一种三维四元酸铜配合物的合成、结构及其性质
2016-01-14邵彩云,刘孟岩,练晨等
一种三维四元酸铜配合物的合成、结构及其性质
邵彩云1,刘孟岩1,练晨1,王力册1,许珍焕1,郭旭1,龙银双1,路云霞2,杨立荣1*
(1. 河南大学 化学化工学院,河南省多酸化学重点实验室,分子与晶体工程研究所,河南 开封 475004;
2. 河南大学图书馆,河南 开封 475001)
摘要:通过水热法合成了一种具有三维孔状结构的金属有机框架物{[Cu(BPTC)0.5·(bpy)]·H2O}∞ (BPTC = 3,3′,4,4′-苯甲酮-四甲酸和 bpy = 4,4′-联吡啶). 运用元素分析、红外光谱、X射线单晶衍射以及X射线粉末衍射等对其进行了结构表征, 利用热重分析评价了其热稳定性. X射线单晶衍射分析表明标题配合物通过一维右手螺旋链联接成为二维层状结构, 进而经由4,4′-联吡啶联接成为三维孔状结构. 当该孔状结构中包容的水分子被移除后,该金属有机框架物依然保持三维孔状结构. 此外,实验还研究了标题配合物的磁学和固态荧光性质.
关键词:有机-金属框架物;水热合成;磁性;荧光性质
Received date: 2015-05-28.
Foundation item: The Natural Science Foundation of Henan Province (13A15006 and 15NB005).
Biography: SHAO Caiyun (1969-), female, senior experimentalist, main direction is coordination chemistry.*Corresponding author, E-mail: lirongyang@henu.edu.cn.
Studies on metal-organic frameworks (MOFs) are of considerable interest due to their fascinating network topologies and potential applications in molecular sieves, sensors, gas storage, ion exchange, size-selective separation, molecular magnetism, photoluminescence and heterogeneous catalysis[1-6]. A large number of MOFs with plentiful structures and unique properties have been designed and synthesized. The construction of MOFs is highly influenced by some certain factors,such as the coordination tendencies of metal ions, the structural characters of organic ligands, the properties of solvent and pH value, and so on[7-12].
Among various organic ligands, multidentate organic ligands like multicarboxylate acids are often used as the linkers of metal ions to construct diversified MOFs. Certainly, 3,3′,4,4′ -benzophenone-tetracarboxylate was selected as multifunctional organic ligands based on several intriguing characters: i) quadruple carboxylic dentate arms are benefit to forming helical structures; ii) eight potential donor oxygen atoms contribute to construct multi-dimensional frameworks; iii) the noncovalent interactions, such as hydrogen bonds and π…π stacking exist in the benzene rings; iv) the pH values may exert an effect on the deprotonated degree of the ligands. What′s more, auxiliary ligands (for example, 4,4′-bipyridine) may lead to abundant network topology as linkers[13-18].
Here in, we report the hydrothermal synthesis, characteristics, thermal analysis and the crystal structure of the 3D porous coordination complex of Cu(II) center with H4BPTC and 4,4′-bipyridine ligand, namely, {[Cu(BPTC)0.5·(bpy)]·H2O}∞. The typical coordination mode of H4BPTC is summarized in Fig. 1. Additionally, we investigated the thermal analysis, FT-IR spectroscopy and X-ray powder diffraction of the coordination complex, and found that the 3D porous framework remains intact after the guest water molecules are removed.
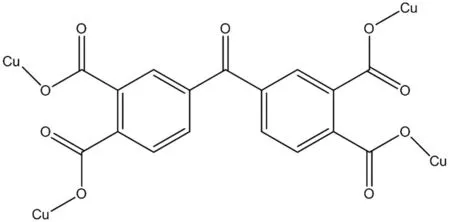
Fig.1 The coordination mode of the BPTC ligand in the complex
1Experimental
1.1 Reagent and instrument
All reagents were analysis grade. Elemental analysis (C, H and N) was performed by a Perkin-Elmer 2400-II CHNS/O analyzer. Infrared (IR) spectrum was recorded on a Bruker VERTEX 70 IR spectrometer in the range of 4 000-400 cm-1(using KBr in pellets). XRD data were recorded on a DX-2700 instrument with Cu Kαradiation (λ= 0.154 056 nm) in the angular range 2θ= 545oat 293 K. Magnetic susceptibility measurements were carried out by using a Quantum Design MPMS-5 magnetometer in the temperature range of 2-300 K. TG analysis was measured on a Perkin-Elmer TGA7 instrument with a heating rate of 10 ℃/min from 25 to 1 000 ℃ in N2flow. The photoluminescence property was performed on a HITACHI F-7000 fluorescence spectrophotometer in the solid state at the room temperature. Single crystals for X-ray structure analysis was performed on Bruker CCD Apex-II diffractometer with Mo Kαradiation (λ= 0.071 073nm) at 296 K. The structure of the complex was solved by direct methods and further refined by full-matrix least-squares refinements onF2using the SHELXL-97 software and an absorption correction was performed by the SADABS program[19].
1.2 Synthesis of the title complex
The title complex was synthesized from the reaction mixture of copper acetate (0.25 mmol, 49.9 mg), 3,3′,4,4′-benzophenone-tetracarboxylate (0.25 mmol, 89.6 mg), 4,4′-bipyridine (0.25 mmol, 39.0 mg ) in 10 mL distilled water and was adjusted to pH = 5 with 1 mol/L NaOH solution. The resultant mixture was homogenized under stirring for 30 min at ambient temperature, transferred into 25 mL Teflon-lined stainless steel autoclave under autogenous pressure at 160 ℃ for 4 d, and then cooled to room temperature at a rate of 5 ℃/h. After filtration, the product was washed with distilled water and then dried, and yellowish transparent block crystals suitable for X-ray diffraction analysis were obtained. Anal. Calc. for C19H10CuN2O5(409.83, %): C 55.68, H 2.46, N 6.84; Found(%): C 54.75, H 2.98, N 6.90. Selected IR (cm-1): 3 563(w), 3 420(w), 3 146(w), 2 946 (w), 1 654 (w), 1 629(s), 1 612 (s), 1 528(m), 1 414(s), 1 385(s), 1 368 (s), 1 296 (w), 1 277(m), 1 244 (m) 1 244(m), 1 083(w), 1 071(m), 1 012(w), 912(w), 849(w), 833(m), 803(w), 765(m), 754(w), 726(w), 704(w), 666(w), 643(m), 604(w), 515(m), 441(w), 418(w).
2Results and Discussion
2.1 Structural description of the complex
The crystal data and structure renement detail for the complex are summarized in Table 1. Single crystal X-ray diffraction reveals that the complex crystallizes in the orthorhombic space groupF-ddd. Each Cu(II) atom is coordinated by two oxygen atoms from two BPTC ligands (Cu(1)-O(1) 0.194 3(6) nm, Cu(1)-O(4) 0.195 8(6) nm, respectively) and two nitrogen atoms from two bpy ligands (Cu(1)-N(1) 0.203 8(7) nm and Cu(1)-N(2) 0.203 0(8) nm), as shown in Fig. 2a. Weak interaction occur between the carboxylic oxygen atoms and central Cu(II) ion (Cu(1)-O(2) 0.270 5(7) nm and Cu(1)-O(5) 0.277 7(7) nm) owing to the Jahn-Teller effect. The bond length data (see Table 2) in the present work are consistent with those in previous work covering Cu(II) ions coordination polymers[20-21].

Table 1 Crystal data and structure refinement parameters for complex
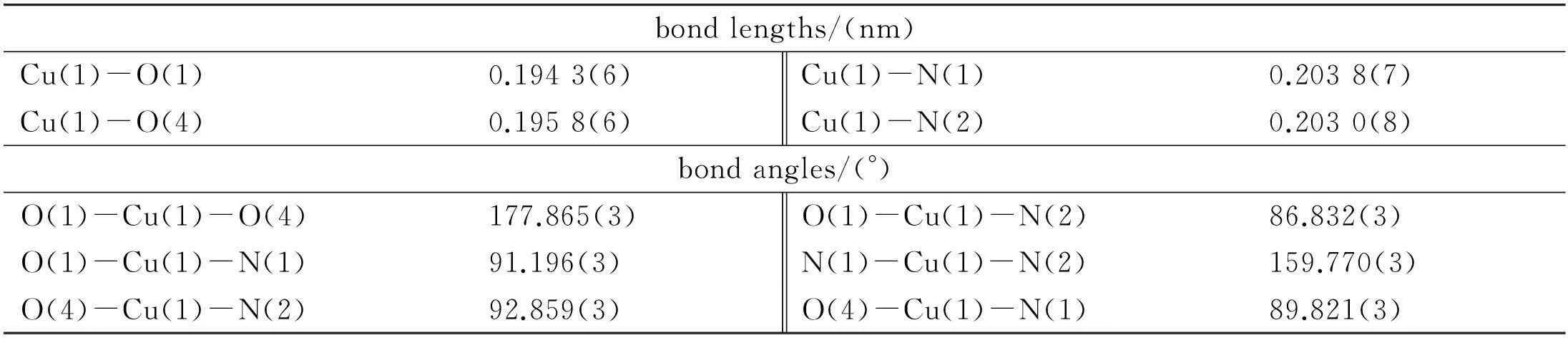
Table 2 Selected bond lengths and bond angles for complex
It is noteworthy that each BPTC anion coordinates to four Cu(II) cations to generate a 2D chiral layer alongadirection to form rhombic windows with dimensions of 1.145 1 nm×2.000 9 nm (Fig. 2b). Within the layer, right-handed helical chains formedviacarbonyl groups of BPTC anions with the pitches of 1.145 2 nm are observed. At the same time, bpy pillars connect Cu(II) ions to generate a 1D chain [-bpy-Cu(1)-bpy-Cu(1)-]∞(Fig. 2c). Furthermore, the adjacent Cu-BPTC 2D layers are linked by bpy linkers to form a cavities-containing 3D framework, as illustrated in Fig. 2d, in which the lattice water molecules are encapsulated in the cavities.
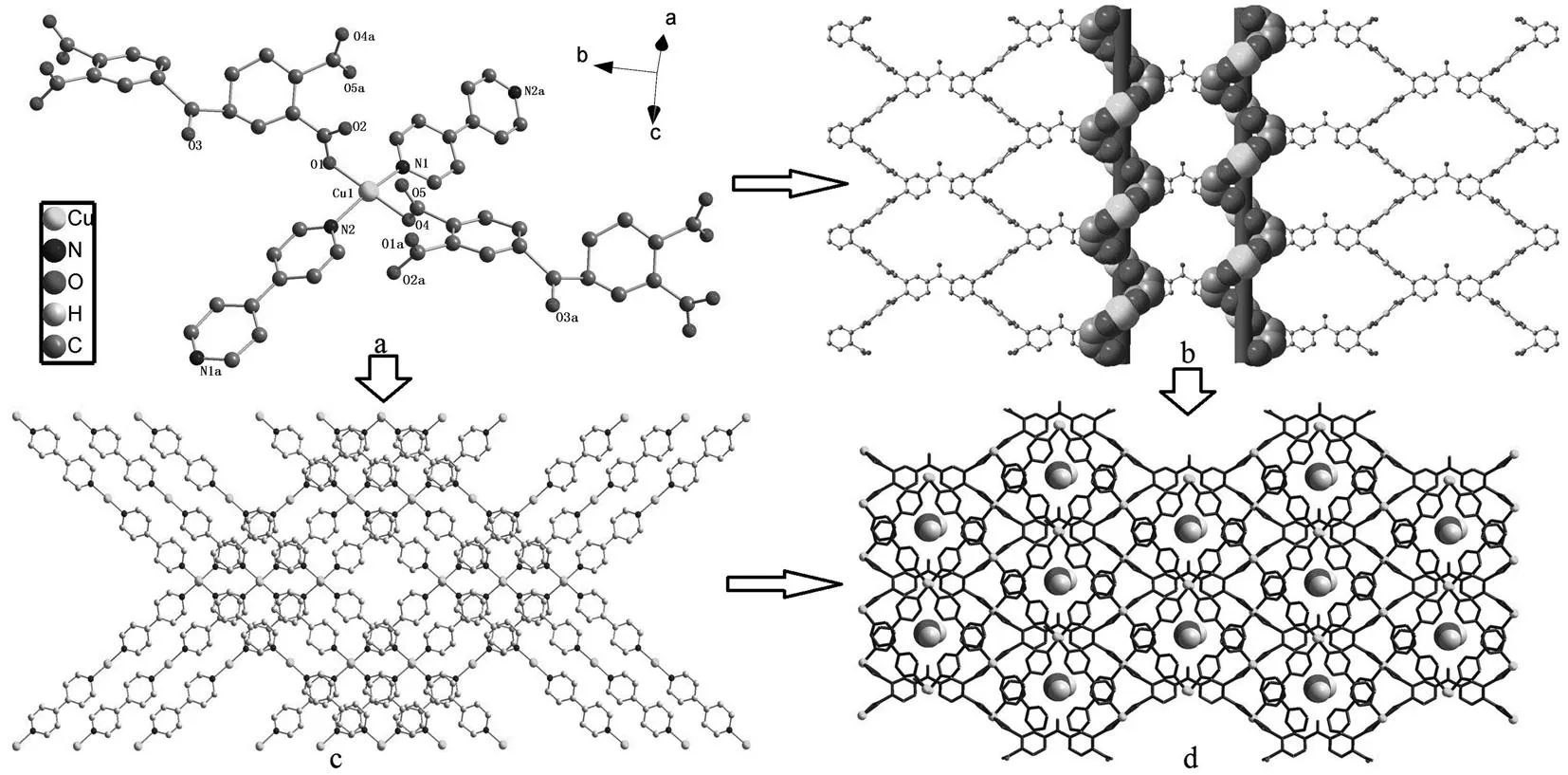
Fig.2 a) Coordination environment of the complex, the asymmetric unit and the related coordination atoms are labeled, lattice water and hydrogen atoms are omitted for clarity; b) the 1D ribbon of [bpy-Cu-bpy-Cu] ∞ in the complex; c) the 2D layer of Cu-BPTC. insert: left- and right-Cu-phthalate helical chain; d) the 3D framework of the complex
2.2 FT-IR spectroscopy and X-ray powder diffraction
The title complex are insoluble in common solvents such as CH3COCH3, CH3CH2OH, CH3CN and THF, but slight soluble in DMF. The structure of the complex is identied by satisfactory elemental analysis as well as FT-IR and single crystal X-ray diffraction. The strong vibrations appeared at about 1 606 and 1 395 cm-1in the complex are ascribed to the coordinated carboxylates. The values of Δ[νas-νs] is 211 cm-1, which indicates that the carboxyl groups are coordinated with the metal ionsvia monodentate mode[22-23]. The sharp peaks ofδO-C-Ovibration in plane emerge in the range of 660-760 cm-1. The absence of the characteristic bands ranging from 1 725 to 1 785 cm-1indicates that the H4BPTC ligands are completely deprotonated in the form of BPTC4-anions upon reaction with the metal ions[24-25]. The results are in good agreement with the single-crystal X-ray structural analysis and well match to the literature reports.
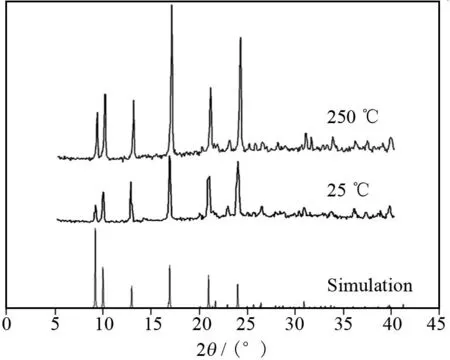
Fig.3 The simulated and experimental (25 ℃ and 250 ℃) powder XRD patterns of the complex
To confirm the purity of the complex and further investigate whether the 3D porous framework would collapse upon removal of the guest water molecules, the original as-synthesized complex and the processed sample were characterized by X-ray powder diffraction (XRD) at the same conditions, the corresponding XRD patterns are shown in Fig. 3. The guest water molecules can be removed by heating at 250 ℃ for 24 h. By comparison, the corresponding positions and intensities of peaks in the as-synthesized patterns of the complex were unchanged when it was heated over 250 ℃, which suggests that the 3D porous framework remains intact after the guest water molecules are removed. This result may also be evidenced by the IR spectra. The corresponding characteristic peaks of the as-synthesized complex do not shift after the guest water molecules are removed by heating to 250 ℃ (see Fig.4).
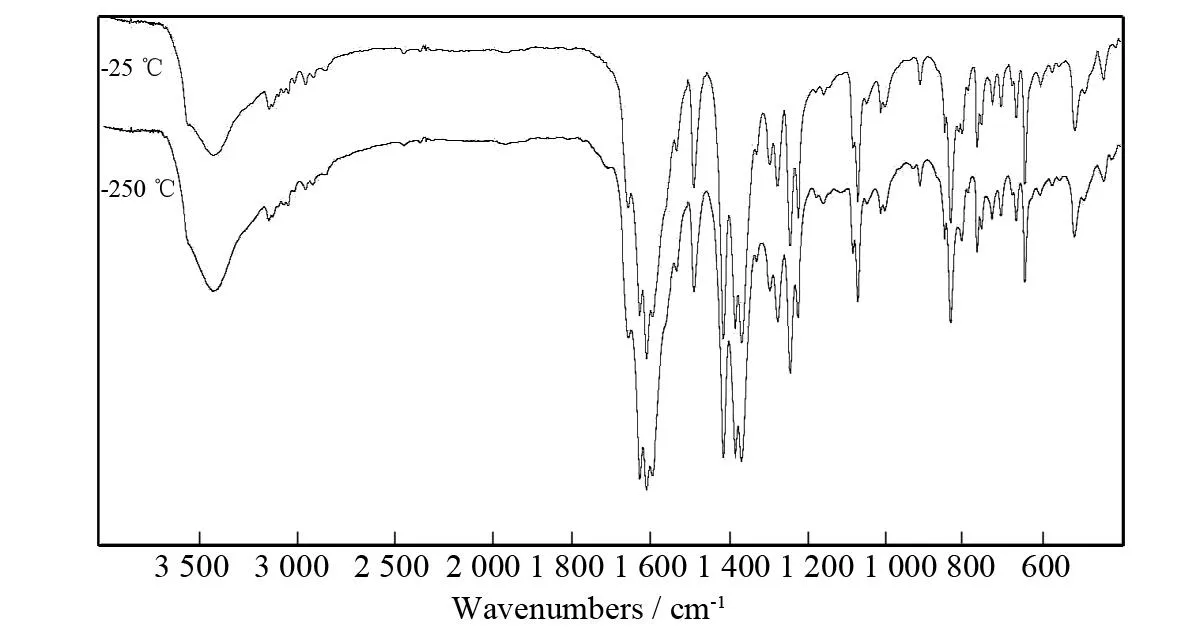
Fig.4 IR spectrum of the complex
2.3 Photoluminescent properties
The solid-state photoluminescence spectrum of the complex was investigated at room temperature intersecting with incidence at an angle of 45° (see Fig.5). The complex exhibits two strong emission bands centered at 483 and 590 nm upon excitation at 374 nm. From the band position and shape, it is reasonable to speculate that the emissions of the complex is tentatively assigned to the mixture effects of intraligand charge transfer and ligand-to-metal-charge-transfer (LMCT) transitions[26-27].
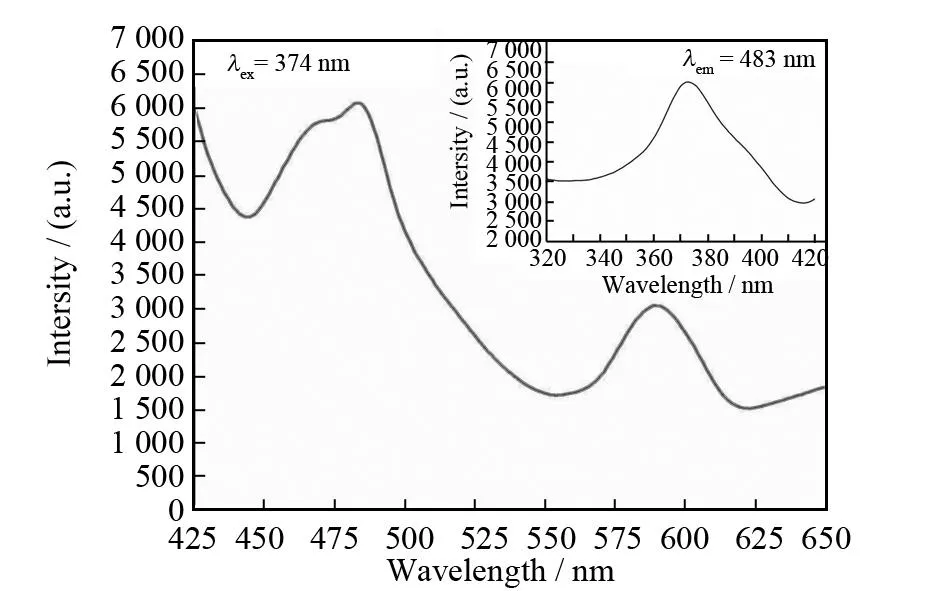
Fig.5 Emission and excitation spectrum for the complex at room temperature
2.4 Thermal analysis
The thermal analysis of the complex has been measured from room temperature to 1 000 ℃ with a heating rate of 10 ℃·min-1in N2flow (see Fig. 6), which indicates that the complex decomposes in two steps, giving a total weight loss of 81.80%. The first weight loss of 3.11% between room temperature and 247 ℃, which is attributed to the loss of the lattice water (calc. 4.40%). The second stage weight loss (78.69%) of 250-1 000 ℃ conform to the segmental decomposition of 4,4′-bipyridine and 3,3′,4,4′-benzophenone-tetracarboxylate ligands. The remnant of the complex is 19.20%, which suggests that CuO is the final product (calc. 19.40%).
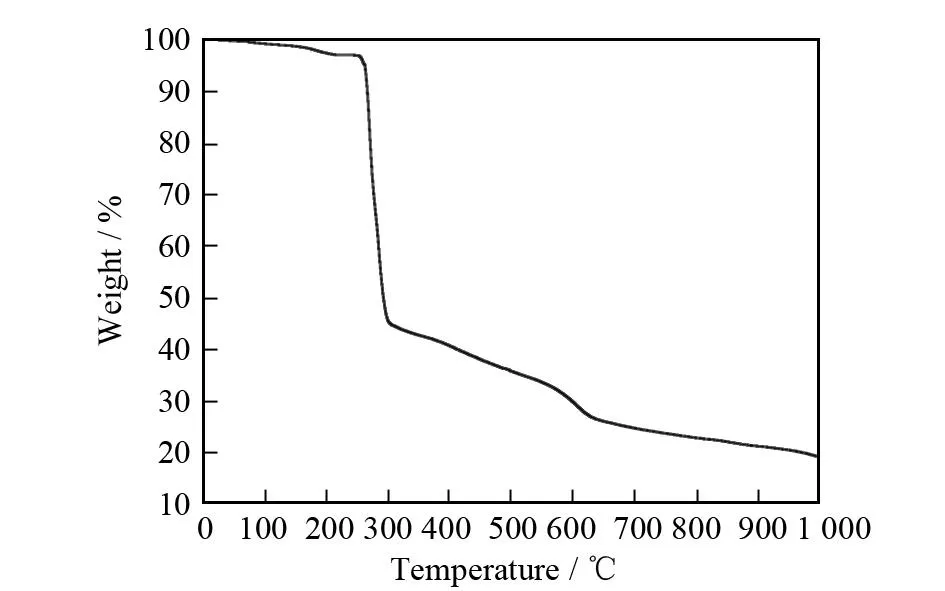
Fig.6 Thermogravimetric analysis of the complex from 25 to 1 000 ℃
2.5 Magnetic Properties
Variable-temperature magnetic susceptibility of the complex is measured in the 2.0-300 K. The variation of the ratio of the magnetic,χM-1andχMTare shown in Fig. 7. The thermal evolution ofχM-1obeys the Curie-Weiss law,χM=C/(T-θ) in the range of 2-300 K with a Weiss constantθ=-12.668 K and a Curie constantC= 0.390 cm3·K·mol-1, respectively. At 300 K, theχMTvalue is 0.069 cm3·mol-1·K (0.744μB), which is lower than the expected value (0.375 cm3·K·mol-1, 1.731μB) for magnetically isolated high-spin Cu(II) (SCu= 1/2,g=2.0). With the temperature decreasing, theχMTvalue changes smoothly to a minimum of 0.011 cm3·K·mol-1at 2 K. The negativeθvalue and theχMTvsTcurve reveal typical antiferromagnetic interactions between the Cu(II) centers. The shortest Cu…Cu distance across the (O2C-C-C-CO2)2bridge (0.523 0(1) nm) is shorter than the bpy bridge (1.115 8(3) nm), suggesting that the observed antiferromagnetic interaction should arise from the magnetic super exchange through the (O2C-C-C-CO2)2bridges.
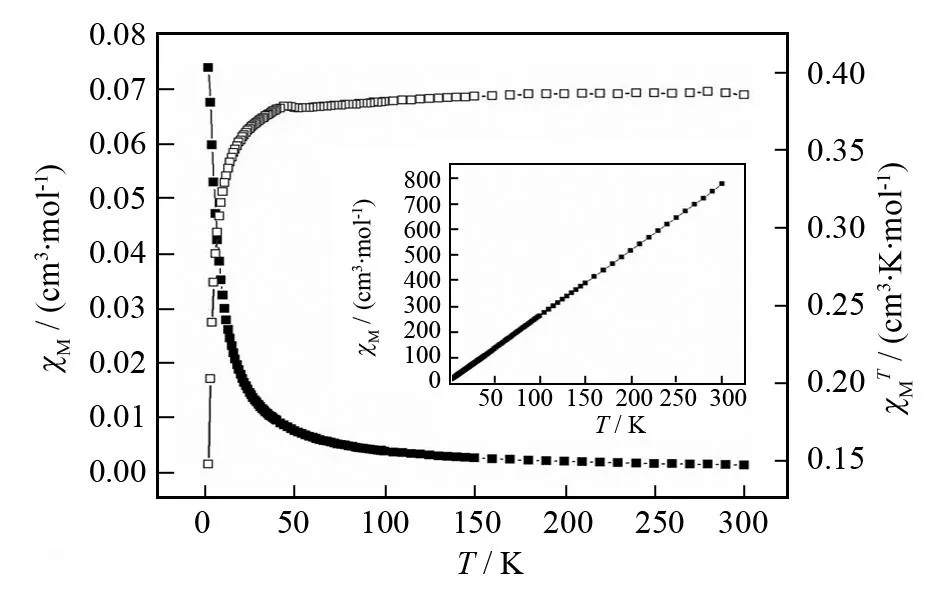
Fig.7 Thermal variation of χ M and χ MT for the complex. Insert: Plot of thermal variation of χ M -1
3Conclusions
In summary, we report here a novel 3D porous metal-organic framework generated from 3,3′,4,4′-benzophenone-tetracarboxylate in the presence of auxiliary 4,4′-bipyridine ligand, and the 3D framework remains intact after the guest water molecules encapsulated in the cavities are removed. The 2D layer in the complex constitutes of right-handed helical chains. Moreover, the complex presents antiferromagnetic behavior and photoluminescence property, the result suggest that the as-synthesized complex may be potential multifunctional materials in photoluminescence and magnetism.
CCDC-995728 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
References:
[1] ZHAO X B, XIAO B, FLETCHER A J, et al. Hysteretic adsorption and desorption of hydrogen by nanoporous metal-organic frameworks [J]. Science, 2004, 306(5698): 1012-1015.
[2] YAGHI O M, O′KEEFFE M, OCKWIG N W, et al. Reticular synthesis and the design of new materials [J]. Nature, 2003, 423(6941): 705-714.
[3] LEE J Y, FARHA O K, ROBERTS J, et al. Metal-organic framework materials as catalysts [J]. Chem Soc Rev, 2009, 38(5): 1450-1459.
[4] KRENO L E, LEONG K, FARHA O K, et al. Metal-organic framework materials as chemical sensors [J]. Chem Rev, 2011, 112(2): 1105-1125.
[5] MULFORT K L, HUPP J T. Chemical reduction of metal-organic framework materials as a method to enhance gas uptake and binding [J]. J Am Chem Soc, 2007, 129(31): 9604-9605.
[6] FÉREY G, MELLOT-DRAZNIEKS C, SERRE C, et al. A chromium terephthalate-based solid with unusually large pore volumes and surface area [J]. Science, 2005, 309(5743): 2040-2042.
[7] YANG G P, WANG Y Y, MA L F, et al. Hydrothermal syntheses and characterizations of three coordination polymers based on mixed organic ligands [J]. Eur J Inorg Chem, 2007, 2007(24): 3892-3898.
[9] LI S L, LAN Y Q, MA J C, et al. Metal-organic frameworks based on different benzimidazole derivatives: effect of length and substituent groups of the ligands on the structures [J]. Cryst Growth Des, 2010, 10(3): 1161-1170.
[10] SU C Y, GOFORTH A M, SMITH M D, et al. Exceptionally stable, hollow tubular metal-organic architectures: synthesis, characterization, and solid-state transformation study [J]. J Am Chem Soc, 2004, 126(11): 3576-3586.
[11] BIRADHA K, HONGO Y, FUJITA M. Crystal-to-crystal sliding of 2D coordination layers triggered by guest exchange [J]. Angew Chem Int Ed, 2002, 41(18): 3395-3398.
[12] LEONG W L, VITTAL J J. One-dimensional coordination polymers: complexity and diversity in structures, properties, and applications [J]. Chem Rev, 2010, 111(2): 688-764.
[13] YANG L R, ZHANG H M, YOU Q, et al. Coordination polymers based on 3,3′,4,4′-benzophenone-tetracarboxylate and N-containing pillars: syntheses, structure, characterization and properties [J]. CrystEngComm, 2013, 15(37): 7505-7514.
[14] ZHANG J, LI Z J, KANG Y, et al. Hydrothermal syntheses, crystal structures, and properties of a novel class of 3,3′,4,4′-benzophenone-tetracarboxylate (BPTC) polymers [J]. Inorg Chem, 2004, 43(25): 8085-8091.
[15] LI S L, LAN Y Q, MA J F, et al. Structures and luminescent properties of seven coordination polymers of zinc (II) and cadmium (II) with 3, 3′, 4, 4′-benzophenone tetracarboxylate anion and bis (imidazole) [J]. Cryst Growth Des, 2008, 8(2): 675-684.
[16] WANG H, WANG Y Y, YANG G P, et al. A series of intriguing metal-organic frameworks with 3, 3′, 4, 4′-benzophenonetetracarboxylic acid: structural adjustment and pH-dependence [J]. CrystEngComm, 2008, 10(11): 1583-1594.
[17] XIAO D R, WANG E B, AN H Y, et al. A bridge between pillared-layer and helical structures: a series of three-dimensional pillared coordination polymers with multiform helical chains [J]. Chem Eur J, 2006, 12(25): 6528-6541.
[18] ZHANG Y N, WANG H, LIU J Q, et al. Novel silver (I) compounds assembled from hybrid ligands based on linear or T-shaped coordination environment [J]. Inorg Chem Commun, 2009, 12(7): 611-614.
[19] SHELDRICK G M. SHELXL-97 program for crystal structure solution and refinement [CP]. Göttingen: University of Göttingen, 1997.
[20] LOGANATHAN R, RAMAKRISHNAN S, SURESH E, et al. Mixed ligandμ-phenoxo-bridged dinuclear copper (II) complexes with diimine co-ligands: efficient chemical nuclease and protease activities and cytotoxicity [J]. Dalton Trans, 2014, 43(16): 6177-6194.
[21] QU Z K, YU K, ZHAO Z F, et al. An organic-inorganic hybrid semiconductor material based on Lindqvist polyoxomolybdate and a tetra-nuclear copper complex containing two different ligands [J]. Dalton Trans, 2014, 43(18): 6744-6751.
[22] TANCREZ N, FEUVRIE C, LEDOUX I, et al. Lanthanide complexes for second order nonlinear optics: evidence for the direct contribution of f electrons to the quadratic hyperpolarizability1 [J]. J Am Chem Soc, 2005, 127(39): 13474-13475.
[23] LI X F, HAN Z B, CHENG X N, et al. Studies on the radii dependent lanthanide self-assembly coordination behaviors of a flexible dicarboxylate ligand [J]. Inorg Chem Commun, 2006, 9(11): 1091-1095.
[24] LIU M S, YU Q Y, CAI Y P, et al. One-, two-, and three-dimensional lanthanide complexes constructed from pyridine-2, 6-dicarboxylic acid and oxalic acid ligands [J]. Cryst Growth Des, 2008, 8(11): 4083-4091.
[25] AGHABOZORG H, MOGHIMI A, MANTEGHI F, et al. A nine-coordinated ZrIVcomplex and a self-assembling system obtained from a proton transfer compound containing 2, 6- pyridinedicarboxylate and 2, 6-pyridinediammonium; synthesis and X-ray crystal structure [J]. Z Anorg Allg Chem, 2005, 631(5): 909-913.
[26] LAN Y Q, LI S L, WANG X L, et al. Self-assembly of polyoxometalate-based metal organic frameworks based on octamolybdates and copper-organic units: from CuII, CuI, II to CuI via changing organic amine [J]. Inorg Chem, 2008, 47(18): 8179-8187.
[27] NIU J, ZHANG S, CHEN H, et al. 1-D, 2-D, and 3-D organic-inorganic hybrids assembled from Keggin-type polyoxometalates and 3d-4f heterometals [J]. Cryst Growth Des, 2011, 11(9): 3769-3777.
[责任编辑:任铁钢]
