Oncogenic role of microRNA-423-5p in hepatocellular carcinoma
2015-12-24
Hangzhou, China
Oncogenic role of microRNA-423-5p in hepatocellular carcinoma
Li-Ming Wu, Jian-Song Ji, Zhe Yang, Chun-Yang Xing, Ting-Ting Pan, Hai-Yang Xie, Feng Zhang, Li Zhuang, Lin Zhou and Shu-Sen Zheng
Hangzhou, China
BACKGROUND: It has been found that microRNA-423-5p (miR423-5p) is an oncogenic factor and frequently upregulated in gastric carcinoma. However, the involvement of miR423-5p in hepatocellular carcinoma (HCC) has been rarely reported. The aim of this study was to assess whether miR423-5p is aberrantly expressed in HCC tissues, and to characterize its roles in the cancerous biology of HCC.
METHODS: HCC and corresponding nonmalignant tissues were obtained from 115 patients during liver transplantation to detect the expression level of miR423-5p. The miR423-5p mimic and inhibitor were transfected into LM3 cell line. Cell viability assay, cell cycle analysis, transwell invasion and migration experiments were used to evaluate the oncogenic role of miR423-5p.
RESULTS: miR423-5p was significantly upregulated in HCC compared with nonmalignant tissues, and this upregulation was negatively associated with recurrence-free survival. For patients beyond the Milan criteria, low expression of miR423-5p was correlated with better prognosis. Functional analysis showed that miR423-5p enhanced the proliferative, invasive and migratory capacity of HCC cells.
CONCLUSIONS: miR423-5p contributed to the tumorigenesis and progression of HCC. It could be a new predictor in HCC patients beyond the Milan criteria and would help to improve patient outcomes and enlarge recipient pools of liver transplantation.
(Hepatobiliary Pancreat Dis Int 2015;14:613-618)
hepatocellular carcinoma;
miR423-5p;
proliferation;
invasion;
oncogene
Introduction
Hepatocellular carcinoma (HCC) is among the
most common cancers worldwide, which leads
to an estimate of 695 900 deaths annually.[1,2]Although the treatment of HCC has been dramatically improved, its prognosis remains dismal. Early diagnosis is crucial to the treatment and new biomarkers are helpful for diagnosis and clinical treatment of HCC patients.
MicroRNAs (miRNAs) are about 22 nucleotides, noncoding RNAs.[3]They act mainly as negative regulators of gene expression through sequence-specific base pairing with target mRNAs[4]and involve in various of biological pathways.[5,6]Although the molecular mechanism of miR NAs remains far from completely understood, growing evidences have shown that there is a strong association between cancer and miRNAs.[7,8]It is well documented that miRNAs play important roles in oncologic related processes, such as proliferation, apoptosis, invasion and differentiation.[9-12]
MicroRNA-423 (miR423) is a recently identified new member of the miRNAs family that was first cloned from neuroblastoma.[13]Liu et al[14]demonstrated that miR423 inhibited trefoil factor 1 (TFF1) expression, and regulated proliferative and invasive ability of gastric cancer cells in a TFF1-dependent manner. In breast cancer, it hasfound that CC homozygote inrs6505162of miR423 is a protective factor for carcinogenesis of breast cancer.[15]The high miR423-5p expression increased the response to sorafenib treatment in HCC patients involving in autophagy regulation in HCC cells.[16]Though miR423 has been identified as an oncogene in gastric cancer, its role in HCC is rarely studied. The aim of this study was to assess whether miR423-5p was aberrantly expressed in HCC tissues, and characterize its roles in the cancerous biology of HCC.
Methods
Patients
HCC and corresponding nonmalignant tissues were obtained from 115 patients who underwent liver transplantation from 2001 to 2010 in our hospital. Written consent was obtained from patients enrolled in this study. This study was approved by the Ethics Committee of the First Affiliated Hospital of Zhejiang University School of Medicine.
Cell culture
Cell culture was performed as we previously described.[17]Briefly, human HCC cell line MHCC-LM3 was grown in DMEM medium (Gibco-Invitrogen, California, USA) containing 10% FBS (SAFC Biosciences, Victoria, Australia), and incubated at 37 ℃ in a 5% carbon dioxide incubator. Cells were passaged with trypsin (Gibco-Invitrogen) when they reached 90% confluency.Transfection of mimic or inhibitor of miR423-5p
To transfect miRNA mimic (Qiagen, Hilden, Germany) and miRNA inhibitors (Qiagen) into HCC cells, we used Lipofectamine 2000 (Invitrogen) according to the manufactuer's instructions. Briefly, 1×106cells were added into each well of a 6-well plate filled with complete culture medium and grown to 50% confluency. Then the culture medium was replaced with transfection medium (Opti-MEM containing Lipofectamine 2000 and 200 pmol of miR423-5p inhibitor or 5 pmol of miR423-5p mimic). Six hours later, the transfection medium was changed to complete culture medium. The cells were then incubated for another 72 days before use.
Quantitative real-time PCR analysis
Trizol (Invitrogen) and miScript II RT Kit (Qiagen) were used to extract RNA from cells. Quantitative realtime PCR was conducted using miScript SYBR Green Kit (Qiagen) on Applied Biosystems ABI 7700 System according to the manufacturer's instructions. ΔΔCt analysis was used to analyze the PCR data.
Cell growth analysis
CCK-8 kit (Dojindo Laboratories, Kumamoto, Japan) was used to measure the cell growth as we previously described.[17]Briefly, 24 hours after transfection with miR423-5p mimic or inhibitor, 2×103cells were plated in each experimental well of the 96-well plates and culture for 24, 48, 72 and 96 hours. Then the culture media was replaced with DMEM containing 10% CCK-8 and cultured at 37 ℃ for 1 hour. The absorbance was detected at 450 nm.
Cell cycle analysis
Cell cycle analysis was conducted as previously reported.[17]Briefly, 72 hours after transfected with miR423-5p mimic or inhibitor, cells were collected and treated with DNA PREP kit (Beckman Coulter, Florida, USA) according to the instruction. FC-500 flow cytometer (Beckman Coulter) was used to detect the percentage of cells in each phase.
Transwell invasion and migration experiments
Transwell invasion and migration experiments were conducted as previously reported.[18]Briefly, the polycarbonate membrane of the upper chamber was coated with Matrigel for invasion experiments. Seventy-two hours after transfection with miR423-5p mimic or inhibitor, cells were collected for transwell experiments. Cells (1×104) were plated in the upper chamber containing DMEM without FBS. DMEM with 10% FBS was added into the lower compartment. After 18 (for migration experiments) or 36 (for invasion experiments) hours, the cells attached to the upper side of the membrane were cleaned, and the rest cells were counted using microscopy.
Statistical analysis
Pearson's product-moment correlation coefficient and the Chi-square test were used to assess the association of miR423-5p expression with clinical parameters. To compare difference between the two groups, independent Student'sttest was used. The Kaplan-Meier method was used to analyze the association of miR423-5p expression and recurrence-free survival. APvalue <0.05 was considered statistically significant.
Results
Association of miR423-5p expression with clinical characteristics of HCC
To evaluate the expression level of miR423-5p in 115HCC patients, quantitative real-time PCR was performed. Patients were segregated into miR423-5p low/high expression groups based on receiver operating characteristics analysis in terms of overall survival [cut-off point 1.7, which can achieve the most optimal predictive sensitivity (71.7%) and specificity (60.0%)]. Our results showed that compared with corresponding nonmalignant tissues, 65 (56.5%) of the 115 HCC tissues showed higher miR423-5p expression. However, this overexpression of miR423-5p in HCC was not associated with any tumor features such as vascular invasion, tumor differentiation, tumor size and so on (Table 1). The Kaplan-Meier method was used to assess whether miR423-5p could influence the recurrence-free survival of HCC patients. The results showed that miR423-5p was negatively associated with recurrence-free survival (P<0.002, Fig. 1A). Since the Milan criteria are most frequently used to predict the survival of HCC patients who received liver transplantation and select candidates with HCC for liver transplantation,[20]we analyzed the prognostic utility of miR423-5p for patients based on the Milan criteria. We found that miR423-5p expression level had no significant effect on recurrence-free survival for patients within the Milan criteria (P=0.158, Fig. 1B). But for patients beyond the Milan criteria, lower miR423-5p expression was correlated with longer recurrence-free survival (P=0.001, Fig. 1C). We thus evaluated the risk indicators for the tumor recurrence (Table 2). Cox-multivariate analysis revealed that portal vein tumor thrombus, tumor size, AFP level and miR423-5p expression were prognostic factors independent of the Milan criteria (Table 3).
miR423-5p promoted the growth of HCC
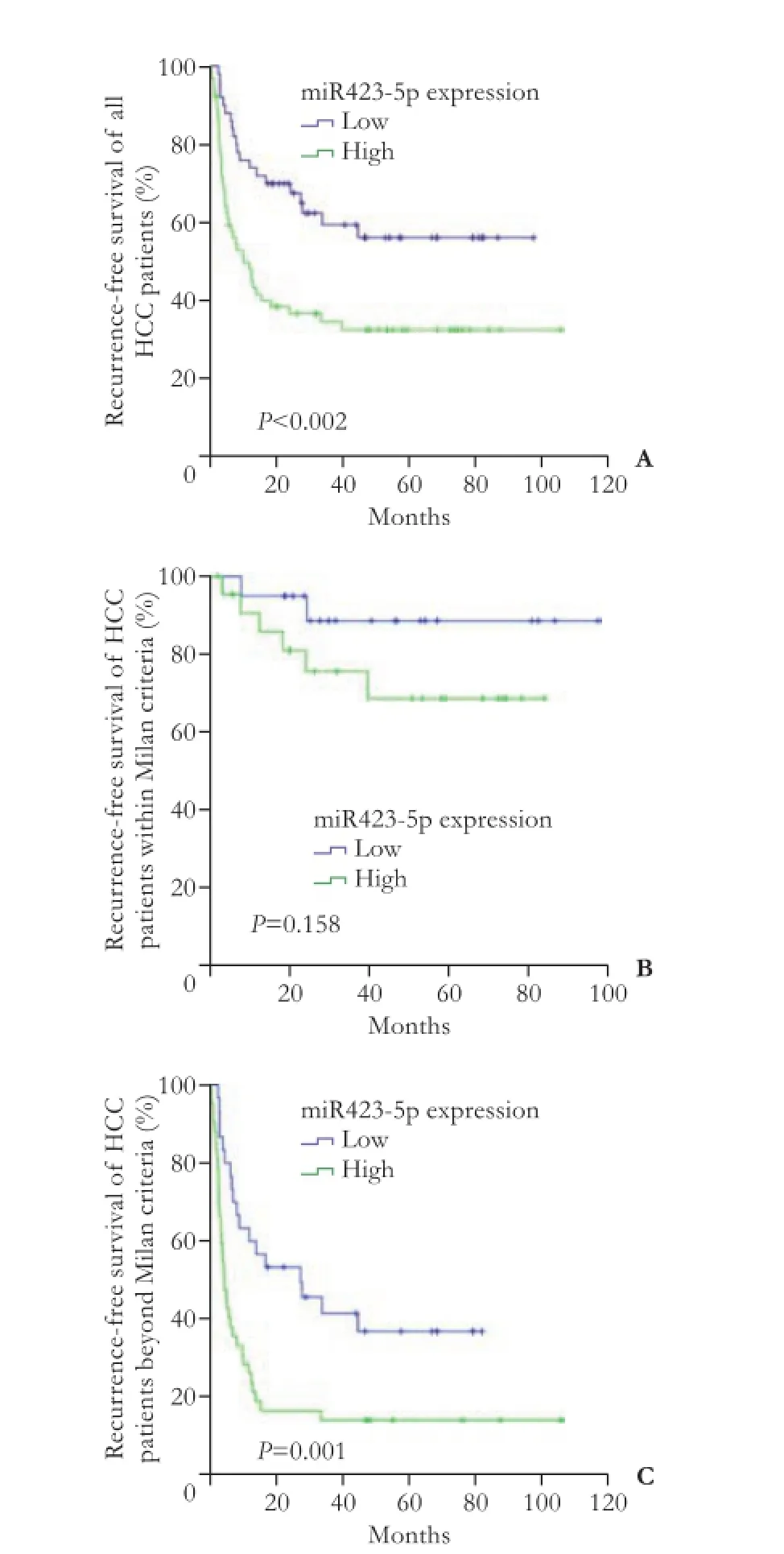
Fig. 1. The association of miR423-5p expression with recurrencefree survival in all HCC patients (A) and HCC patients within or beyond the Milan criteria (B and C).
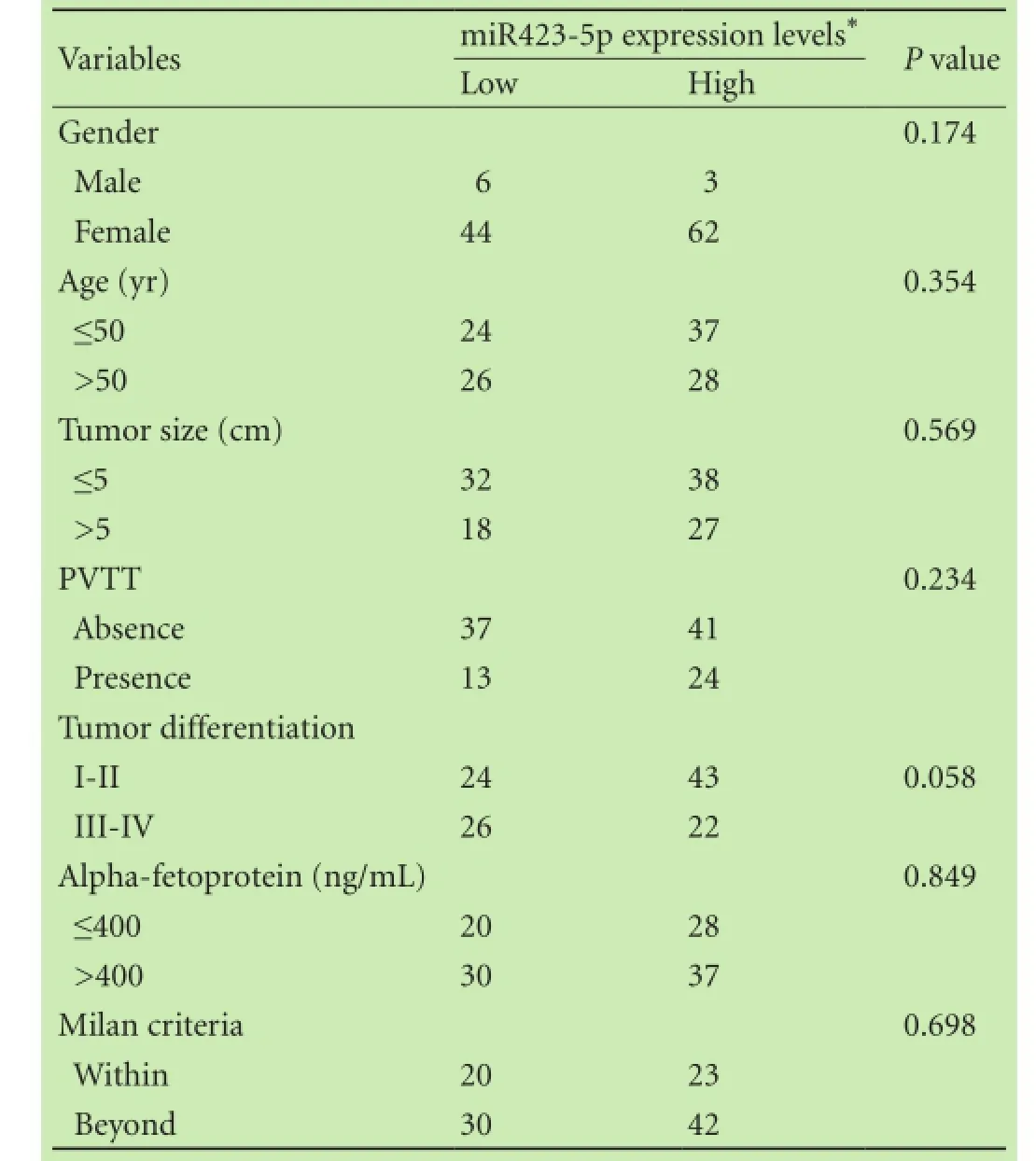
Table 1. Correlations between miR423-5p expression and clinicopathological features
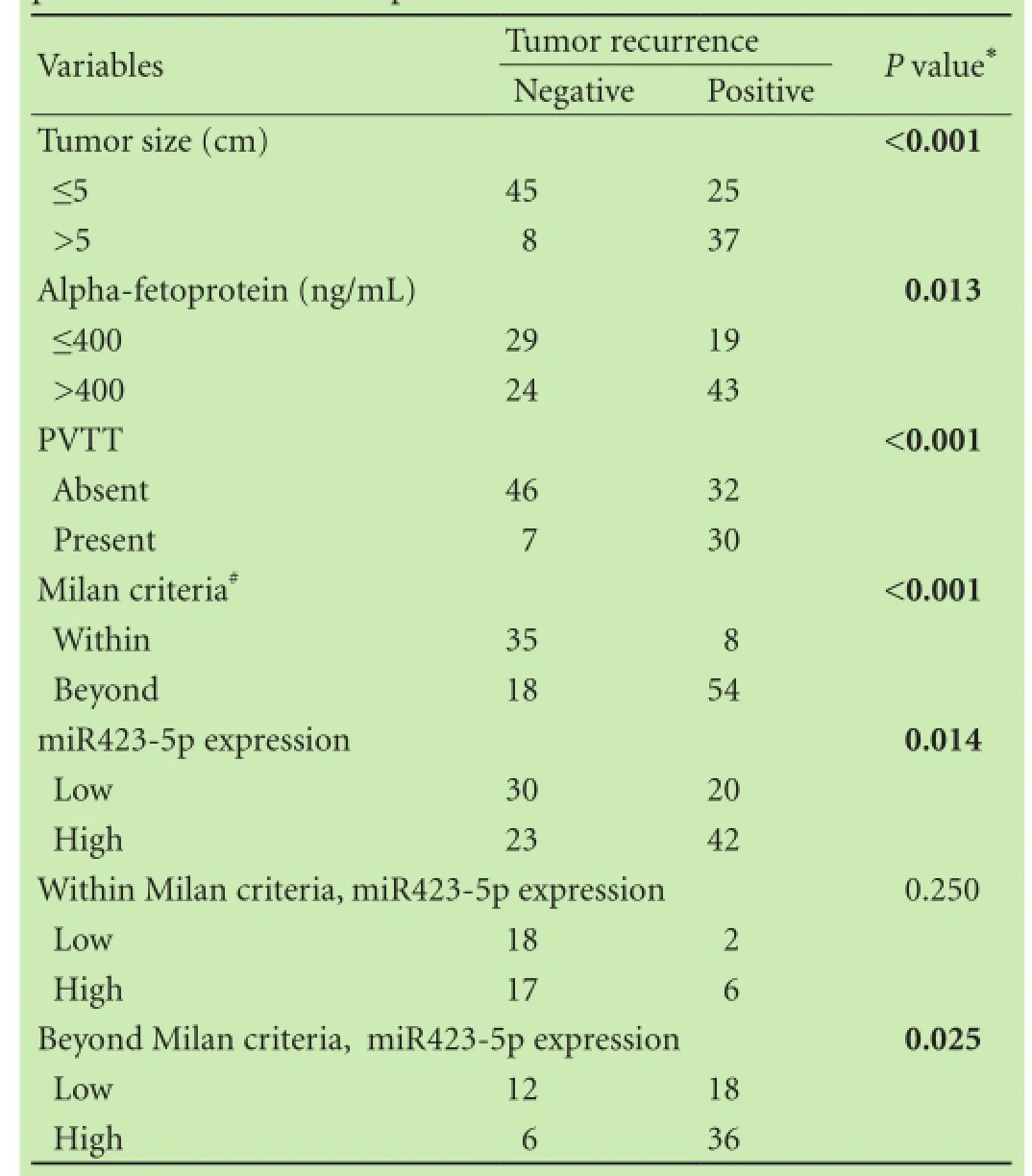
Table 2. Univariate analyses of predictors of recurrence in HCC patients after liver transplantation
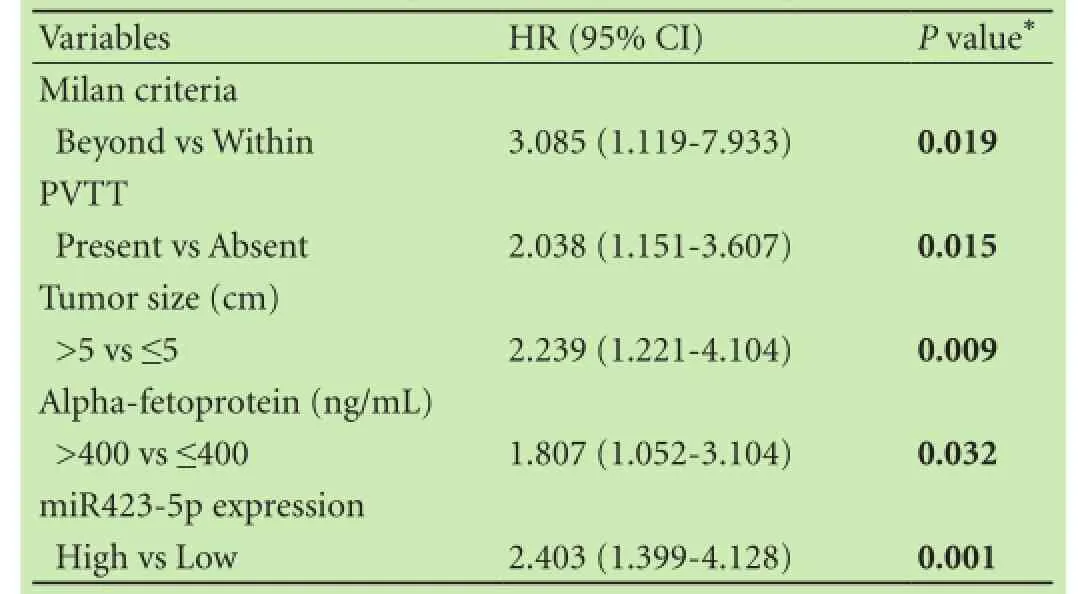
Table 3. Cox-multivariate analyses of independent predictive factor for recurrence in patients after liver transplantation
Quantitative real-time PCR analysis showed that miR423-5p mimic and inhibitor altered the expression of miR423-5p effectively (Fig. 2). Transient overexpression of miR423-5p increased the rate of cell growth and vice versa (Fig. 3). Cell cycle experiments showed overexpression of miR423-5p decreased G1 and increased G2/M cells. Reverse result was obtained by depressing miR423-5p expression with miR423-5p inhibitor, further consolidate our finding that miR423-5p has the ability to promote the growth of HCC cells by facilitating G1/S and S/G2 transition (Fig. 4).
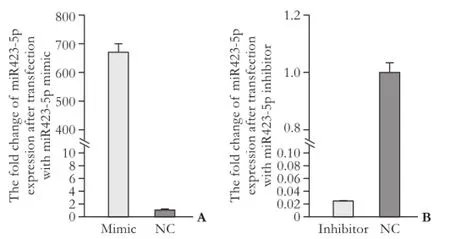
Fig. 2. The fold change of miR423-5p expression after transfection with miR423-5p mimic (A) or miR423-5p inhibitor (B).
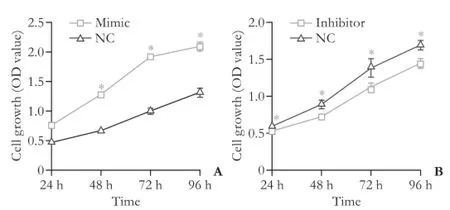
Fig. 3. The impact of transfection of miR423-5p mimic or miR423-5p inhibitor on cell proliferation. *:P<0.05, compared with the control (NC) group.
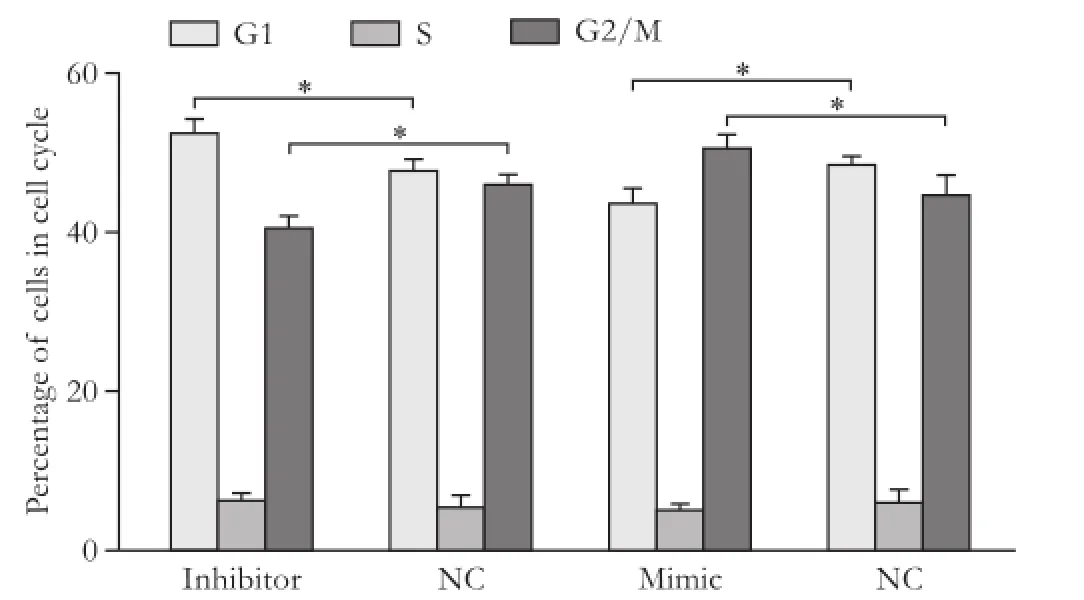
Fig. 4. The impact of transfection of miR423-5p mimic or miR423-5p inhibitor on cell cycles. *:P<0.05, compared with the control (NC) group.
miR423 increased the invasive ability of HCC cells
The expression of miR423-5p was positively associated with the growth of HCC cells; overexpression of miR423-5p significantly increased the invasion of LM3 cells, while miR423-5p inhibition impaired the invasion of LM3 cells (Fig. 5A). Compared with the control group,cell migration ability was significantly promoted after overexpression of miR423-5p and vice versa (Fig. 5B).
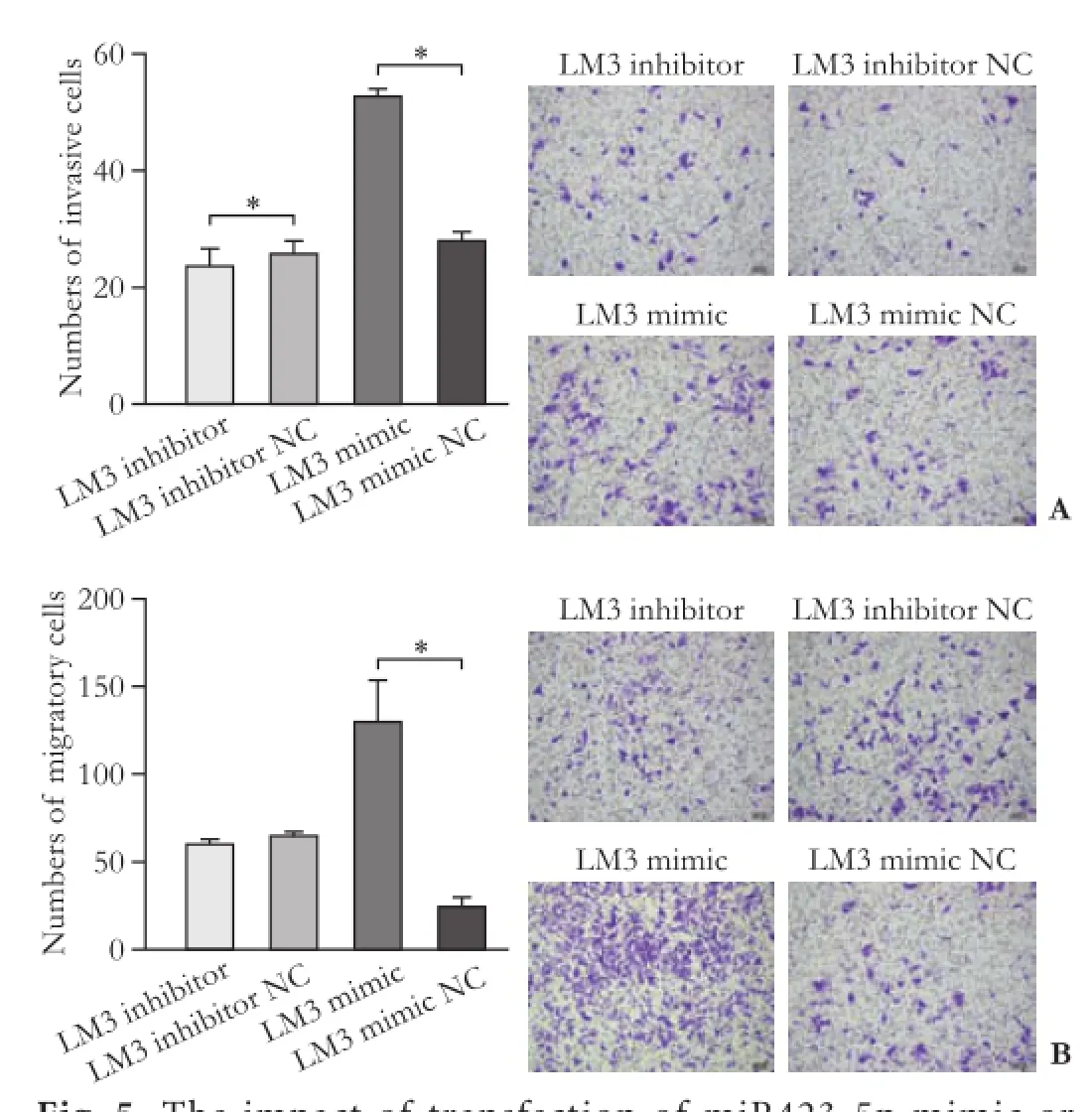
Fig. 5. The impact of transfection of miR423-5p mimic or miR423-5p inhibitor on invasive (A) and migratory (B) abilities of LM3 cells. *:P<0.05, compared with the control (NC) group.
Discussion
Though miR423-5p was found to be involved in the carcinogenesis of gastric cancer,[14]its role in HCC has rarely been studied. The present study showed that miR423-5p was commonly upregulated in HCC tissues, and this upregulation was associated with a lower recurrence-free survival rate of patients after liver transplantation.
The Milan criteria are frequently used for patient selection of liver transplantation. More recently, many researchers have argued that these criteria are too strict, and may leave out some patients with a favorable prognosis.[19,20]Thus, it is important to find new molecular indicators to classify HCC patients out of the Milan criteria. The results of our study indicated that for patients beyond the Milan criteria, lower miR423-5p contributed to longer recurrence-free survival, indicating that miR423-5p could be a new predictor in HCC patients out of the Milan criteria.
To evaluate the molecular functions of miR423-5p in HCC, we investigated whether altered miR423-5p expression affected the malignant behaviors of HCC. The pro-proliferative effects of miR423-5p have been well established in gastric cancer.[14]In this study, we found that miR423-5p could enhance the proliferation of HCC cells. Furthermore, our results demonstrated that overexpression of miR423-5p facilitated G1/S and S/G2 transition, matched well with the previous study in gastric cancer.[14]The underlying mechanism of miR423-5p on cell proliferation is still not clear. miR423-5p may play its role through the TFF1 dependent pathway. It has been reported that miR423-5p could negatively regulated the expression of TFF1. Liu et al[14]found that the inhibition of miR423-5p expression increased the mRNA expression of TFF1, and suppressed cyclin D1 and cyclin D3 expression in a TFF1 dependent manner, which are major regulators of the cell cycle transition.[21,22]Cyclin D could form complexes with CDK4 and CDK6, and phosphorylate retinoblastoma to the inactive form, causing the upregulation of genes (like cyclin E) involved in cell cycle progression.[23,24]
Metastasis is a hallmark of malignant process of HCC.[25]The clinical data of our study showed a positive association between miR423-5p expression and metastatic ability of HCC cells. We found that cell invasion was significantly increased after overexpression of miR423-5p and vice versa. This finding could also be explained by the negative regulation of TFF1 by miR423-5p. It has been reported that TFF1 increased the invasive ability of human HCT8/S11 cells, and the overexpression of TFF1 showed a migratory and invasive phenotype in epithelial cells.[26,27]There was a negative correlation between the TFF1 and β-catenin, and transfection with the miR423-5p mimics caused the inhibition of TFF1 expression and the increase of β-catenin expression,[14]which plays an important role in both cell adhesion and proliferation. However, whether miR423-5p plays its role through other pathways independent of TFF1 awaits further studies.
In conclusion, miR423-5p commonly upregulated in HCC cells is associated with poor prognosis. It plays an important role in the proliferation and invasion of HCC cells, and inhibition of miR423-5p reverts the malignant phenotype of HCC.
Contributors:ZSS proposed the study. WLM and JJS performed research and wrote the first draft. YZ, XCY and PTT collected and analyzed the data. WLM and JJS contributed equally to the article. All authors contributed to the design and interpretation of the study and to further drafts. ZSS is the guarantor.
Funding:This study was supported by grants from the Fundamental Research Funds for the Central Universities (2015FZA7013), the Natural Science Foundation of Zhejiang Province (LY15H160021), the National Natural Science Foundation of China (81101840 and 81302074), the Medical Science and Technology Project of Zhejiang Province (201472813), the Medical and Health Platform Backbone Personnel Plan of Zhejiang Province (2012RCB015 and 2013KYB107), the Innovative Research Group of the National Natural Science Foundation of China (81421062).
Ethical approval:This study was approved by the Ethics Commit-
tee of the First Affiliated Hospital of Zhejiang University School of Medicine.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011;61:69-90.
2 Lau WY, Lai EC. Hepatocellular carcinoma: current management and recent advances. Hepatobiliary Pancreat Dis Int 2008;7:237-257.
3 Henry JC, Azevedo-Pouly AC, Schmittgen TD. MicroRNA replacement therapy for cancer. Pharm Res 2011;28:3030-3042.
4 Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell 2009;136:215-233.
5 Ambros V. The functions of animal microRNAs. Nature 2004;431:350-355.
6 Alvarez-Garcia I, Miska EA. MicroRNA functions in animal development and human disease. Development 2005;132:4653-4662.
7 Garzon R, Marcucci G, Croce CM. Targeting microRNAs in cancer: rationale, strategies and challenges. Nat Rev Drug Discov 2010;9:775-789.
8 Ryan BM, Robles AI, Harris CC. Genetic variation in microRNA networks: the implications for cancer research. Nat Rev Cancer 2010;10:389-402.
9 Yamakuchi M, Lotterman CD, Bao C, Hruban RH, Karim B, Mendell JT, et al. P53-induced microRNA-107 inhibits HIF-1 and tumor angiogenesis. Proc Natl Acad Sci U S A 2010;107: 6334-6339.
10 Boyerinas B, Park SM, Hau A, Murmann AE, Peter ME. The role of let-7 in cell differentiation and cancer. Endocr Relat Cancer 2010;17:F19-36.
11 Wu Q, Jin H, Yang Z, Luo G, Lu Y, Li K, et al. MiR-150 promotes gastric cancer proliferation by negatively regulating the pro-apoptotic gene EGR2. Biochem Biophys Res Commun 2010;392:340-345.
12 Song B, Wang C, Liu J, Wang X, Lv L, Wei L, et al. MicroRNA-21 regulates breast cancer invasion partly by targeting tissue inhibitor of metalloproteinase 3 expression. J Exp Clin Cancer Res 2010;29:29.
13 Afanasyeva EA, Hotz-Wagenblatt A, Glatting KH, Westermann F. New miRNAs cloned from neuroblastoma. BMC Genomics 2008;9:52.
14 Liu J, Wang X, Yang X, Liu Y, Shi Y, Ren J, et al. miRNA423-5p regulates cell proliferation and invasion by targeting trefoil factor 1 in gastric cancer cells. Cancer Lett 2014;347:98-104.
15 Smith RA, Jedlinski DJ, Gabrovska PN, Weinstein SR, Haupt L, Griffiths LR. A genetic variant located in miR-423 is associated with reduced breast cancer risk. Cancer Genomics Proteomics 2012;9:115-118.
16 Stiuso P, Potenza N, Lombardi A, Ferrandino I, Monaco A, Zappavigna S, et al. MicroRNA-423-5p promotes autophagy in cancer cells and is increased in serum from hepatocarcinoma patients treated with sorafenib. Mol Ther Nucleic Acids 2015;4: e233.
17 Xing C, Zhou W, Ding S, Xie H, Zhang W, Yang Z, et al. Reversing effect of ring finger protein 43 inhibition on malignant phenotypes of human hepatocellular carcinoma. Mol Cancer Ther 2013;12:94-103.
18 Lai MC, Yang Z, Zhou L, Zhu QQ, Xie HY, Zhang F, et al. Long non-coding RNA MALAT-1 overexpression predicts tumor recurrence of hepatocellular carcinoma after liver transplantation. Med Oncol 2012;29:1810-1816.
19 Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol 2009;10: 35-43.
20 Yao FY, Ferrell L, Bass NM, Bacchetti P, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: comparison of the proposed UCSF criteria with the Milan criteria and the Pittsburgh modified TNM criteria. Liver Transpl 2002;8:765-774.
21 Bienvenu F, Jirawatnotai S, Elias JE, Meyer CA, Mizeracka K, Marson A, et al. Transcriptional role of cyclin D1 in development revealed by a genetic-proteomic screen. Nature 2010;463: 374-378.
22 Sankaran VG, Ludwig LS, Sicinska E, Xu J, Bauer DE, Eng JC, et al. Cyclin D3 coordinates the cell cycle during differentiation to regulate erythrocyte size and number. Genes Dev 2012;26: 2075-2087.
23 Weinberg RA. The retinoblastoma protein and cell cycle control. Cell 1995;81:323-330.
24 Musgrove EA, Caldon CE, Barraclough J, Stone A, Sutherland RL. Cyclin D as a therapeutic target in cancer. Nat Rev Cancer 2011;11:558-572.
25 Schafer DF, Sorrell MF. Hepatocellular carcinoma. Lancet 1999;353:1253-1257.
26 Emami S, Le Floch N, Bruyneel E, Thim L, May F, Westley B, et al. Induction of scattering and cellular invasion by trefoil peptides in src- and RhoA-transformed kidney and colonic epithelial cells. FASEB J 2001;15:351-361.
27 Rodrigues S, Nguyen QD, Faivre S, Bruyneel E, Thim L, Westley B, et al. Activation of cellular invasion by trefoil peptides and src is mediated by cyclooxygenase- and thromboxane A2 receptor-dependent signaling pathways. FASEB J 2001;15:1517-1528.
Received March 14, 2015
Accepted after revision August 30, 2015
Author Affiliations:Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, Hangzhou 310003, China (Wu LM, Yang Z, Xing CY, Xie HY, Zhang F, Zhuang L, Zhou L and Zheng SS); Key Laboratory of Combined Multi-organ Transplantation, Ministry of Public Health; Key Laboratory of Organ Transplantation of Zhejiang Province; Division of Hepatobiliary and Pancreatic Surgery, First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, China (Wu LM, Yang Z, Xing CY, Pan TT, Xie HY, Zhang F, Zhuang L, Zhou L and Zheng SS); Lishui Hospital of Zhejiang University, Lishui 323000, China (Ji JS)
Shu-Sen Zheng, MD, PhD, FACS, Division of Hepatobiliary and Pancreatic Surgery, First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, China (Tel: 86-571-87236570; Fax: 86-571-87236466; Email: shusenzheng@zju.edu.cn)
© 2015, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(15)60038-8
Published online November 18, 2015.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Gut microbiota and non-alcoholic fatty liver disease
- Risk factors of metabolic syndrome after liver transplantation
- Combined Hangzhou criteria with neutrophillymphocyte ratio is superior to other criteria in selecting liver transplantation candidates with HBV-related hepatocellular carcinoma
- Warm HTK donor pretreatment reduces liver injury during static cold storage in experimental rat liver transplantation
- Intrahepatic distant recurrence following complete radiofrequency ablation of small hepatocellular carcinoma: risk factors and early MRI evaluation
- Ankaflavin ameliorates steatotic liver ischemiareperfusion injury in mice
