松嫩平原盐碱化羊草群落中AM真菌物种资源及侵染率研究
2016-01-28张义飞毕琪杨允菲张忠辉胡长群杨雨春赵珊珊王相刚
张义飞,毕琪,杨允菲,张忠辉,胡长群,杨雨春,赵珊珊,王相刚
(1.吉林省林业科学研究院,吉林 长春 130033;2.东北师大附属中学,吉林 长春 130022;3.东北师范大学生命科学学院,
吉林 长春 130024;4.敦化市明星特产科技开发有限公司,吉林 敦化133000)
松嫩平原盐碱化羊草群落中AM真菌物种资源及侵染率研究
张义飞1*,毕琪2,杨允菲3,张忠辉1,胡长群1,杨雨春1,赵珊珊1,王相刚4
(1.吉林省林业科学研究院,吉林 长春 130033;2.东北师大附属中学,吉林 长春 130022;3.东北师范大学生命科学学院,
吉林 长春 130024;4.敦化市明星特产科技开发有限公司,吉林 敦化133000)
摘要:本研究在松嫩平原西部12个地区15个重度盐碱化草地中,调查了羊草群落天然斑块中AM真菌的种类和分布、AM真菌羊草根系的侵染能力及土壤pH值的影响。共分离出AM真菌4属11种,其中球囊霉属(Glomus)占总物种数的72.42%,在各调查样点中出现频度最高,其中摩西球囊霉(G. mossea)出现频度达100.0%。土壤pH强烈抑制盐碱化草地中羊草天然群落斑块中AM真菌的物种丰富度,但对孢子密度的影响未达到显著水平。AM真菌对羊草根系的侵染频率和侵染强度显著正相关。AM真菌对羊草根系的侵染频率和侵染强度随着AM物种数量的增加而增强,随着土壤pH的增加而下降。在盐碱化羊草地中存在较丰富的侵染羊草根系的AM真菌资源,研究结果为筛选和利用耐盐碱AM真菌菌株以恢复和重建松嫩盐碱化羊草草地生态系统提供了理论依据。
关键词:丛枝菌根真菌;盐碱化草地;羊草;土壤pH;物种丰富度
Arbuscular mycorrhizal fungi diversity in saline-alkalineLeymuschinensisgrasslands on the Songnen Plain
ZHANG Yi-Fei1*, BI Qi2, YANG Yun-Fei3, ZHANG Zhong-Hui1, HU Chang-Qun1, YANG Yu-Chun1, ZHAO Shan-Shan1, WANG Xiang-Gang4
1.JilinAcademyofForestryScience,Changchun130033,China; 2.HighSchoolAttachedtoNortheastNormalUniversity,Changchun130022,China; 3.SchoolofLifeScience,NortheastNormalUniversity,Changchun130024,China; 4.DunhuaStarLocalProductsTechnologyDevelopmentCo.Led.,Dunhua133000,China
Abstract:Arbuscular mycorrhizal fungi (AMF) are beneficial microorganisms, distributed widely in many different soil types. The investigation of species diversity of AMF in extreme environments is a rapidly developing area of research because of the potential benefits for ecosystem restoration. Screening for effective arbuscular mycorrhizal fungi species is regarded as an important approach to successful revegetation. The identification of arbuscular mycorrhizal species and their distribution were investigated in 15 natural saline-alkaline Leymus chinensis grasslands in 12 regions of the western Songnen Plain. The occurrence frequency, species richness and spore density were also investigated. The ability of AMF to infect roots of L. chinensis, including colonization rate and infection intensity, and the effect of soil pH were measured. In total, 11 species from 4 different families were identified; 72.4% of species belonged to Glomus. One species, G. mossea, was found at all sites. High soil pH strongly decreased AMF species richness in natural saline-alkaline communities of L. chinensis, but did not affect spore density. Disturbance of soil through erosion may be an important factor influencing spore density in soil because AMF spores were seldom detected in bare soil where the surface had been significantly disturbed. Root colonization rate was positively correlated with infection intensity. The infection of L. chinensis roots was increased with increasing AMF species richness, depressed by increasing soil pH. Our research indicated that there was an abundance of AMF species in saline-alkaline grassland able to infect roots of L. chinensis, and suggested approaches for screening saline-alkaline tolerant AMF species with the potential to help restore the degraded grassland ecosystem on the Songnen Plain.
Key words:arbuscular mycorrhizal fungi; saline-alkaline grassland; Leymus chinensis; soil pH; species richness
丛枝菌根(arbuscular mycorrhizal,AM)真菌是一类广泛分布于土壤中的有益微生物[1-3]。由于进化历程和生存条件的差异,不同区域的AM真菌己适应了当地环境条件和寄主植物,其种类和分布表现出地域和生境差异,形成丰富的种质资源[4-7]。近年来,在盐碱地、退化草地、重金属污染土壤、荒漠土地、工业污染区等极端生境中调查AM真菌资源成为研究热点[8-10]。极端环境中的AM真菌因具有独特的生物学特性,可能具有较高的应用价值,如利用AM真菌修复退化生态系统[11-14]。了解AM真菌的资源分布,是进一步开发利用该类微生物的基础。
吉林西部草地位于松嫩草地西端,因过度开垦放牧及全球气候变暖等因素的影响,该区草原生态系统严重退化,主要表现为不同程度的盐碱化。羊草(Leymuschinensis)作为该区草地的优势物种,其优势地位随盐碱化程度的加深不断下降,恢复难度也随盐碱化程度的加深而增加。人们发现土壤中缺乏AM真菌可能是某些植被恢复困难的原因之一[15],因此筛选适于土壤条件的AMF有效菌种对成功应用菌根技术恢复植被十分重要[16]。有研究表明,从盐碱生境中筛选的AM菌种往往具有较高的耐盐性和生长能力,这些具有高耐盐能力的AM菌种,在促进重度盐碱化草地的植被恢复中具有应用潜力[17]。因此,本研究在松嫩平原西部多个重度盐碱化草地中,调查了羊草天然群落斑块中AM真菌的种类和分布;AM真菌羊草根系的侵染能力及土壤pH值的影响,以期为筛选和利用耐盐碱AM真菌菌株,恢复和重建松嫩盐碱化羊草草地生态系统提供理论依据。
1材料与方法
1.1 采样地点
2005年9月调查了吉林省西部12个地区15个群落(图1,表1):松原地区的乌兰图嘎羊草群落、红星牧场羊草群落、长岭种马场羊草群落、查干湖畔蒙古屯羊草群落、大遐牧场羊草群落、东三家子羊草群落和盐碱化羊草群落,白城地区的姜家甸羊草群落和羊草+杂类草群落、通榆前程羊草群落、北大岗贝加尔针茅(Stipabaicalensis)+羊草群落和羊草+杂类草群落、镇赉种羊场羊草群落、青山村羊草群落,内蒙古兴安盟羊草群落。
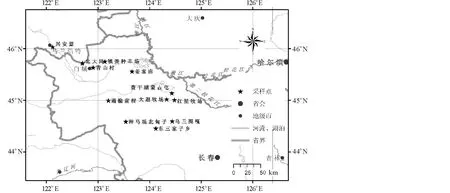
图1 采样地点地理分布Fig.1 The distribution of investigation sites
1.2 采样方法
选取当地羊草代表性群落,因选取的群落较为均匀一致,故取样面积为25 cm×25 cm,3个重复样方。移去羊草地上部后,去掉约2 cm厚的表土层。考虑到羊草根系的分布深度,取20~30 cm根系和土壤。挑出根系后即刻置于FAA固定液中备用。混匀土样后取约2 kg土装入袋中。记录采样人、采样时间、地点、周围环境等。
1.3 羊草根系透明、染色和侵染情况
采用KOH透明-乳酸甘油酸性品红染色法染色。首先将根系用蒸馏水冲洗2~3次,然后切成1 cm左右长度的根段。将根段放入10% KOH溶液中,水浴(90℃)60 min,蒸馏水冲洗2次。随后放入碱性双氧水中软化20 min,水洗后在2%的盐酸中酸化3~4 min。然后在90℃水浴锅中用酸性品红染色30 min。取出样根,放在乳酸甘油(1∶1)中浸泡脱色。随机选取50条根段,压片,在显微镜下观察每条根的侵染长度(以mm记录),并记录被侵染的根系数量。依据下列公式计算AM菌对羊草根系的侵染情况[18]:
侵染频率(colonization rate,CR%)=(侵染根段数/总根段数)×100
侵染强度(infection intensity,II%)=(侵染根长/总根长)×100
采样点Sampling site:乌兰图嘎 Wulantuga,红星牧场 Hongxing Pasture,种马场北甸子 North Meadow of Studhorse Farm,查干湖畔蒙古屯 Mongolia Village near Chagan Lake,大遐牧场 Daxia Pasture,东三家子 Dongsanjiazi,姜家甸 Jiangjiadian,通榆前程 Qiancheng of Tongyu,北大岗Beidagang,镇赉种羊场 Zhenglai Stud Farm,青山村 Qingshan Village,兴安盟 Hinggan League.
城市 City:长春 Changchun,哈尔滨 Harbin,乌兰浩特 Ulanhot,白城 Baicheng,大庆 Daqing,吉林 Jilin.
河流 River:嫩江 Nenjing,松花江 Songhua River,第二松花江 Second Songhua River,西辽河 West Liaohe River.
图标 Icon:采样点 Sample spot,省会 Provincial capital,地级市 Prefecture-level city,河流、湖泊 River and lake,省界 Provincial boundary.
1.4 孢子分离、检测及鉴定
采用湿筛倾析-蔗糖离心法[19]。用四分法取20 g土样,放入大烧杯中加水搅拌。静置10 s后过筛。将筛出物装入玻璃离心管中,以3000 r/min转速离心3 min。离心后去掉上清液,加入50%蔗糖搅匀,再次以3000 r/min转速离心1.5 min。上清液过400 目筛后获得孢子。在体视显微镜下于培养皿内分格计数,测定孢子密度(spore density,SD)。
在体视显微镜下先观察并记录孢子的颜色、大小、连孢菌丝特征等。再用吸管挑取孢子于载玻片上,加浮载剂封片后在综合显微镜下观察并测定孢子的颜色、大小、孢壁颜色、类型、厚度、内含物性质等特征。使用Melzer’s试剂,观察孢子的特异反应,对有代表性或特异性的特征进行拍照。最后根据 Schenck和Perez[20](1988)的“VA 菌根真菌鉴定手册”,国际丛枝菌根真菌保藏中心(INVAM)网站上提供的物种描述,参阅鉴定材料和近几年发表的新种、新记录种等资料进行AM真菌种属的检索和鉴定。对于难以确定的种或可能的新种、新记录种作单孢培养,在获得大量同源孢子后,按照上述步骤重新进行鉴定。
1.5 AM真菌多样性指标计算方法
依据调查数据,计算了AM真菌的出现频度(F),即某菌种在所有样点总体中的出现率[21];物种丰富度(SR),即统计某样点60 g土壤中含有的AM真菌物种的数目[22];孢子密度(SD),即计数每20 g观测土样内所有AM真菌物种的孢子总数。
1.6 土壤pH测量
取10 g风干土,用10 mL去离子水浸泡(1∶1水土比),振荡3 min后静止30 min,取上清液测量pH值(PHS-3C型精密pH计)。
1.7 统计分析
使用SPSS 19.0进行数据统计,SigmaPlot 10绘图。采用线性回归法,分析了侵染频率与侵染强度之间的关系;土壤pH对物种丰富度、孢子密度、侵染频率和侵染强度的影响;以及物种丰富度对侵染频率和侵染强度的影响。孢子密度数据进行平方根转换,以保证数据的正态分布。所有检验的显著性水平为P=0.05。

表1 羊草根际土壤AM真菌资源调查地点信息
2结果与分析
2.1 AM真菌物种资源及分布
从吉林省西部盐碱化羊草天然群落的45个土样中,共分离出AM真菌4属11种。其中球囊霉属(Glomus)8种,占总种数的72.42%;无梗囊霉属(Acaulospora)1种,类球囊霉属(Paraglomus)1种,盾巨孢囊霉属(Scutellospora)1种,各个物种的地理分布见表2。
在所有调查样点中,东三家子盐碱化羊草草地的AM真菌物种丰度最小,只有1个物种。北大岗保护良好的贝加尔针茅+羊草草地中AM真菌物种丰度最大,在羊草根际土壤中鉴定出6个物种。在所有鉴定出的菌种中,球囊霉属物种出现频度最高,其中摩西球囊霉(G.mossea)出现在所有调查样点中,频度达100.0%,其次是近明球囊霉(G.claroideum)和地球囊霉(G.geosporum),频度均为66.7%。球囊霉属的微丛球囊霉(G.microaggregatum)和盾巨孢囊霉属的透明盾巨孢囊霉(S.pellucida)的频度最低,仅为 6.7%(表2)。各调查样点的孢子密度变化较大,在6~280个/20 g土之间变化,其中东三家子盐碱化羊草草地中的孢子密度最低,姜家甸的羊草+杂类草群落中的孢子密度最高,各样点孢子密度平均值为(98.55±13.53)个/20 g土(表2)。
土壤pH强烈抑制了盐碱化草地中羊草天然群落斑块中AM真菌的物种丰富度(SR=23.59-2.22×pH,r2=0.53,P<0.001)(图2A)。随着pH的增加,AM真菌的物种数量由最高6种下降为仅1种。虽然pH值越高,孢子密度越低,但土壤pH对土壤中孢子密度的影响未达到显著水平(P=0.198)(图2B)。
表2羊草根际土壤AM真菌资源及地区分布
Table 2 The resources and distribution of AM fungi in rhizosphere of L. chinensis

侵染频率=(侵染根段数/总根段数)×100%;侵染程度=(侵染根长/总根长)×100%;孢子密度=A M真菌所有种的孢子数量/20 g土壤。
Colonization rate=(nu m ber of rootinfected/nu m ber of root m easured)×100%;Infection intensity=(length of rootinfected/length of root m easured)×100%;Spore density=spore nu m ber of all A M F speciesin 20 g soil.
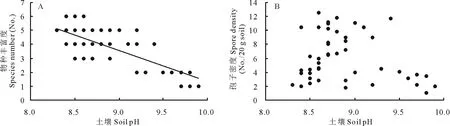
图2 土壤pH对AM真菌物种丰富度(A)和孢子密度(B)的影响Fig.2 The effects of soil pH on species richness (A) and spore density (B) of AM fungi
2.2 AM菌的侵染情况及影响因素
在所调查的羊草群落中,各样点AM真菌对羊草根系的侵染频率为30%~76%,平均(53.40±3.07)%。侵染强度为4%~36%,平均(17.50±1.70)%。侵染频率(CR)与侵染强度(II)显著正相关(II=0.47×CR-7.47,r2=0.71,P<0.001),即AM菌侵染的羊草根系数量越多,其在每条根上侵染的长度越长(图3)。
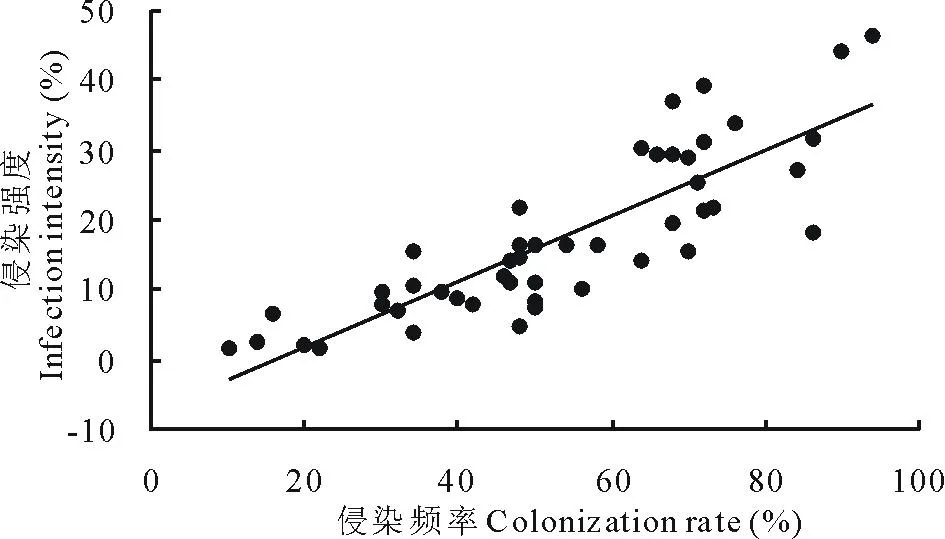
图3 羊草根系AM菌根的侵染频率和侵染强度的关系Fig.3 The relationships between colonization rate and infection intensity of AM fungi to L. chinensis root
AM真菌对羊草根系的侵染频率(CR=31.08+5.83×SR,r2=0.13,P=0.011 )和侵染强度(II=6.50+2.87×SR,r2=0.11,P=0.025)均与土壤中AM真菌的物种丰富度显著正相关(图4A),表明AM真菌的物种数量越多,对羊草根系的侵染活动越强。随着土壤盐碱化强度的增加,AM真菌对羊草根系的侵染能力变差,表现为土壤pH与AM真菌的侵染频率(CR=213.98-18.06×pH,r2=0.14,P=0.010)和侵染强度(II=87.86-7.92×pH,r2=0.09,P=0.046)显著负相关(图4B)。
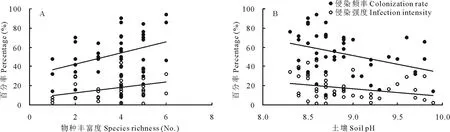
图4 侵染频率和侵染强度与AM真菌物种丰富度(A)和土壤pH(B)的关系Fig.4 The relationships of colonization rate and infection intensity with species richness of AM fungi (A) and soil pH (B)
3讨论
盐碱化草地存在较丰富的AM真菌资源。早在1928年,Mason[23]就报道了盐碱土壤环境下的植物菌根。随后的研究发现,在盐碱土这一特殊的生态系统中,丛枝菌根真菌几乎侵染所有植物。唐明等[24](2007)在内蒙古盐碱土13种主要植物的根际土壤中分离出3属26种AM真菌;王桂君[25](2005)在吉林省西部盐碱化草地的20种典型植物的根际土壤中鉴定出29种AM菌根真菌。本研究仅针对羊草这一物种,在松嫩平原西部盐碱化草地中共检测到能够侵染羊草根系的4属11种菌根真菌(表2)。一般而言,AM真菌在自然生态系统具有较高的多样性,而在人类频繁干扰的生态系统中,如农田和放牧场,AM真菌多样性降低[26]。除了受宿主植物影响外,环境因子,如气候、土壤、土壤微生物、土地利用方式等,都影响着AM真菌的资源分布[6,27-28],如人们发现土壤pH对AM真菌种类分布的影响具有一定的规律性[29-30]。此外,宿主植物对环境因子变化的响应,也会通过影响AM真菌与宿主植物的亲和性而影响AM菌的存在与分布[31-32]。
球囊霉属(Glomus)菌种在大多数调查样地中占居优势(表2),这与其他盐碱环境中的调查结果相同[24,33-34],目前普遍认为球囊霉属多出现在中性和 pH 较高的土壤中[35]。球囊霉属的摩西球囊霉(G.mossea)在本次调查中出现频率最多,每个地段都有。作为球囊霉属中分布最广的物种,摩西球囊霉的发生频度往往随盐碱胁迫的增加而增加[35]。地球囊霉(G.geosporum)、近明球囊霉(G.claroideum)在频率和孢子密度上都表现为较高水平。一些研究证明摩西球囊霉和地球囊霉是重度盐土中最优势的物种[36-37],并且地球囊霉也是重度退化地区的优势种[33,38],甚至出现在pH值为11的盐土中[39]。无梗囊霉属(Acaulospora)的光壁无梗囊霉(A.laevis)、类球囊霉属(Paraglomus)的隐类球囊霉属(P.occultum)和盾巨孢囊霉属(Scutellospora)的透明盾巨孢囊霉属(S.pellucida)在某些地区数量很多,并成为该地区优势种,但是在其他地区出现较少。
作为繁殖体,土壤中AM真菌孢子的多少对菌化植物的形成意义重大。我们的研究表明,虽然各个调查样点的孢子密度变化较大,但土壤pH并不是影响孢子密度的主要因素(图2B)。而在土层受到严重干扰的裸碱斑土壤中很少检测到AM真菌侵染现象,并且很少找到AM真菌孢子[15,40],因此,强烈的土壤干扰和侵蚀可能是影响AM真菌繁殖体数量的主要原因之一。
AM真菌对羊草根系的侵染频率和侵染强度在各个调查样点间变化较大(表2)。不同AM真菌对相同植物的侵染速度和侵染率不同[41],这种差异与植物和AM真菌的亲和性有关[42],也与环境因子,特别是影响根系生长的土壤条件有很大关系[43],如土壤pH、水分、盐分含量、养分水平等[44]。由于盐碱胁迫不利于孢子萌发和菌丝生长[45],因此,土壤 pH 不仅直接影响 AM 真菌的发生和种群分布[46],也对AM真菌侵染羊草根系的频率和强度产生负面影响(图4),降低了真菌对羊草根系的侵染活动。虽然已有研究表明AM真菌能够加强植物对营养元素的选择性吸收,降低盐碱胁迫对生长的影响[47-48],但只有耐盐能力强的AM菌种才能在重度盐碱化草地植被恢复中发挥积极作用[17]。我们认为在盐碱化羊草地中存在较丰富的侵染羊草根系的AM真菌资源,研究结果为筛选和利用耐盐碱AM真菌菌株、恢复和重建松嫩盐碱化羊草草地生态系统提供了理论依据。
References:
[1]Mangan S A, Eom A H, Adler G H,etal. Diversity of arbuscular mycorrhizal fungi across a fragmented forest in Panama: Insular spore communities differ from mainland communities. Oecologia, 2004, 141(4): 687-700.
[2]Wu Q S, Yuan F Y, Fei Y J,etal. Effects of arbuscular mycorrhizal fungi on aggregate stability, GRSP, and carbohydrates of white clover. Acta Prataculturae Sinica, 2014, 23(4): 269-275.
[3]Wu Q S, Yuan F Y, Fei Y J,etal. Effects of arbuscular mycorrhizal fungi on root system architecture and sugar contents of white clover. Acta Prataculturae Sinica, 2014, 23(1): 199-204.
[4]Jansa J, Mozafar A, Anken T,etal. Diversity and structure of AMF communities as affected by tillage in a temperate soil. Mycorrhiza, 2002, 12(5): 225-234.
[5]Lugo M A, Cabello M N. Native arbuscular mycorrhizal fungi (AMF) from mountain grassland (Córdoba, Argentina) I. Seasonal variation of fungal spore diversity. Mycologia, 2002, 94(4): 579-586.
[6]Entry J A, Fuhrmann J J, Sojka R E,etal. Influence of irrigated agriculture on soil carbon and microbial community structure. Environmental Management, 2004, 33(SUPPL. 1): 363-373.
[7]Cai B P, Chen J Y, Zhang Q X,etal. Three new records of arbuscular mycorrhizal fungi associated withPrunusmumein China. Mycosystema, 2008, 27(4): 538-542.
[8]Wu C H, Chen X, Wang Z Q. Lead absorption by weeds from lead-polluted soil. Chinese Journal of Applied Ecology, 2004, 15(8): 1451-1454.
[9]Greipsson S, El-Mayas H. Arbuscular mycorrhizae ofLeymusarenariuson coastal sands and reclamation sites in Iceland and response to inoculation. Restoration Ecology, 2000, 8(2): 144-150.
[10]McHugh J M, Dighton J. Influence of mycorrhizal inoculation, inundation period, salinity, and phosphorus availability on the growth of two salt marsh grasses,SpartinaalternifloraLois. andSpartinacynosuroides(L.) Roth., in nursery systems. Restoration Ecology, 2004, 12(4): 533-545.
[11]Richter B S, Stutz J C. Mycorrhizal inoculation of big sacaton: Implications for grassland restoration of abandoned agricultural fields. Restoration Ecology, 2002, 10(4): 607-616.
[12]Cordoba A S, De Mendonca M M, Stuermer S L,etal. Diversity of arbuscular mycorrhizal fungi along a sand dune stabilization gradient: A case study at Praia da Joaquina, Ilha de Santa Catarina, South Brazil. Mycoscience, 2001, 42(4): 379-387.
[13]Kiers E T, Lovelock C E, Krueger E L,etal. Differential effects of tropical arbuscular mycorrhizal fungal inocula on root colonization and tree seedling growth: Implications for tropical forest diversity. Ecology Letters, 2000, 3(2): 106-113.
[14]Yang H X, Liu R J, Guo S X. Effects of arbuscular mycorrhizal fungusGlomusmosseaeon the growth characteristics ofFestucaarundinaceaunder salt stress conditions. Acta Prataculturae Sinica, 2014, 23(4): 195-203.
[15]Dodd J C, Thomson B D. The screening and selection of inoculant arbuscular-mycorrhizal and ectomycorrhizal fungi. Plant and Soil, 1994, 159(1): 149-158.
[16]Kaya C, Ashraf M, Sonmez O,etal. The influence of arbuscular mycorrhizal colonisation on key growth parameters and fruit yield of pepper plants grown at high salinity. Scientia Horticulturae, 2009, 121(1): 1-6.
[17]Smith S E, Facelli E, Pope S,etal. Plant performance in stressful environments: Interpreting new and established knowledge of the roles of arbuscular mycorrhizas. Plant and Soil, 2010, 326(1): 3-20.
[18]Zhang M Q, Wang Y S, Xing L J. The ecological distribution of AM fungi community in south and east coast of China. Mycosystema, 1998, 17(3): 274-277.
[19]Sheng M, Tang M, Zhang F F,etal. Effect of soil factors on arbuscular mycorrhizal fungi in saline alkaline soils of Gansu, Inner Mongolia and Ningxia. Biodiversity Science, 2011, 19(1): 85-92.
[20]Schenck N C, Perez Y. Manual for Identification of Vesicular Arbuscular Mycorrhizal Fungi[M]. Gainesville: University of Florida, 1988.
[21]Mao S C, Xing J S, Song M J,etal. Reaction of two indigenous strains of VAMF on cotton. Cotton Science, 1994, 6(4): 237-242.
[22]Sanders I R, Fitter A H. Evidence for differential responses between host-fungus combinations of vesicular-arbuscular mycorrhizas from a grassland. Mycologist Resarch, 1992, 96(6): 415-419.
[23]Mason E. Note on the presence of mycorrhiza in the roots of salt marsh plants. New Phytologist, 1928, 27(3): 193-195.
[24]Tang M, Huang Y H, Sheng M,etal. Diversity and distribution of arbuscular mycorrhizal fungi in saline alkaline soil, Inner Mongolia. Acta Pedologica Sinica, 2007, 44(6): 1104-1110.
[25]Wang G J. The Symbiotic Diversity of Arbuscular Mycorrhizal in SalinizedLeymuschinensisGrassland in Western Jilin Province[D]. Changchun: Northeast Normal University, 2005.
[26]Cai B P, Zhang Y, Chen Y J,etal. Three new records of arbuscular mycorrhizal fungi associated with wildPrunusmumefrom Tibet in China. Mycosystema, 2007, 26(1): 36-39.
[27]Miller S P. Arbuscular mycorrhizal colonization of semi-aquatic grasses along a wide hydrologic gradient. New Phytologist, 2000, 145(1): 145-155.
[28]Nilsson L O, Giesler R, Bååth E,etal. Growth and biomass of mycorrhizal mycelia in coniferous forests along short natural nutrient gradients. New Phytologist, 2005, 165(2): 613-622.
[29]Peng Y L, Yang M N, Cai X B. Influence of soil factors on species diversity of arbuscular mycorrhizal(AM) fungi inStipasteppe of Tibet Plateau. Chinese Journal of Applied Ecology, 2010, 21(5): 1258-1263.
[30]Hou X F, He X L. The spatio-temporal distribution of arbuscular mycorrhizal fungi fromHedysarumleaveMaxim. Journal Publishing Department of Agricultural University of Hebei, 2007, 133(4): 50-54.
[31]Phillings J M, Hayman D S. Improves procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Transactions of the British Mycological Society, 1970, 55(1): 158-160.
[32]Giovannetti M, Mosse B. An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytologist, 1980, 84(3): 489-500.
[33]Liu R J, Liu P Q, Xu K,etal. Ecological distribution of arbuscular mycorrhizal fungi in saline alkaline soils of China. Chinese Journal of Applied Ecology, 1999, 10(6): 721-724.
[34]Carvalho L M, Caçador I, Martins-Loução M. Temporal and spatial variation of arbuscular mycorrhizas in salt marsh plants of theTagusestuary(Portugal). Mycorrhiza, 2001, 11(6): 303-309.
[35]Sengupta A, Chaudhuri S. Arbuscular mycorrhizal relations of mangrove plant community at the Ganges river estuary in India. Mycorrhiza, 2002, 12(4): 169-174.
[36]Al-Karaki G N. Salt stress response of salt-sensitive and tolerant durum wheat cultivars inoculated with mycorrhizal fungi. Acta Agronomica Hungarica, 2001, 49(1): 25-34.
[37]Thrall P H, Bever J D, Slattery J F. Rhizobial mediation ofAcaciaadaptation to soil salinity: Evidence of underlying trade-offs and tests of expected patterns. Journal of Ecology, 2008, 96(4): 746-755.
[38]Beauchamp V B, Stromberg J C, Stutz J C. Interactions betweenTamarixramosissima(saltcedar),Populusfremontii(cottonwood), and mycorrhizal fungi: Effects on seedling growth and plant species coexistence. Plant and Soil, 2005, 275(1): 221-231.
[39]Jeffries P, Gianinazzi S, Perotto S,etal. The contribution of arbuscular mycorrhizal fungi in sustainable maintenance of plant health and soil fertility. Biology and Fertility of Soils, 2003, 37(1): 1-16.
[40]Juniper S, Abbott L K. A change in the concentration of NaCl in soil alters the rate of hyphal extension of some arbuscular mycorrhizal fungi. Canadian Journal of Botany, 2004, 82(8): 1235-1242.
[41]Smith F A, Smith S E. Multalism and parasitism: Diversity in function and structure in the arbuscular(VA) mycorrhizal symbiosis. Advances in Botanical Research, 1996, 22: 1-43.
[42]Gai J P, Liu R J. Effects of soil factors on arbuscular mycorrhizae(AM) fungi around roots of wild plants. Chinese Journal of Applied Ecology, 2003, 14(3): 470-472.
[43]Zhang Y, Guo L D, Liu R J. Diversity and ecology of arbuscular mycorrhizal fungi in Dujiangyan. Chinese Journal of Plant Ecology, 2003, 27(4): 537-544.
[44]Peason J N, Simth S E, Smith F A. Effect of photon irradiance on the development and activity of VA mycorrhizal infection inAlliumporrum. Mycological Research, 1991, 101(3): 1063-1071.
[45]Carvalho L M, Correia P M, Caçador I,etal. Effects of salinity and flooding on the infectivity of salt marsh arbuscular mycorrhizal fungi inAstertripoliumL. Biology and Fertility of Soils, 2003, 38(3): 137-143.
[46]Li X L, Feng G. Ecology and Physiology of VA Mycorrhizaes[M]. Beijing: Huawen Press, 2001.
[47]Feng G, Li X L, Zhang F S,etal. Effect of phosphorus and arbuscular mycorrhizal fungus on response of maize plant to saline environment. Journal of Plant Resources and Environment, 2000, 9(2): 22-26.
[48]Sheng M, Tang M, Chen H,etal. Influence of arbuscular mycorrhizae on the root system of maize plants under salt stress. Canadian Journal of Microbiology, 2009, 55(7): 879-886.
参考文献:
[2]吴强盛, 袁芳英, 费永俊, 等. 菌根真菌对白三叶根际团聚体稳定性、球囊霉素相关土壤蛋白和糖类物质的影响. 草业学报, 2014, 23(4): 269-275.
[3]吴强盛, 袁芳英, 费永俊, 等. 丛枝菌根真菌对白三叶根系构型和糖含量的影响. 草业学报, 2014, 23(1): 199-204.
[7]蔡邦平, 陈俊愉, 张启翔, 等. 梅根际丛枝菌根真菌三个中国新记录种. 菌物学报, 2008, 27(4): 538-542.
[8]吴春华, 陈欣, 王兆骞. 铅污染土壤中杂草对铅的吸收. 应用生态学报, 2004, 15(8): 1451-1454.
[14]杨海霞, 刘润进, 郭绍霞. AM真菌摩西球囊霉对盐胁迫条件下高羊茅生长特性的影响. 草业学报, 2014, 23(4): 195-203.
[18]张美庆, 王幼珊, 邢礼军. 我国东南沿海地区AM真菌群落生态分布研究. 菌物系统, 1998, 17(3): 274-277.
[19]盛敏, 唐明, 张峰峰, 等. 土壤因子对甘肃、宁夏和内蒙古盐碱土中AM真菌的影响. 生物多样性, 2011, 19(1): 85-92.
[21]毛树春, 邢金松, 宋美珍, 等. 棉花对两种VA菌根真菌的反应. 棉花学报, 1994, 6(4): 237-242.
[24]唐明, 黄艳辉, 盛敏, 等. 内蒙古盐碱土中AM真菌的多样性与分布. 土壤学报, 2007, 44(6): 1104-1110.
[25]王桂君. 吉林省西部盐碱化羊草草原的丛枝菌根共生多样性[D]. 长春: 东北师范大学, 2005.
[26]蔡邦平, 张英, 陈俊愉, 等. 藏东南野梅根际丛枝菌根真菌三个我国新记录种(英文). 菌物学报, 2007, 26(1): 36-39.
[29]彭岳林, 杨敏娜, 蔡晓布. 西藏高原针茅草地土壤因子对丛枝菌根真菌物种多样性的影响. 应用生态学报, 2010, 21(5): 1258-1263.
[30]侯晓飞, 贺学礼. 荒漠植物羊柴根际AM真菌时空分布研究. 河北农业大学学报, 2007, 133(4): 50-54.
[33]刘润进, 刘鹏起, 徐坤, 等. 中国盐碱土壤中AM菌的生态分布. 应用生态学报, 1999, 10(6): 721-724.
[42]盖京苹, 刘润进. 土壤因子对野生植物AM真菌的影响. 应用生态学报, 2003, 14(3): 470-472.
[43]张英, 郭良栋, 刘润进. 都江堰地区丛枝菌根真菌多样性与生态研究. 植物生态学报, 2003, 27(4): 537-544.
[46]李晓林, 冯固. 丛枝菌根生态生理[M]. 北京: 华文出版社, 2001.
[47]冯固, 李晓林, 张福锁, 等. 施磷和接种AM真菌对玉米耐盐性的影响. 植物资源与环境学报, 2000, 9(2): 22-26.
张义飞,毕琪,杨允菲,张忠辉,胡长群,杨雨春,赵珊珊,王相刚.松嫩平原盐碱化羊草群落中AM真菌物种资源及侵染率研究. 草业学报, 2015, 24(9): 80-88.
ZHANG Yi-Fei, BI Qi, YANG Yun-Fei, ZHANG Zhong-Hui, HU Chang-Qun, YANG Yu-Chun, ZHAO Shan-Shan, WANG Xiang-Gang. Arbuscular mycorrhizal fungi diversity in saline-alkalineLeymuschinensisgrasslands on the Songnen Plain. Acta Prataculturae Sinica, 2015, 24(9): 80-88.
通讯作者*Corresponding author. E-mail:yifeii@hotmail.com
作者简介:张义飞(1972-),男,吉林长春人,助理研究员,博士。E-mail:yifeii@hotmail.com
基金项目:国家自然科学基金资助项目(30270260,30770397),林业公益性行业科研专项(201104040)和吉林省科技发展计划(201205065)资助。
收稿日期:2014-10-13;改回日期:2014-12-10
DOI:10.11686/cyxb2014426http://cyxb.lzu.edu.cn
