Enhancement of The Photovoltaic Performance of CH3NH3PbI3 Perovskite Solar Cells by Using Polyvinylpyrrolidone Additive
2016-02-05LIJianfengZHAOChuangZHANGHengTONGJunfengZHANGPengYANGChunyanXIAYangjun
LI Jian-feng, ZHAO Chuang, ZHANG Heng, TONG Jun-feng, ZHANG Peng, YANG Chun-yan, XIA Yang-jun
(Key Lab of Optoelectronic Technology and Intelligent Control of Education Ministry, Lanzhou Jiaotong University, Lanzhou 730070, China)*Corresponding Author, E-mail: xiayangjun2015@126.com
Enhancement of The Photovoltaic Performance of CH3NH3PbI3Perovskite Solar Cells by Using Polyvinylpyrrolidone Additive
LI Jian-feng, ZHAO Chuang, ZHANG Heng, TONG Jun-feng, ZHANG Peng, YANG Chun-yan, XIA Yang-jun*
(KeyLabofOptoelectronicTechnologyandIntelligentControlofEducationMinistry,LanzhouJiaotongUniversity,Lanzhou730070,China)*CorrespondingAuthor,E-mail:xiayangjun2015@126.com
We demonstrate a new one-step solution approach to prepare perovskite CH3NH3PbI3films by adding polyvinylpyrrolidone (PVP) to the standard CH3NH3PbI3precursor solution. The film morphologies, crystallinities, and optical properties of CH3NH3PbI3perovskite layers are carefully studied by SEM, XRD, and UV-Vis. The results reveal that the perovskite film properties can be manipulated by incorporating a small amount of PVP. The absorbance of the film with PVP additive is significantly higher than the pristine film and the absorption peak is red shift by 20 nm, indicating the perovskite film with additive possessing better crystal structures. The use of PVP strongly affects the crystallization process of forming pure CH3NH3PbI3and helps the formation of smooth film, leading not only to enhanced crystallization of CH3NH3PbI3but also to significantly improved coverage of CH3NH3PbI3on a planar substrate. The optimized power conversion efficiency (PCE) of CH3NH3PbI3solar cells improved from 1.30% to 8.38% for the planar cell structure after the addition of 1% mass fraction of PVP. These results suggest that this new one-step solution approach is promising for controlling CH3NH3PbI3growth to achieve high-performance perovskite solar cells.
perovskite solar cells; polyvinylpyrrolidone; additive
1 Introduction
Organic-inorganic hybrid perovskite materials have gained much attention in the past years because of their excellent optical properties that are tunable by managing chemical compositions[1], ambipolar charge transport[2], and very long electron-hole diffusion lengths[3-4]. Organo-lead iodide perovskites such as CH3NH3PbI3have been applied as light absorbers in solid state mesoscopic solar cells where they were found to act not only as a light harvester, but also as an electron and hole conductor. The ambipolar semiconducting nature of perovskite enabled the feasible construction of planar device architectures, which can be fabricated at low temperature. It is fully compatible with roll-to-roll process for large area and flexible solar cells fabrication. Guoetal.[5]first reported the planar heterojunction perovskite solar cells, in which PEDOT∶PSS was used as the hole transporter and fullerene derivatives as the electron transporter. Seoketal.[6]reported a planar heterojunction solar cell with an optimized electron transporting layer, which was composed of a layer of PCBM and a very thin layer of LiF. The device efficiency was improved to 14.1%.
At present, one of the main challenges of planar heterojunction perovskite solar cells encountered in perovskite thin film fabrication is the control of the crystallization process and its impact on film quality. Poor perovskite morphology has been cited as very detrimental to device performance because it not only causes electrical shorting but also deleteriously impacts charge dissociation/transport/recombination[7-10]. Because of the sensitive dependence of growth kinetics on interfacial energy, solution concentration, precursor composition, solvent choice, additive used, and deposition temperature, improving perovskite morphology and coverage through controlling crystallization during film deposition and annealing is an attractive route to device optimization. It is possible to achieve optimal perovskite film morphology by finding effective ways to manipulate its nucleation and growth. Jenetal.[11]utilized 1% (mass fraction) of 1,8-diiodooctance (DIO) as an additive in the CH3NH3PbI3-xClxthin film with the planar structure to facilitate homogeneous nucleation and improve the coverage of perovskite film. Also, Chenetal.[12]added 1-chloronaphthalene (CN) in the precursor solution can effectively regulate the crystallization of perovskite and form more homogenous films with fewer pin-holes and voids. Besides, Suetal.[13]first reported utilizing polymer as an additive to control the morphology of the perovskite layer, and successfully enhanced the PCE of the n-i-p planar heterojuntion perovskite solar cell with FTO/TiO2/CH3NH3PbI3-xClx/spiro-OMe-TAD/Au structure from 10.58% to 13.20% by adding 1% (mass fraction) of poly(ethylene glycol) in the perovskite film.
Here, we report a simple and very effective method to control the perovskite growth and film- forming properties by introducing polyvinylpyrrolidone (PVP) additives to the standard CH3NH3PbI3precursor solution to improve the crystallinity and morphology of perovskite layers, and the performance of solar cells. The effects of PVP additive on the crystallinity and morphology of perovskite layers were studied by XRD and SEM. The PVP additive helped to form flawless perovskite nanocrystals and also helped to enhance the CH3NH3PbI3coverage on planar substrates, leading to significant improvement of device performance. An one-step solution-processed device with the structure of ITO/PEDOT∶PSS/CH3NH3PbI3/PC61BM/Al was studied. The device fabricated using PVP as an additive gave a PCE around 8.38%.This research provides a facile way to fabricate a high efficiency perovskite solar cell by the low temperature solution process (<160 ℃), it is fully compatible with roll-to-roll process for large area and flexible solar cells fabrication.
2 Experiments
The methylammonium iodide (MAI) was synthesized according to the report of Kimetal.[14]. The CH3NH3PbI3perovskite precursor solution was prepared by mixing as-synthesized MAI powder and PbI2(99.999%, Aldrich) at 40% (mass fraction) in 1 mL DMF with appropriate mass fractions of PVP (0%, 0.5%, 1%, 3%, and 5%) and stirring continuously overnight at 60 ℃ in the dark. The perovskite solar cells were fabricated with a configuration of indium tin oxide (ITO)/PEDOT∶PSS/CH3NH3PbI3/PC61BM/Al (Fig.1). Patterned indium tin oxide(ITO)-coated glass with a sheet resistance of 15-20 Ω/□ were cleaned by a surfactant scrub, then underwent a wet-cleaning process inside an ultrasonic bath, beginning with deionized water, followed by acetone and isopropanol. After oxygen plasma cleaning for 14 min, a 40 nm thick poly(3,4-ethylene- dioxythiophene)∶poly(styrene sulfonate) (PEDOT∶PSS) (Bayer Baytron 4083) anode buffer layer was spin-casted onto the ITO substrate and then dried at 160 ℃ for 30 min. Prior to deposition of the CH3NH3PbI3layer, The precursor solutions containing different amounts of PVP additiv were preheated at 80 ℃ for 20 min and then deposited on top of the PEDOT∶PSS coated ITO substrates by spin-coating and followed by thermal annealing in nitrogen at 100 ℃ for 20 min. PC61BM was spin-coated from a chlorobenzene solution (15 mg·mL-1). Finally, a 100 nm Al layer was evaporated with a shadow mask in sequence in a vacuum chamber at a pressure of 6×10-5Pa. The overlapping area between the cathode and anode defined a pixel size of 0.1 cm2. The power conversion efficiencies (PCEs) of the resulting perovskite solar cells were measured under 1 sun, AM 1.5G (Air mass 1.5 global) condition using a solar simulator (XES-70S1, San-EI Electric Co.) with irradiation of 100 mW·cm-2. The current density-voltage(J-V) characteristics were recorded with a Keithley 2400 source-measurement unit. The spectral responses of the devices were measured with a commercial EQE/incident photon to charge carrier efficiency (IPCE) setup (7-SCSpec Ⅲ, Bejing 7-star Opt. In. Co.).
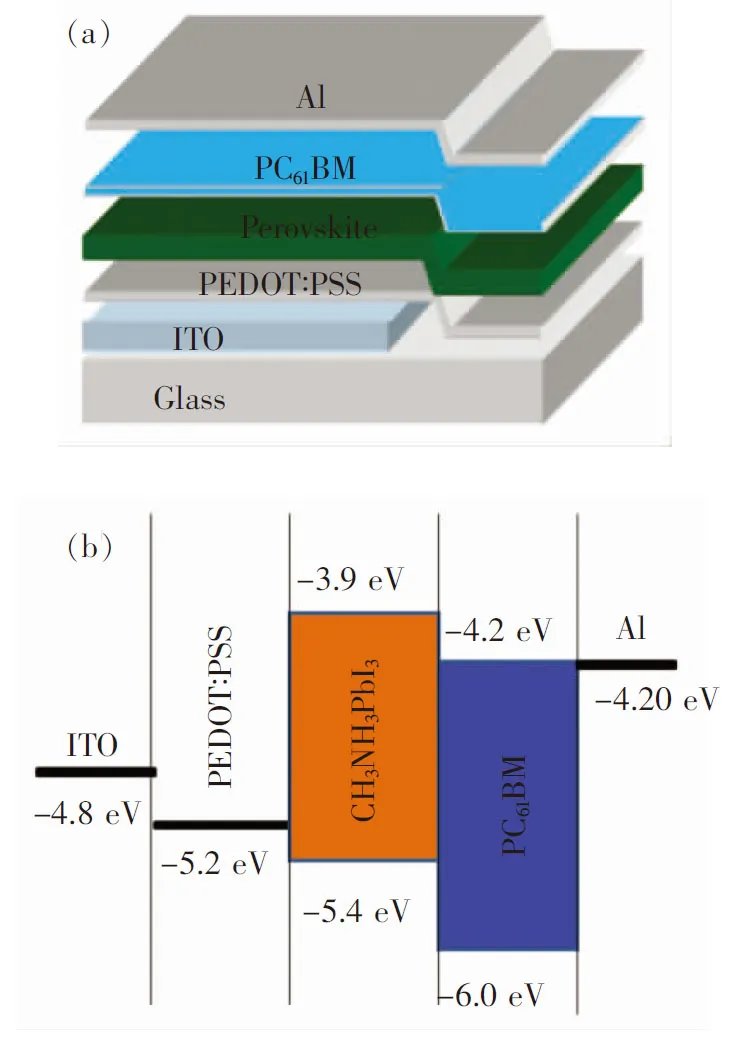
Fig.1 Device configuration(a) and energy-level diagram of the materials (b)
3 Results and Discussion
To study the effects of PVP additive on the morphology, absorption, and crystallization of CH3NH3PbI3films, we fabricated such films under the same conditions as those described above, but with different mass fractions (0%, 0.5%, 1%, 3%, and 5%) of PVP in the precursor solution. We first investigated the effect of additives on light absorption and crystallization of the CH3NH3PbI3film through UV-Vis spectra and X-ray diffraction (XRD). All the films for absorption and XRD measurements were prepared under the same conditions except the difference of additives in perovskite precursor solution (in DMF). The film thickness isca. 180 nm. As shown in Fig. 2, all films show absorption onsets atca. 780 nm. Films prepared using PVP additive exhibit an absorption peak atca. 385 nm and a shoulder atca. 477 nm. The shape of the UV-Vis spectra is similar to the reported results[15]. The red shift of the main absorption band and the larger absorbance of film with PVP indicated that the perovskite film with PVP possesses better crystal structures[16]. The more intense absorption of the films with additive should also be partially attributed to the more homogenous morphology and better surface coverage, which were supported by SEM images of the film.
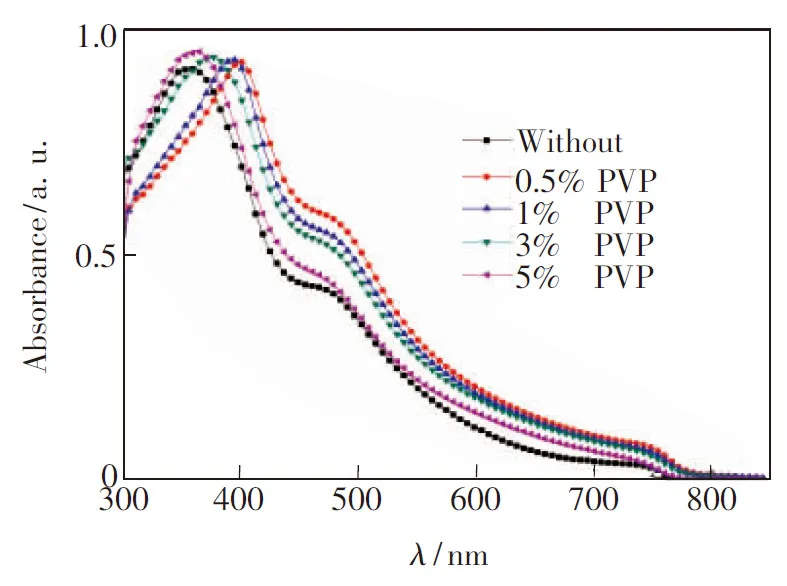
Fig.2 UV-Vis absorption spectra of CH3NH3PbI3films processed with different mass fraction of PVP
The XRD peak positions for all CH3NH3PbI3films on PEDOT∶PSS coated glass substrates are nearly the same (Fig.3). The diffraction peaks of the CH3NH3PbI3film at 14.28°, 28.62°, and 43.42° can be assigned to (110), (220), and (330) planes, respectively[17]. However, the diffraction intensities for CH3NH3PbI3films prepared using different additives content are quite different. The diffraction peaks of the film prepared using no additive are very weak, suggesting that the additives favor CH3NH3PbI3crystallization is more effective. Strong diffraction peaks for a thin CH3NH3PbI3film (180 nm) prepared using the PVP additive indicate that CH3NH3PbI3is highly crystalline.
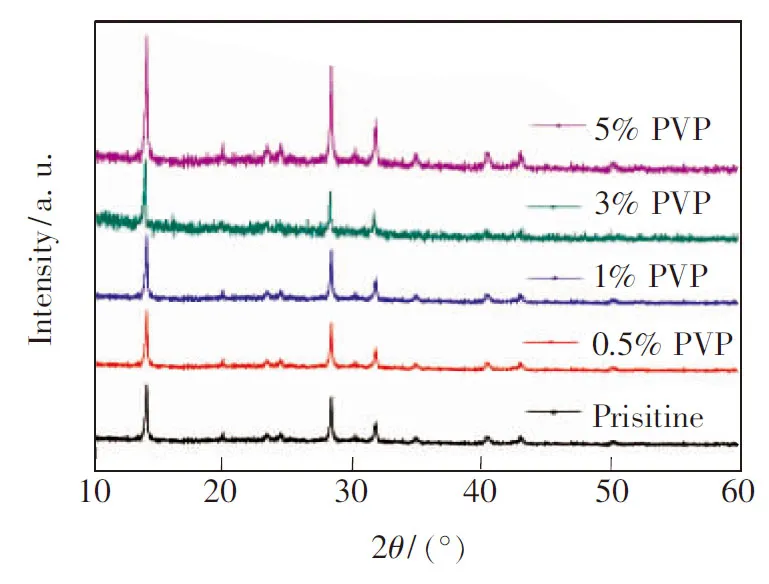
Fig.3 XRD patterns of CH3NH3PbI3films processed with different mass fraction of PVP
Perovskitefilm morphology and surface coverage are crucial to the performance of planar heterojunction perovskite solar cells[18]. So the scanning electron microscopy (SEM) was employed to observe the changes of surface morphology and coverage of the perovskite layer by varying the amount of PVP. Fig.4 shows SEM images of CH3NH3PbI3films prepared using different additives. For the pristine perovskite film prepared using no additive, the crystals grew and aggregated into large domains with large voids and poor coverage. Areas without perovskite coverage can be clearly seen as shown in Fig.4(a). On the other hand, the film containing 0.5% PVP, a continuous film with decreased size of domains and voids is observed. The coverage of the perovskite film was significantly improved. when the mass fraction of PVP was increased to 3%, nearly no voids can be seen in the film. This phenomenon is more obvious for the film with 5% PVP. We speculate that the PVP additive helps the spread of precursor solution on the substrate and retards the growth and aggregation of perovskite crystal to have a continuous film with adequate domain sizes. The residual PVP would fill in the space between the perovskite grains.
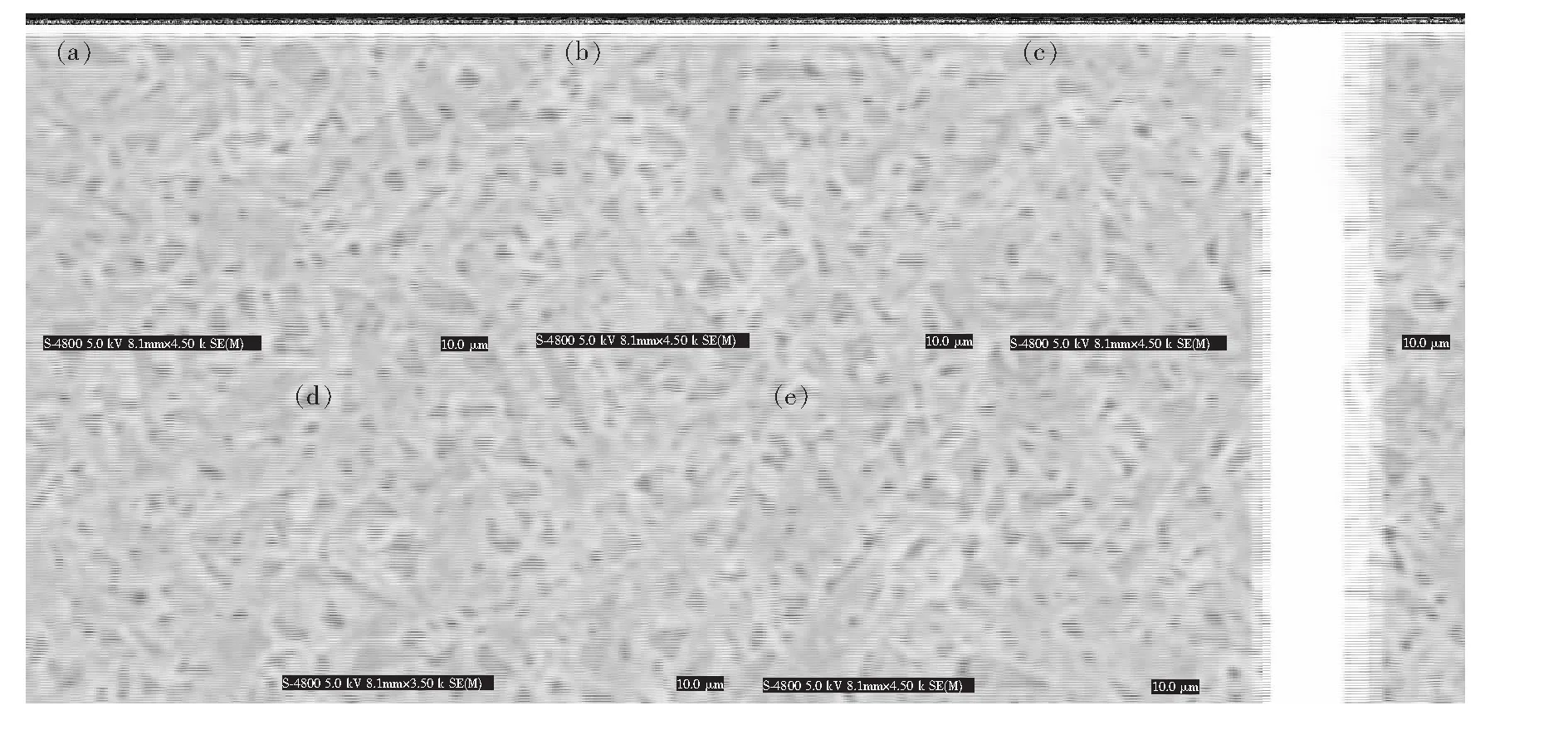
Fig.4 SEM images of perovskite films with different mass fractions of PVP additive. (a) Pristine. (b) 0.5%. (c) 1%. (d) 3%. (e) 5%.
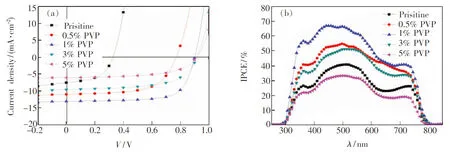
Fig.5 J-V (a) and IPCE (b) curves of the devices with different mass fractions of PVP additive
Tab.1 Characteristics of perovskite solar cells with different mass fractions of PVP additive
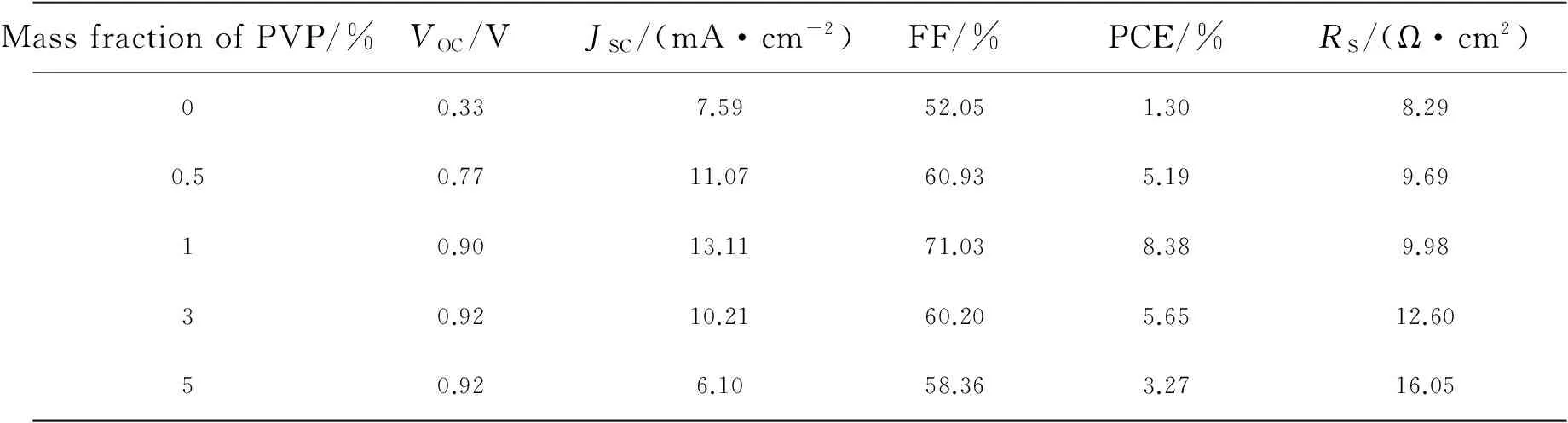
MassfractionofPVP/%VOC/VJSC/(mA·cm-2)FF/%PCE/%RS/(Ω·cm2)00.337.5952.051.308.290.50.7711.0760.935.199.6910.9013.1171.038.389.9830.9210.2160.205.6512.6050.926.1058.363.2716.05
Solar cells with structure ITO/PEDOT∶PSS/CH3NH3PbI3/PC61BM/Al were fabricated to investigate the effect of additives on photovoltaic performance. The current density-voltage curves of the devices are shown in Fig.5(a), and their performance parameters are summarized in Tab.1. The device fabricated using no additive exhibited an open-circuit voltage (VOC) of 0.33 V, a short-circuit current density (JSC) of 7.59 mA·cm-2, a fill factor (FF) of 52.05% and a power conversion efficiency (PCE) of 1.30%. The performance of the device fabricated using no additive being inconsistent with previous reports is due to the thickness difference between CH3NH3PbI3films. The CH3NH3PbI3film fabricated in this work using no additive is thinner (ca. 150 nm), and the imperfect crystallinity and morphology will create severe trapping or shorting sites for charge recombination. Furthermore, they will affect the exciton diffusion and transportation length and the charge dissociation process, which lead to poor device performance. Then, the device with 0.5% PVP in the perovskite film showed significant improvement inVOCof 0.77 V,JSCof 11.07 mA/cm2, FF of 60.93%, and PCE of 5.19%. When the mass fraction of PVP was improved to 1%, theVOC,JSC, FF, and PCE of the device were improved to 0.90 V, 13.11 mA/cm2, 71.03%, and 8.38%, respectively. By further increasing the mass fraction of PVP to 3%, however, theJSCand FF were decreased and the PCE was only 5.65%. When the perovskite layer contained 5% PVP, theJSCof the device decreased rapidly. The results are due to the insulating characteristics of PVP. The insulating PVP would hinder the charge transportation in the perovskite layer. Once the amount of PVP additive is overloaded, the charges cannot effectively transport to either PEDOT∶PSS or PC61BM layer and would accumulate or recombine in the perovskite layer. Therefore, theJSCand FF are decreased. In a short summary, the PVP additive has dual functions to affect theJSCof the devices. The first one is on the positive side from improving the extent of coverage of perovskite film on PEDOT∶PSS layer. The PVP additive can retard the growth and aggregation of perovskite crystals, thus, a continuous film with good coverage can be obtained. This full coverage perovskite crystalline film can facilitate charge transport. The second one is on the negative side from increasing theRSof devices with increasing amount of PVP due to its insulating characteristic. As a result, even though the coverage of perovskite film with 5% additive is better than that of the pristine one, its highRSproduces poorJSCperformance. Thus, the device with 1% PVP reveals the bestJSCperformance by the compromise between continuous film formation and increasingRS. To confirm the accuracy of the PCE measurements, we also measured the incident photon to charge carrier efficiencies (IPCEs) of our devices. The IPCE responses of these devices reveal (Fig.5(b)) a significant contribution at wavelengths between 300 and 800 nm, consistent with the absorption spectra of PVSK films, with the maximum EQE appearing near 500 nm. Convolution of the spectral responses with the photon flux AM 1.5G spectrum allowed us to estimateJSCvalues of 7.67, 11.19, 13.36, 10.71, and 6.18 mA·cm-2, respectively, for the devices prepared at the mass fraction of PVP from 0 to 5% under irradiation.
4 Conclusion
In summary, an innovative approach by using PVP additive to enhance the performance of planar heterojuntion perovskite solar cells was developed. The effects of PVP additives on perovskite film properties and photovoltaic performance were investigated. The PVP additive can tune the morphology of the perovskite layer by retarding the growth and aggregation of perovskite crystals, and promoting the formation of continuous films with fewer pin-holes and voids, which were supported by SEM measurement. The better film quality of perovskite film with PVP additive can enhanceJSC, FF, and thus PCE of the devices. Compared with the corresponding normal device, we observed an improvement in the PCE from 1.30% to 8.38% for the device prepared using 1% (mass fraction) of PVP as an additive.
[1] NOH J H, IM S H, HEO J H,etal.. Chemical management for colorful, efficient, and stable inorganic-organic hybrid nanostructured solar cells [J].NanoLett., 2013, 13:1764-1769.
[2] HEO J H, IM S H, NOH J H,etal.. Efficient inorganic-organic hybrid heterojunction solar cells containing perovskite compound and polymeric hole conductors [J].Nat.Photon., 2013, 7:486-491.
[3] STRANKS S D, EPERON G E, GRANCINI G,etal.. Electron-hole diffusion lengths exceeding 1 micrometer in an organometal trihalide perovskite absorber [J].Science, 2013, 342:341-344.
[4] XING G, MATHEWS N, SUN S,etal.. Long-range balanced electron- and hole-transport lengths in organic-inorganic CH3NH3PbI3[J].Science, 2013, 342:344-347.
[5] Jeng J Y, Chiang Y F, Lee M H,etal.. CH3NH3PbI3perovskite/fullerene planar-heterojunction hybrid solar cells [J]. Adv. Mater., 2013, 25:3727-3732.
[6] SEO J, PARK S, KIM Y C,etal.. Benefits of very thin PCBM and LiF layers for solution-processed p-i-n perovskite solar cells [J].EnergyEnviron.Sci., 2014, 7:2642-2646.
[7] SUN S, SALIM T, MATHEWS N,etal.. The origin of high efficiency in low-temperature solution-processable bilayer organometal halide hybrid solar cells [J].EnergyEnviron.Sci., 2014, 7:399-407.
[8] EPERON G E, BURLAKOV V M, DOCAMPO P,etal.. morphological control for high performance, solution-processed planar heterojunction perovskite solar cells [J].Adv.Funct.Mater., 2013, 24:151-157.
[9] DOCAMPO P, BALL J M, DARWICH M,etal.. Efficient organometal trihalide perovskite planar-heterojunction solar cells on flexible polymer substrates [J].Nat.Commun., 2013, 4:2761-1-6.
[10] CONINGS B, BAETEN L, DOBBELAERE C D,etal.. Perovskite-based hybrid solar cells exceeding 10% efficiency with high reproducibility using a thin film sandwich approach [J].Adv.Mater., 2014, 26:2041-2046.
[11] LIANG P W, LIAO C Y, CHUEHC C,etal.. Additive enhanced crystallization of solution-processed perovskite for highly efficient planar-heterojunction solar cells [J].Adv.Mater., 2014, 26:3748-3754.
[12] SONG X, WANG W, SUN P,etal.. Additive to regulate the perovskite crystal film growth in planar heterojunction solar cells [J].Appl.Phys.Lett., 2015, 106:033901-1-5.
[13] CHANG C Y, CHU C Y, HUANG Y C,etal.. Tuning perovskite morphology by polymer additive for high efficiency solar cell [J].ACSAppl.Mater.Interf., 2015, 7:4955-4961.
[14] KIM H S, LEE C R, IM J H,etal.. Lead iodide perovskite sensitized all-solid-state submicron thin film mesoscopic solar cell with efficiency exceeding 9% [J].Sci.Rep., 2012, 2:591-1-7.
[15] ZUO C, DING L. An 80.11% FF record achieved for perovskite solar cells by using the NH4Cl additive [J].Nanoscale, 2014, 6:9935-9938.
[16] WANG Q, DONG Q, XIAO Z,etal.. Large fill-factor bilayer iodine perovskite solar cells fabricated by a low-temperature solution-process [J].EnergyEnviron.Sci., 2014, 7:2359-2365.
[17] HOU F, SU Z, JIN F,etal.. Efficient and stable planar heterojunction perovskite solar cells with an MoO3/PEDOT∶PSS hole transporting layer [J].Nanoscale, 2015, 7:427-9432.
[18] EPERON G E, BURLAKOV V M, DOCAMPO P,etal.. Morphological control for high performance, solution-processed planar heterojunction perovskite solar cells [J].Adv.Funct.Mater., 2014, 24:151-157.

李建丰(1976-),男,湖南湘潭人,副教授,硕士生导师,2009年于兰州大学获得博士学位,主要从事有机光电功能材料与器件的研究。
E-mail: ljfpyc@163.com

夏养君(1973-),男,甘肃天水人,教授,博士生导师,2006年于华南理工大学获得博士学位,主要从事有机光电功能材料与器件的研究。
E-mail: xiayangjun2015@126.com
1000-7032(2016)01-0056-07
2015-09-18;
2015-11-10
国家自然科学基金(61264002,61166002,61404067,51463011); 甘肃省自然科学基金(1308RJZA159); 教育部新世纪人才计划(NCET-13-0840); 甘肃省高等学校科研项目(2014A-0042); 兰州交通大学博士后专项基金资助项目
利用PVP添加剂提高钙钛矿太阳能电池光伏性能
李建丰, 赵 创, 张 恒, 同军锋, 张 鹏, 杨春燕, 夏养君*
(兰州交通大学 光电技术与智能控制教育部重点实验室, 甘肃 兰州 730070)
研究了聚乙烯吡络烷酮( PVP) 作为添加剂对CH3NH3PbI3钙钛矿基太阳能电池光电性能的影响。通过SEM、XRD和UV-Vis等手段,研究了不同浓度PVP掺杂CH3NH3PbI3钙钛矿前驱体对薄膜的表面形貌、结晶度和光学性能的影响。结果表明,少量的PVP添加可以调控钙钛矿薄膜的质量,添加了PVP的钙钛矿薄膜的吸收性能明显得到提高,且吸收峰红移了20 nm;同时,不仅增加了CH3NH3PbI3的结晶度,而且还明显提高了钙钛矿薄膜的覆盖率,减少了钙钛矿薄膜中的针孔结构。在CH3NH3PbI3前驱体溶液中添加质量分数为1%的PVP,得到的钙钛矿太阳能电池的能量转换效率达到8.38%。与未加PVP 的标准电池器件效率(1.30%)相比,效率提高了544%。这些结果表明,通过添加剂来调控一步法CH3NH3PbI3的晶体生长和薄膜形貌来获取高性能的钙钛矿太阳能电池是很有前途的。
钙钛矿太阳能电池; 聚乙烯吡咯烷酮; 添加剂
O649.4; TM914.4 Document code: A
10.3788/fgxb20163701.0056
