (cat#4440041; Applied Biosystems [ABI], Foster City, CA, USA), 0.2 μM each probe and each primer (cat# 4351379; ABI), and 50–100 ng genomic DNA. PCR programs had an initial denaturation of 10 minutes at 95°C, followed by 40 cycles of 15 seconds at 95°C and 1 minute at 60°C. Data analysis for allele discrimination was performed using iCycler iQ software (Bio-Rad). Controls were included in each run for quality control, and repeated genotyping of random 5% subsets yielded 100% identical genotypes. Additionally, sequencing analyses performed using the ABI 3730XL DNA Sequencing System with universal primers (5′-GGCGC GGACA TGGAG GAC-3′ and 5′-GCCCC GGCCT GGTAC ACT-3′) for both polymorphisms yielded 100% identical genotypes.
Statistical analysis
Results were analyzed using SPSS 18.0 software (SPSS Institute, Chicago, IL, USA). Comparison of variables among groups was performed using Student’st-test, one-way analysis of variance, chi-square test, or Fisher’s exact test. Spearmanrtest was used to analyze correlation between genotypes and other variables. Using a frequently matched design, non-conditional logistical regression was performed to estimate odds ratios (OR) and 95% confidence intervals (CI) for PSD risk.Statistical significance was considered atα= 0.05.
Results
Characteristics of subjects
One hundred and sixty-four patients (113 males and 51 females; mean age 60.8 ± 11.9 years) completed the 6-month follow-up. Overall, 76 patients were ultimately diagnosed as PSD cases, producing an incidence of 46.3%. The remaining 88 cases were included as NPSD. Distribution of demographic and clinical characteristics (including age, sex, smoking and drinking status, and coronary heart disease, hypertension, and diabetes mellitus history) was not significantly different between PSD and NPSD cases (Table 1). However, compared with controls without cerebral infarction, cerebral infarction cases had a higher frequency of risk factors including hypertension, diabetes mellitus, and blood glucose (Table 1).
APOErs429358 polymorphism increases PSD risk
Analysis ofAPOErs429358 and rs7412 polymorphisms in control individuals showed a genotype distribution consistent with Hardy-Weinberg equilibrium (P> 0.05). Meanwhile, logistic regression analysis indicated that only the rs429358 polymorphism significantly correlated with PSD risk (Table 2). Compared with rs429358-T allele homozygotes (rs429358-TT), rs429358-T and -C allele heterozygotes (rs429358-TC) had a higher risk of PSD (adjustedOR[95%CI], 2.93 [1.50–5.72] for PSDversusNPSD; 3.31 [1.27–8.64] for PSD versus controls; and 3.17 [1.42–7.05] for PSDversuscombined NPSD and control groups). The correspondingOR(95%CI) for rs429358-C allele homozygotes (rs429358-CC) were 9.80 (1.95–49.24), 11.98 (1.60–89.44), and 11.24 (2.98–42.40), respectively. Altogether, these results show that PSD risk is associated with the number of rs429358-C alleles.
Indeed, a higher frequency of rs429358-C alleles was found in cerebral infarction cases compared with healthy controls (18.1%versus11.9%). Logistic regression analysis showed that the risk of rs429358-C alleles for brain infarction was 1.86 (1.14–3.04). However, this risk was not significant after adjusting for other risk variables (including smoking and drinking, and hypertension, heart disease, and diabetes mellitus history) (P> 0.05;Table 2).
APOErs429358 polymorphism correlates with clinical features of PSD cases
Next, we analyzed the effect ofAPOErs429358 polymorphism on clinical features of PSD cases (Table 3). We found rs429358 was significantly related to increasing levels of serum glucose (rs429358-TC/CCversusrs429358-TT, 7.03 ± 3.40versus5.35 ± 0.84 mM; relative coefficient, 0.24), and also total cholesterol (6.87 ± 3.05versus5.29 ± 2.49 mM; relative coefficient, 0.37). Moreover, rs429358 affected the degree of depression for PSD cases (P= 0.01).
Effect ofAPOErs429358 polymorphism on rCBF in PSD cases
Previous studies have shown that rCBF positively correlates with brain function and depression (Paschali et al., 2009, 2010; Cantisani et al., 2015; Simpson et al., 2016). Therefore, to determine whether theAPOErs429358 polymorphism affects rCBF, we examined rCBF in regions of interest (including the frontal lobe, temporal lobe, parietal lobe, occipital lobe, basal ganglia, and thalamus) in all PSD and NPSD cases (Tables 4and5). Our results show lower rrCBF in the left temporal and parietal lobes compared with the corresponding right lobes among PSD cases (0.71versus0.77 for temporal lobe and 0.72versus0.80 for parietal lobe;P< 0.05), but not among NPSD cases. Furthermore, comparative analysis showed significantly decreasing rrCBF in the left temporal lobe of PSD and NPSD cases (P= 0.04) (Table 4).
To further investigate the relationship betweenAPOErs429358 polymorphism and rCBF, we performed a stratified analysis (Table 5). Because of the small number of subjects withAPOErs429358-CC, genotypes, rs429358-CC and rs429358-TC were combined into a single group (i.e., rs429358-TC/CC), withAPOEgenotypes then divided into two strata. In PSD cases withAPOErs429358-TC/CC genotypes, decreasing rrCBF was still observed in the left temporal and parietal lobes, but not in those with rs429358-TT genotypes. Comparative analysis of cases with and withoutAPOErs429358-TC/CC showed that rs429358 polymorphism is significantly associated with rrCBF in the left temporal lobe (0.74 ± 0.15 for rs429358-TT and 0.67 ± 0.16 for rs429358-TC/CC,r= –0.26). Taken together, these findings suggest that theAPOErs429358 polymorphism may modify brain function.
Discussion
To date, no studies have examined the role ofAPOEpolymorphism in PSD risk. Here, we examined association betweenAPOEpolymorphisms and PSD risk among the Guangxi population of China. Accordingly, we found thatAPOEgenotypes with rs429358-C alleles are associated with increased PSD risk among this population (OR, 3.17 for rs429358-TC; 11.24, for rs429358-CC).
Stroke, such as cerebral infarction, is one of the leading causes of morbidity and long-term disability worldwide (Robinson and Jorge, 2015). Epidemiological studies have shown that approximately 40% of stroke patients develop PSD after acute stroke, and thus PSD is a common and serious psychiatric complication of stroke (Robinson and Jorge, 2015). Compared with stroke patients without depression, PSD typically leaves patients with severer deficits in daily living activities, a worsen functional outcome, severer cognitive deficits, and higher mortality (Fang and Cheng, 2009; Dwyer Hollender, 2014; Eum and Yim, 2015; Robinson and Jorge, 2015). In this study, approximately 46% of our cerebral infarction cases had depression and ultimately developed PSD. Furthermore, we found that PSD cases had higher levels of serum total cholesterol compared with NPSD cases, and that increasing serum total cholesterol levels increased both PSD and NPSD risk. These results suggest a strong biological relationship between PSD and stroke.
APOEis an important apolipoprotein gene located onchromosome 19q13.2. The protein encoded byAPOEconsists of 299 amino acid residues (relative molecular weight, 34 kDa), and contains amphipathic α-helical lipid-binding structural domains that enableAPOEto interact with members of the low-density lipoprotein receptor family. Through this interaction,APOEdisplays a key role in lipid transport in both plasma and the central nervous system (Hatters et al., 2006). Accumulating evidence shows that dysregulation ofAPOEexpression and genetic variance ofAPOEaffectsAPOEfunction, and eventually leads to pathogenesis of certain nervous and cardiovascular diseases (Hatters et al., 2006; Cosentino et al., 2008; Jofre-Monseny et al., 2008; Lagos et al., 2015; Luckhoff et al., 2015; Yuan et al., 2015; Lu et al., 2016).
With regards genetic variance, more than 5,000 single-nucleotide polymorphisms inAPOEhave been identified. Two of these (namely rs429358 and rs7412) influence functional and structural properties of theAPOEprotein, and are associated with development of neurodegenerative disease (Cosentino et al., 2008; Agarwal and Tripathi, 2014; Giau et al., 2015; Luckhoff et al., 2015), coronary heart disease (Chouinard-Watkins and Plourde, 2014; Yousuf and Iqbal, 2015), and liver disease (Shen et al., 2015). For example, Cosentino et al. (2008) investigated the effect ofAPOEpolymorphisms on Alzheimer’s disease development, and found that the ε4 allele (which is derived from a combination of rs429358 and rs7412) is a risk biomarker for Alzheimer’s disease.APOErs429358 and rs7412 polymorphisms also affect antioxidant and anti-inflammatory properties ofAPOE, and the ε4 allele is associated with relatively higher oxidative stress and a higher pro-inflammatory state (Jofre-Monseny et al., 2008; Yuan et al., 2015). In this study, theAPOErs429358 polymorphism was associated not only with increased PSD risk, but also more severe depression in PSD cases. Interestingly, our results also show that patients with more severe deficits are more likely to develop PSD. Supporting our results, several recent reports have shown that differential functional status ofAPOE(resulting from genetic polymorphic status and expression levels) is related to cognitive performance (Zhang et al., 2012; Rajan et al., 2014; Wang et al., 2014; Feng et al., 2015) and PSD pathogenesis (Zhang et al., 2013).
To investigate the effect ofAPOErs429358 polymorphism on PSD risk, rCBF in stroke cases was examined. This was mainly performed as rCBF is a valuable marker for brain function, including neural regeneration and activity (Venkat et al., 2016), therefore any disruption in rCBF may affect neural repair after stoke and impact upon PSD pathogenesis. Recent evidence has shown that rCBF significantly correlates with depressive disorder, and may reflect pathological-psychological change in PSD (Cantisani et al., 2015). Here, we found a decreasing rCBF score in the left temporal lobe of PSD cases that was modified byAPOErs429358 polymorphism. Additionally, rs429358 was associated with increasing serum glucose and total cholesterol levels. Consistent with these results, a recent meta-analysis found that theAPOErs429358 polymorphism is associated with lipid profile changes and increased serum total cholesterol levels (Lu et al., 2016). Collectively, these results suggest that rs429358 may modulate PSD risk by modifying brain function and lipid profiles. Hence,APOEscreening may be helpful for identifying PSD patients from those with abnormal brain function, or glucose and lipid profiles.
There are several limitations to our study. First, despite analyzing two polymorphisms, we did not examine any otherAPOEpolymorphisms. Second, a potential selection bias may have occurred through selection of hospital-based control subjects. Third, the risk value of the rs429358 polymorphism may be underestimated because stroke risk is slightly associated with this polymorphism, and stroke as a variable might be a potential confounding factor. Fourth, our findings are based on a relatively small number of subjects, and limited further by the small number of stratified subjects. Finally, although we investigated the effect ofAPOErs429358 polymorphism on clinical features, with rCBF as a brain function marker of PSD, the underlying mechanism(s) remain largely unknown. Therefore, additional functional analyses based on larger samples and combiningAPOEand brain function (including neural regeneration and repair) are required.
In conclusion, this is the first report to investigate association betweenAPOEpolymorphisms and PSD risk in the Guangxi population of China. Further, our findings provide evidence that the rs429358 polymorphism ofAPOEis essential to PSD etiology. Similarly, our findings support the hypothesis thatAPOEpolymorphisms contribute to PSD risk. Understanding different genetic and clinical values in individual patients will allow more informed counseling with regards screening, prevention, treatment options, follow-up plans, and secondary prevention approaches. Therefore, in combination with information on functional neural regeneration during stoke, our findings may improve identification of high-risk populations of PSD.
Declaration of patient consent:The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/ have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Author contributions:XBL and ADX conceived and designed the study. JMH, LQM, and RYH performed the experiments. XBL, JW, and ADX analyzed the data. JW and JLW provided materials. XBL wrote the paper. All authors approved the final version of the paper.
Conflicts of interest:None declared.
Plagiarism check:This paper was screened twice using CrossCheck to verify originality before publication.
Peer review:This paper was double-blinded and stringently reviewed by international expert reviewers.
Agarwal R, Tripathi CB (2014) Association of apolipoprotein E genetic variation in Alzheimer’s disease in Indian population: a meta-analysis. Am J Alzheimers Dis Other Demen 29:575-582.
Cantisani A, Koenig T, Stegmayer K, Federspiel A, Horn H, Müller TJ, Wiest R, Strik W, Walther S (2015) EEG marker of inhibitory brain activity correlates with resting-state cerebral blood flow in the reward system in major depression. Eur Arch Psychiatry Clin Neurosci doi:10.1007/s00406-015-0652-7.
Chouinard-Watkins R, Plourde M (2014) Fatty acid metabolism in carriers of apolipoprotein E epsilon 4 allele: is it contributing to higher risk of cognitive decline and coronary heart disease? Nutrients 6:4452-4471.
Cosentino S, Scarmeas N, Helzner E, Glymour MM, Brandt J, Albert M, Blacker D, Stern Y (2008) APOE ε4 allele predicts faster cognitive decline in mild Alzheimer’s disease. Neurology 70:1842-1849.
Dwyer Hollender K (2014) Screening, diagnosis, and treatment of poststroke depression. J Neurosci Nurs 46:135-141.
Eum Y, Yim J (2015) Literature and art therapy in post-stroke psychological disorders. Tohoku J Exp Med 235:17-23.
Fang J, Cheng Q (2009) Etiological mechanisms of post-stroke depression: a review. Neurol Res 31:904-909.
Feng F, Lu SS, Hu CY, Gong FF, Qian ZZ, Yang HY, Wu YL, Zhao YY, Bi P, Sun YH (2015) Association between apolipoprotein E gene polymorphism and depression. J Clin Neurosci 22:1232-1238.
Giau VV, Bagyinszky E, An SS, Kim SY (2015) Role of apolipoprotein E in neurodegenerative diseases. Neuropsychiatr Dis Treat 11:1723-1737.
Hatters DM, Peters-Libeu CA, Weisgraber KH (2006) Apolipoprotein E structure: insights into function. Trends Biochem Sci 31:445-454.
洋桔梗易感染土生病害,可采用噁霉灵+福美双熏蒸消毒法对土壤进行处理,具体方法为:先进行旋耕整地,将70%噁霉灵、50%福美双可湿性粉剂分别按30 kg·hm-2、100 kg·hm-2混合拌匀,然后盖上塑料薄膜进行熏闷,7 d后揭膜待药味散尽即可。另深翻暴晒、放水泡地等方法也简便可行。
Jofre-Monseny L, Minihane AM, Rimbach G (2008) Impact of apoE genotype on oxidative stress, inflammation and disease risk. Mol Nutr Food Res 52:131-145.
Kessler RC, Üstün TB (2004) The World Mental Health (WMH) Survey Initiative Version of the World Health Organization (WHO) Composite International Diagnostic Interview (CIDI). Int J Methods Psychiatr Res 13:93-121.
Lagos J, Zambrano T, Rosales A, Salazar LA (2015) APOE polymorphisms contribute to reduced atorvastatin response in Chilean Amerindian subjects. Int J Mol Sci 16:7890-7899.
Lu Z, Wu X, Jin X, Peng F, Lin J (2016) Apolipoprotein E ε2/ε3/ε4 variant in association with obstructive sleep apnoea and lipid profile: A meta-analysis. J Int Med Res 44:3-14.
Luckhoff HK, Brand T, van Velden DP, Kidd M, Fisher LR, van Rensburg SJ, Kotze MJ (2015) Clinical relevance of apolipoprotein E genotyping based on a family history of Alzheimer’s disease. Curr Alzheimer Res 12:210-217.
McGuinness B, Carson R, Barrett SL, Craig D, Passmore AP (2010) Apolipoprotein epsilon4 and neuropsychological performance in Alzheimer’s disease and vascular dementia. Neurosci Lett 483:62-66.
Ning G (1986) A application of Hamilton anxiety scale in Chinese neurasthenia. Zhonghua Shenjing Jingshen Ke Zazhi 32:342-344.
Paschali A, Messinis L, Lyros E, Constantoyannis C, Kefalopoulou Z, Lakiotis V, Papathanasopoulos P, Vassilakos P (2009) Neuropsychological functions and rCBF SPECT in Parkinson’s disease patients considered candidates for deep brain stimulation. Eur J Nucl Med Mol Imaging 36:1851-1858.
Paschali A, Messinis L, Kargiotis O, Lakiotis V, Kefalopoulou Z, Constantoyannis C, Papathanasopoulos P, Vassilakos P (2010) SPECT neuroimaging and neuropsychological functions in different stages of Parkinson’s disease. Eur J Nucl Med Mol Imaging 37:1128-1140.
Robinson RG, Jorge RE (2015) Post-stroke depression: A review. Am J Psychiatry 173:221-231.
Seripa D, D’Onofrio G, Panza F, Cascavilla L, Masullo C, Pilotto A (2011) The genetics of the human APOE polymorphism. Rejuvenation Res 14:491-500.
Shen Y, li M, Ye X, Bi Q (2015) Association of apolipoprotein E with the progression of hepatitis B virus-related liver disease. Int J Clin Exp Pathol 8:14749-14756.
Simpson BN, Kim M, Chuang Y-F, Beason-Held L, Kitner-Triolo M, Kraut M, Lirette ST, Windham BG, Griswold ME, Legido-Quigley C, Thambisetty M (2016) Blood metabolite markers of cognitive performance and brain function in aging. J Cereb Blood Flow Metab 36:1212-1223.
Tang WK, Tang N, Liao CD, Liang HJ, Mok VC, Ungvari GS, Wong KS (2013) Serotonin receptor 2C gene polymorphism associated with post-stroke depression in Chinese patients. Genet Mol Res 12:1546-1553.
Venkat P, Chopp M, Chen J (2016) New insights into coupling and uncoupling of cerebral blood flow and metabolism in the brain. Croat Med J 57:223-228.
Wang Z, Ma W, Rong Y, Liu L (2014) The association between apolipoprotein E gene polymorphism and mild cognitive impairment among different ethnic minority groups in China. Int J Alzheimers Dis 2014:150628.
Weaver J, Liu KJ (2015) Does normobaric hyperoxia increase oxidative stress in acute ischemic stroke? A critical review of the literature. Med Gas Res 5:11.
Wu X (1997) The fourth Chinese conference proceedings about cerebrovascular diseases. Cuzhong he Naoxueguan Jibing 4:51-55.
Yi L, Wu T, Luo W, Zhou W, Wu J (2014) A non-invasive, rapid method to genotype late-onset Alzheimer’s disease-related apolipoprotein E gene polymorphisms. Neural Regen Res 9:69-75.
Yousuf FA, Iqbal MP (2015) Review: Apolipoprotein E (Apo E) gene polymorphism and coronary heart disease in Asian populations. Pak J Pharm Sci 28:1439-1444.
Yuan L, Liu J, Dong L, Cai C, Wang S, Wang B, Xiao R (2015) Effects of APOE rs429358, rs7412 and GSTM1/GSTT1 polymorphism on plasma and erythrocyte antioxidant parameters and cognition in old Chinese adults. Nutrients 7:8261-8273.
Zhang J, Shen XH, Qian MC, Sun JS, Zhong H, Yang JH, Lin M, Li L (2012) Effects of apolipoprotein E genetic polymorphism on susceptibility of depression and efficacy of antidepressants. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 34:595-600.
Zhang Z, Mu J, Li J, Li W, Song J (2013) Aberrant apolipoprotein E expression and cognitive dysfunction in patients with poststroke depression. Genet Test Mol Biomarkers 17:47-51.
Zhao Q, Guo Y, Yang D, Yang T, Meng X (2016) Serotonin Transporter gene 5-httlpr polymorphism as a protective factor against the progression of post-stroke depression. Mol Neurobiol 53:1699-1705.
Zhao Y, Li J, Tang Q, Gao J, Chen C, Jing L, Zhang P, Li S (2014) Apolipoprotein E mimetic peptide protects against diffuse brain injury. Neural Regen Res 9:463-473.
Zhou Z, Ding X, Yang Q, Hu J, Shang X, Huang X, Ge L, Zhou T (2015) Association between single-nucleotide polymorphisms of the tyrosine kinase receptor B (TrkB) and post-stroke depression in China. PLoS One 10:e0144301.
Copyedited by James R, Yajima W, Yu J, Li CH, Qiu Y, Song LP, Zhao M
*Correspondence to: Xue-bin Li, M.D. or An-ding Xu, Ph.D., yyfylxb@163.com or tlil@jnu.edu.cn.
#These authors contributed equally to this study.
orcid: 0000-0003-3154-0985 (An-ding Xu)
10.4103/1673-5374.194748
Accepted: 2016-09-28
猜你喜欢
杂志排行
中国神经再生研究(英文版)的其它文章
- Cortical spreading depression-induced preconditioning in the brain
- Nerve growth factor protects against palmitic acidinduced injury in retinal ganglion cells
- Tissue-engineered rhesus monkey nerve grafts for the repair of long ulnar nerve defects: similar outcomes to autologous nerve grafts
- HLA class II alleles and risk for peripheral neuropathy in type 2 diabetes patients
- Rab27a/Slp2-a complex is involved in Schwann cell myelination
- Key genes expressed in different stages of spinal cord ischemia/reperfusion injury