Sero-prevalence and associated risk factors of Toxoplasma gondii infection among pregnant women attending antenatal care at Felege Hiwot Referral Hospital, northwest Ethiopia
2015-12-23KefaleAwokeEndalkachewNibretAbainehMunshea
Kefale Awoke, Endalkachew Nibret, Abaineh Munshea
Biology Department, Bahir Dar University, P.O.Box 79, Bahir Dar, Ethiopia
Sero-prevalence and associated risk factors of Toxoplasma gondii infection among pregnant women attending antenatal care at Felege Hiwot Referral Hospital, northwest Ethiopia
Kefale Awoke, Endalkachew Nibret*, Abaineh Munshea
Biology Department, Bahir Dar University, P.O.Box 79, Bahir Dar, Ethiopia
ARTICLE INFO
Article history:
Received 15 April 2015
Received in revised form 20 May 2015
Accepted 15 June 2015
Available online 20 July 2015
Pregnant women
Seroprevalence
Toxoplasma gondii
Bahir Dar
Ethiopia
Objective: To determine the prevalence of toxoplasmosis and to assess the possible risk factors associated with the infection among pregnant women attending antenatal care center at Felege Hiwot Referral Hospital, Bahir Dar town, northwest Ethiopia. Methods: A hospital based cross-sectional study was designed to determine the prevalence of toxoplasmosis among pregnant women. Three hundred eighty four serum samples were collected from November 2013 to January 2014. Data on socio-demographic and predisposing factors were collected from each study participant with simple random sampling technique. The serum samples were examined for anti- Toxoplasma gondii (T. gondii) antibodies using latex agglutination test. Results: The overall seroprevalence of T. gondii among the pregnant women was 18.5%. All of T. gondii positive cases found to be positive only for IgG antibody. Significant association was observed between seroprevalence and presence of domestic cats [AOR=2.85, 95% CI: 1.66-4.90, P=0.000], consumption of raw or undercooked meat [AOR=1.98, 95% CI: 1.15-2.43, P=0.014] and history of abortion [AOR=2.47, 95% CI: 1.40-4.34, P=0.002]. No significant association was observed between seroprevalence and socio-demographic characters, gestational age, gravidity, consumption of raw vegetable, and blood transfusion. Conclusions: The seroprevalence of toxoplasmosis among pregnant women in Bahir Dar town was relatively high. Presence of domestic cats at home and consumption of raw or undercooked meat were identified as main risk factors for T. gondii infection. Therefore, health education towards avoiding eating raw or undercooked meat and avoiding contact with cats are recommended for prevention of miscarriage or defects during pregnancy.
1. Introduction
Toxoplasmosis is caused by an obligate intracellular tissue protozoan parasite Toxoplasma gondii, (T. gondii) which is able to infect humans as well as other warm blooded domestic and wild animals. The infection has a worldwide distribution with approximately one-third of the world population estimated to be exposed to this parasite[1]. T. gondii is transmitted to humans by eating raw or inadequately cooked infected meat, through ingestion of oocysts that cats have passed in their feces and women can transmit the infection transplacentally to their unborn fetus. Other infection pathways are transfusion, transplantation and direct contamination[2].
The importance of this parasite is mainly in pregnancy as it can cross the placental barrier to infect the foetal tissues and thereby cause congenital deformities. If acquired during pregnancy as a primary infection, the parasite can cross the placenta, leading to spontaneous miscarriage, death of the foetus in utero or severe congenital defects such as hydrocephaly, mental retardation or chorioretinitis[3,4]. Antenatal serological screening of T. gondii infection based on IgG and IgM detection is the mainstay in monitoring the risk for congenital toxoplasmosis. Maternal-fetal intervention for toxoplasmosis can be achieved through the use of drugs such as spiramycine which prevents congenital infection bymore than 60%[5].
The global status of T. gondii seroprevalence varied between regions and is a measure of the accumulated exposure to T. gondii in a particular social setting as well as being an indicator of the relative protection for a woman in the population against primary infection during pregnancy[6]. Seroprevalence in Europe is high, up to 54% in Southern European countries[7], whereas in sub-Saharan Africa the overall seroprevalence of T. gondii infection as high as 92.5% has been reported[8]. Recent evaluation of the epidemiology of T. gondii in different towns of Ethiopia has shown 83.6% among pregnant women in Jimma, 93.3% among HIV/AIDS patients in Addis Ababa and 60% among general population of Nazareth, Ethiopia[9-11].
The chance of acquiring acute infection with T. gondii is high during pregnancy and the infection would have potential tragic outcomes for the mother, the fetus and newborn despite the fact that it can be prevented[12]. In spite of the wide practice of keeping cats as domestic animals and presence of stray cats around, and suitable climatic conditions favoring survival of the parasite in the study area, to our knowledge, there is no regular serological screening of pregnant women for T. gondii infection. Moreover, there is no documented data about the prevalence of the disease and associated risk factors in the study area. It is believed that antenatal data on the prevalence of infectious diseases in the study area would give baseline information about the prevalence of T. gondii in pregnant women and also for planning and implementation of T. gondii control and prevention strategies.
2. Materials and methods
2.1. Study design and area
Hospital based cross–sectional study was conducted from November 2013 to January 2014 to determine the prevalence of toxoplasmosis and assess the possible risk factors associated with the infection among pregnant women attending antenatal care at Felege Hiwot Referral Hospital (FHRH), Bahir Dar, northwest Ethiopia. Bahir Dar is the capital of Amhara National Regional state, located approximately 578 km Northwest of Addis Ababa, capital city of Ethiopia. It is located at 11036' latitude N and 37023' longitude E and an elevation of 1 800 meter above sea level. Based on the 2014 Bureau of Finance and Economics Development of Amhara National Regional State, the population of Bahir Dar including rural kebeles is 284 020 of which 134 818 are males and 149 202 are females. Among females, 93 174 of them are between 15-49 years of age (reproductive age groups)[13].
The source population was all pregnant women who came to antenatal care center at Felege Hiwot Referral Hospital, Bahir Dar, Ethiopia. The study participants were those pregnant women who attended antenatal service at Felege Hiwot Referral Hospital during sample collection period were considered as a study population. Pregnant women who were critically ill, unable to communicate and those who were not willing to provide vital information and blood sample were excluded from the study.
2.2. Sample size determination and sampling technique
In the estimation of the sample size, statistical formula for sample size calculation was considered as a basis[14]. As seroprevalence of T. gondii is not known in the study area, the sample size was calculated with a prevalence of 50% and a total of 384 pregnant women were included in the study.
n= Z2P (1-P) ∕ d2
Where n=sample size
Z=critical value at 5% level (1.96) P= prevalence (50%)
d= margin of error (5%) The method for selecting study participants was simple random sampling in which study subjects were picked during the time of data collection until the required sample size was reached.
2.3. Data collection procedure and laboratory investigation
Information on socio-demographic data, history of exposure for the possible associated factors, and other valuable information were collected by trained antenatal care nurses using structured questionnaire.
A full verbal explanation about the study was given by the investigators to all voluntary participants. After obtaining informed consent, 5 mL of venous blood was drawn from each of the study participant using labeled test tubes by trained medical laboratory technician. Then serum was separated from the whole blood by centrifugation at 3 000 rpm for 5 minutes and transported with ice box from Hospital to Biomedical and Microbiology Laboratory, Biology Department, Bahir Dar University, for investigation. The serum samples were kept at -20 ℃ till serological test was done.
2.4. Principle and performance of the test
Toxo-latex test is a rapid slide agglutination procedure, developed to detect more than 10 IU/mL anti-Toxoplasma antibodies in human serum. The assay was performed by testing a suspension of latex particles coated with antigenic extract of T. gondii against unknown samples. The presence or absence of a visible agglutination indicates the presence or absence of anti-Toxoplasma antibodies in the sample tested [Tulip diagnostics (p) Ltd. Verna, Goa-403722, India].
2.5. Quality control
To assure the quality of the data, half day training was given for data collectors on procedures, techniques, and ways of expressing the questionnaires to collect the necessary information. Every day, the collected data was reviewed and checked for completeness by the principal investigator. For laboratory investigations standardized operating procedures and manufacturer’s instructions were strictly followed. The quality of latex agglutination kits for anti-T. gondii were checked by both positive and negative controls.
2.6. Data analysis and interpretation
Information recorded in the questionnaire and laboratory resultswere entered into computer and analyzed using Statistical Package for the Social Sciences (SPSS version 20.0). The groups were characterized according to the target variables using descriptive statistical analysis methods. Prevalence of toxoplasmosis was defined as the percentage of positive cases for serological tests. Associations between toxoplasmosis and possible risk factors were tested with Chi-square test. The magnitude of associations was assessed using odds ratio (OR) at 95% CI, and multivariate logistic regression models was used to identify the explanatory variables among the confounding risk variables that would explain the occurrence of toxoplasmosis. P-value less than 0.05 was considered statistically significant.
2.7. Ethical considerations
The study was ethically cleared by ethical review board of Bahir Dar University and Amhara Regional Health Bureau. The study subjects were informed about the study and written informed consents were obtained from all of the participants before collecting blood samples. Participation in the study was on voluntary basis and study subjects were free to withdraw from the study before and after collection of blood samples without losing any of the benefits they were supposed to obtain from the hospital. While collecting venous blood, the participants might experience pain and therefore maximum effort was taken to minimize the pain and/or associated complications. All blood samples were collected using new disposable tubes, syringes and needles.
3. Results
3.1. Socio-demographic characteristics
A total of 384 pregnant women were included in this study. The mean age of the study participants was 26.96 ± 4.56. One hundred seventy-six (45.8%) of the study subjects belonged to 25-29 years of age. Majority of the study participants, 340 (88.5%), were urban residents. Two hundred nine (54.4%) of the pregnant women were in their 1st trimester. Regarding their educational status, 122 (31.8%) of the respondents educated in college and above, and followed by secondary school 116 (30.2%). Occupation wise, majority of them 162 (42.2%) were housewives. Of the total study subjects, 167 (43.5%) belonged to primigravidae. With regard to pregnancy related problem, nearly a quarter 95 (24.7%) of the total study subjects had history of abortion (Table 1).
3.2. History of exposures to different risk factors of T. gondii infection
Nearly half of pregnant women, 178 (46.4%), had a habit of eating raw/under cooked meat. Only 126 (32.8%) of the study subjects had cat in their houses. More than half of the pregnant women (59.9%) had a habit of eating raw/under cooked vegetables. Majority of the study participants, 363 (94.5%), reported to use pipe water as a source of drinking. Only 9 (2.3%) of the pregnant women had history of blood transfusion (Table 1).
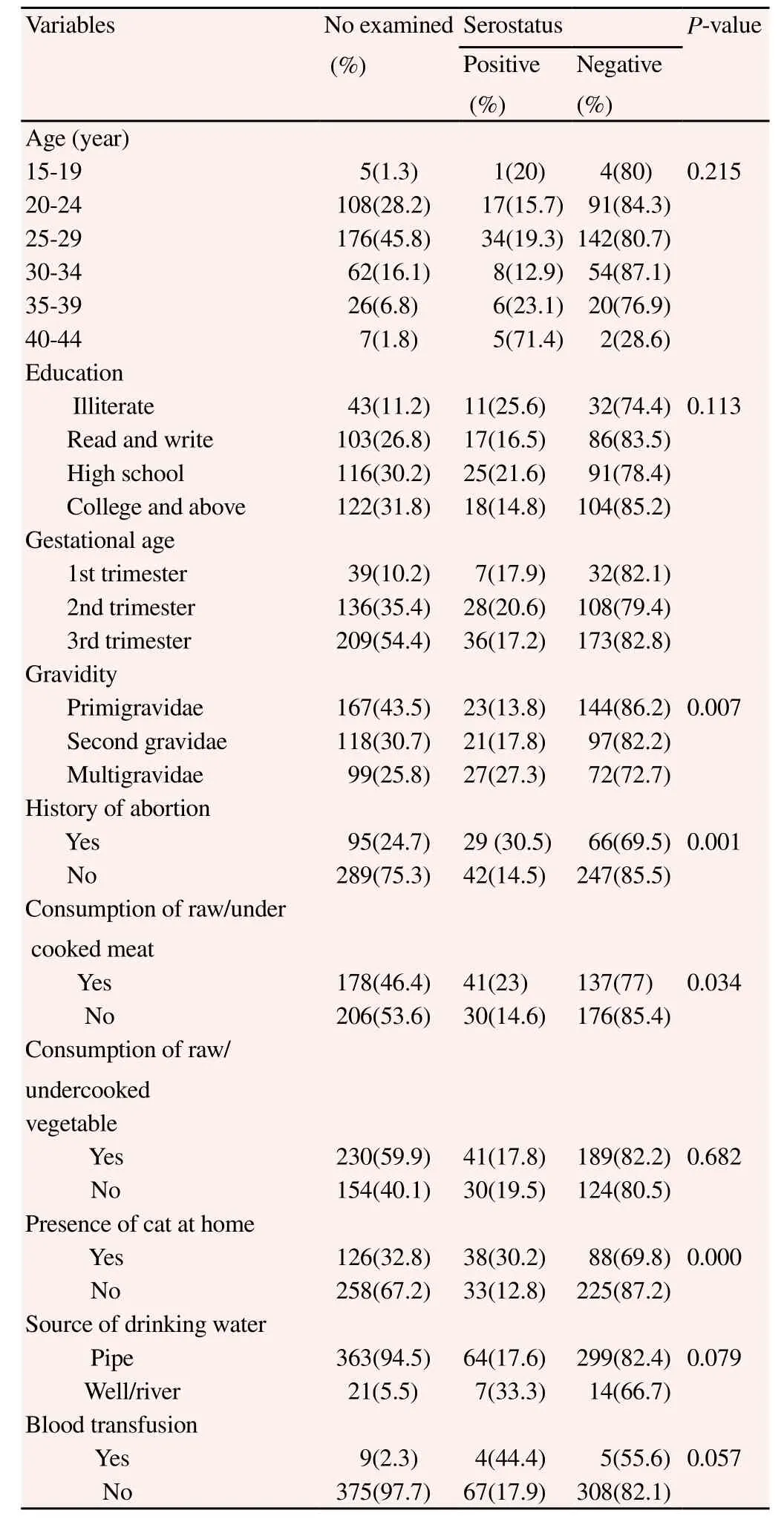
Table 1 Seroprevalence of T. gondii infection in relation to different characteristics among pregnant women at FHRH, Bahir Dar town, from November 2013 to January 2014.
3.3. Seroprevalence of T. gondii
The overall seroprevalence of T. gondii among the pregnant women was 18.5%. All of toxoplasmosis cases were positive only for IgG antibody. Five (71.4%) of pregnant women who were positive for T. gondii were in the age group of 40–44 years. Among T. gondii positive cases, 12 (27.3%) study participants were from rural setting. With regard to educational background, illiterates accounted 11 (25.6%) of all positive cases. From the total 71 T. gondii positive cases, 31 (19.1%) of them were housewives. Comparable results of seropositivity were obtained among 1st, 2nd and 3rd trimesters withprevalence of 17.9%, 20.6% and 17.2%, respectively. With regard to gravidity, 27 (27.3%) positive cases were from multigravidae category (Table 1).
Thirty eight (30.2%) of pregnant women who had cats in their households were found to be positive for T. gondii. Among the study participants who had a habit of eating raw or under cooked meat, 41 (23%) of them were seropositive for T. gondii infection. Seroprevalence among women who used pipe water as a source of drinking water was 64 (17.6%). The result of this study revealed that 41 (17.8%) of T. gondii positive cases were from those who had a habit of eating raw or under cooked vegetables. Among pregnant women who had history of blood transfusion, 4 (44.4%) of them were positive for anti-T. gondii antibody (Table 1).
3.4. Factors associated with seropositivity
The univariate analysis indicated that almost all socio-demographic variables such as age, residence, education, occupation, gestational period and majority of possible risk factors did not show significant association with T. gondii seropositivity. However, gravidity (multigravidae) (COR= 2.35, 95% CI: 1.26-4.38), history of abortion (COR=2.58, 95% CI: 1.50-4.46), having domestic cat at home (COR=2.94, 95% CI: 1.74-4.99) and having a habit of eating raw or under cooked meat (COR=1.76, 95% CI: 1.04-2.96) were significantly associated with T. gondii seropositivity (Table 2).
In multivariate analysis, stepwise logistic regression technique was used and the relative effect of the independent variable on the outcome variable was determined. In doing so, to avoid an excessive number of variables and unstable estimates in the subsequent model, only variables that reached a p-value less than 0.3 were kept in the subsequent analyses[15]. Finally, only three possible risk factors namely history of abortion (AOR=2.47, 95% CI: 1.40-4.34), having domestic cat at home (AOR=2.85, 95% CI: 1.66-4.90) and having a habit of eating raw/undercooked meat (AOR=1.98, 95% CI: 1.15-2.43) remained significant in the final step of multivariate analysis. But, pregnant women who were in multigravidae category was significantly associated with seropositivity of T. gondii during crude analysis was turned to be insignificant after it was adjusted for some of the significant explanatory variables (Table 2).
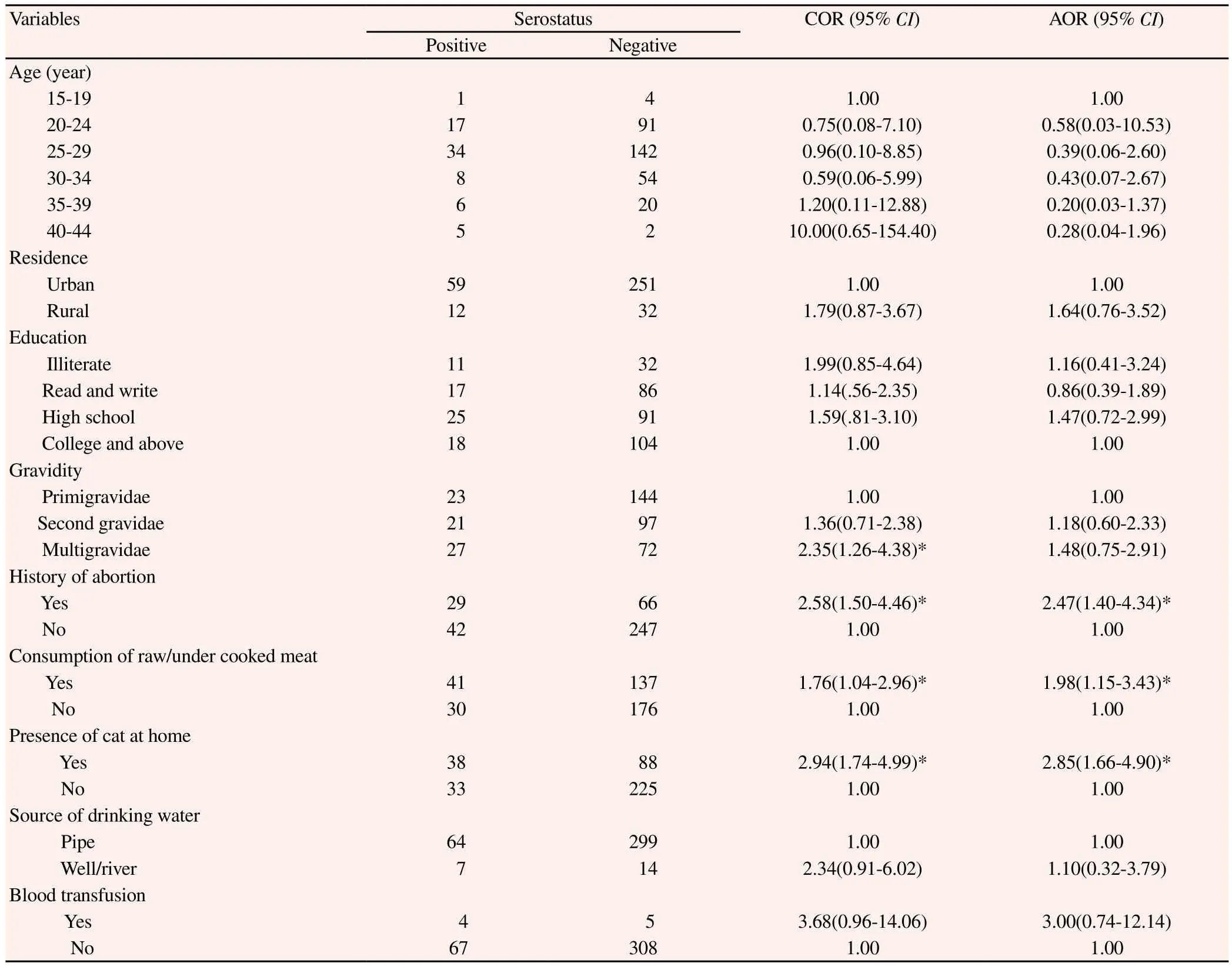
Table 2 Univariate and multivariate analysis of characteristics in pregnant women and their association with T. gondii infection at FHRH, Bahir Dar town, from November 2013 to January 2014.
4. Discussion
A wide variability in the prevalence of toxoplasmosis among pregnant women has been reported worldwide. The present study demonstrated that prevalence of anti-Toxoplasma antibodies in pregnant women was 18.5%. None of the study subjects found to be positive for anti-Toxoplasma IgM antibodies. IgM antibody is usually detected within the first two weeks of infection and reduces to negligible levels within 6 months after exposure[16]. Thus, the absence of IgM antibodies in this study may indicate the absence of acute toxoplasmosis infection.
The prevalence of anti-T. gondii antibody observed in this study is in agreement with the studies that have been conducted in South Africa and Italy, which reported prevalence of 18.1% and 19.1%, respectively[17,18]. However, our finding is lower than seroprevalence reported among pregnant women in Jimma town (83.6%) and among general population in Nazareth town (60%), Ethiopia[9,11]. The observed difference in the rates of infection may be due to the commitment of Ministry of Health of Ethiopia to reduce maternal mortality through awareness creation about the infection using health extension workers or it could be due to the difference in diagnostic method used. It may also be the varied prevalence of the parasite in animals and the type of animals consumed. This is further corroborated by the fact that varied seroprevalence of the disease was reported among domestic animals in Ethiopia with prevalence of 22.9% in sheep, 11.6% in goats, and 6.6% in cattle[19]. However, the present finding is higher than prevalence rates which were reported from China 10.6%, USA 11% and Japan 10.3%[6,20,21]. This variability could be attributed to differences in climatic conditions, feeding habits, socio-economic and literacy status of the study subjects.
The finding of the present study showed significant association between T. gondii infection and presence of domestic cats at home, which was one of the predictors for T. gondii infection in this study. This finding is in agreement with studies reported from Jimma, Ghana and Taiwan[8,9,22]. In contrast, some studies reported absence of association between Toxoplasma infection and presence of domestic cats in the household in Nigeria and in Tanzania[23-25]. This variation among different studies could be the risk of contracting T. gondii infection might not just be the presence of cats in the households but it could be contact of cats’ fecal material while gardening.
The study results showed that Toxoplasma infection may result from consumption of raw or undercooked meat. That means having a habit of eating raw or under cooked meat was found to be a major factor contributing to maternal infection in the study area, which is consistent with studies conducted by Elnahas et al and Mohamed et al in Sudan[26,27]. However, other studies reported from Jimma town, Ethiopia, Ghana and Turkey did not find significant association of raw or undercooked meat consumption with Toxoplasma infection[8,9,28]. The possible reason for the variation could be difference in feeding habits of the study participants.
In relation to histories of abortion, there was significant association between occurrences of previous abortions and seroprevalence for toxoplasmosis. This association does not mean that previous abortion is a risk factor predisposing to infection, but it may indicate that premature termination of pregnancy may expose the women to T. gondii infection. Similar results were also reported from Sudan and India[29,30]. In contrast, some studies reported the absence of significant association between seroprevalence of T. gondii infection and histories of abortion in pregnant women of Jimma town, Ethiopia and that of Assam town, India[9,31]. The possible reason for the cause of variation may be induced abortion in contrast to the findings of the present study.
Some of the risk factors examined, such as blood transfusion, consumption of raw/under cooked vegetable and drinking water source have been documented in different parts of the world to have influence on Toxoplasma transmission[32]. The absence of a statistically significant relationship between the seroprevalence of Toxoplasma infection and these potential factors does not mean that they have no influence on the transmission of toxoplasmosis. However, it may suggest that such factors play a limited role in the study area for the transmission of the parasite in the studied subjects. In the present study, there was a higher seroprevalence of T. gondii in those aged above 35 years than those below 35 years of age. Nevertheless, there was no significant association between toxoplasmosis and mother's age. Statistical association does not necessarily mean that older age is a risk factor predisposing to infection but it might be explained by the fact that the older persons can be exposed to the causative agent for longer time and consequently they may retain a steady level of anti-Toxoplasma IgG in serum for years. Unlike the present study, results that have been reported from Jimma, Nigeria and Brazil have shown statistically significant association between age of pregnant women and seroprevalence of T. gondii infection[9,24,33]. The observed difference in the rates of infection could be due to variation in age classification of the study participants in the present study.
Despite the non-statistical significant association, the present study showed that whenever there is an increase in gravidity, there could be an increase of the probability of T. gondii infection in pregnant women. This situation shows that as the age of the women and the number of pregnancy increases this would in turn lead to an increment of exposure time to an infection. In this study, gravidity, socio-demographic variables such as residence, education, occupation, and gestational age did not show significant association with seroprevalence of T. gondii infection.
It can be concluded that the seroprevalence of T. gondii infection among pregnant women in Bahir Dar town was relatively high. This finding revealed that exposure to T. gondii infection may increase the risk of premature termination of pregnancy. Presence of domestic cats at home and consumption of raw or under-cooked meat were also identified as main risk factors for T. gondii infection. Therefore, implementation of regular serological testing during pregnancy, health education towards avoiding eating undercooked or raw meat, and avoiding contact with cats fecal material during cleaning and gardening should be emphasized by health extension workers and other public health professionals for prevention of the disease.
Conflict of interest statement
We declare that we have no conflict of interest.
[1] Dubey JP. Toxoplasmosis of animals and humans. Beltseville: CRC Press; 2010.
[2] Galvan Ramirez ML, Rodríguez Pérez LR, Ledesma Agraz SY, Sifuentes ávila LM, Armenta Ruíz AS, Corella DB, et al. Sero- epidemiology of toxoplasmosis in high-school students in the metropolitan area of Guadalajara, Jalisco, Mexico. Sci Med 2010; 20: 59–63.
[3] Tenter AM, Heckeroth AR, Weiss LM. Toxoplasma gondii: from animals to humans. Int J Parasitol 2000; 30: 1217–1258.
[4] Sukthana Y. Toxoplasmosis: beyond animals to humans. Trends in Parasitol 2006; 22: 137–142.
[5] Montoya JG, Remington JS. Management of Toxoplasma gondii infection during pregnancy. Clin Infect Dis 2008; 47: 554–566.
[6] Pappas G, Roussos N, Falagas ME. Toxoplasmosis snapshots: Global status of Toxoplasma gondii seroprevalence and implications for pregnancy and congenital toxoplasmosis. Int J Parasitol 2009; 39: 1385–1394.
[7] Cook AJ, Gilbert RE, Buffolano W, Zufferey J, Petersen E, Jenum PA, et al. Sources of Toxoplasma infection in pregnant women: European multicentre case-control study. European Research Network on Congenital toxoplasmosis. BMJ 2000; 321: 142–147.
[8] Ayi I, Edu A, Apea-Kubi K. Sero-epidemiology of toxoplasmosis amongst pregnant women in the greater Accra region of Ghana. Gh Med J 2009; 43: 107–114.
[9] Zemene E, Yewhalaw D, Abera S, Belay T, Samuel A, Zeynudin A. Seroprevalence of Toxoplasma gondii and associated risk factors among pregnant women in Jimma town, Southwestern Ethiopia. BMC Infect Dis 2012; 12: 337.
[10] Shimelis T, Tebeje M, Tadesse E, Tegbaru B, Terefe A. Sero-prevalence of latent Toxoplasma gondii infection among HIV-infected and HIV-uninfected people in Addis Ababa Ethiopia: a comparative cross-sectional study. BMC Res Notes 2009; 2: 213.
[11] Negash T, Tilahun G, Medhin G. Seroprevalence of Toxoplasma gondii in Nazareth town Ethiopia. East Afr J Public Health 2008; 5: 211–214.
[12] Rorman E, Zamir CS, Rilkis I, Ben-David H. Congenital toxoplasmosisprenatal aspects of Toxoplasma gondii infection. Reprod Toxicol 2006; 21: 458–472.
[13] Bureau of Finance and Economics Development (BOFED). Population size by sex and age Group and Urban Rural, Bahir City Administration 2014.
[14] Naing L, Winn T, Rusil BN. Practical issues in calculating sample size for prevalence studies. Arch Orofacial Sci 2006; 1: 9–14.
[15] Victoria CG, Huttly SR, Fuchs SC, Olinto MTA. The role of conceptual frameworks in epidemiological analysis: A hierarchical approach. Int J Epidemiol 1997; 26: 224–227.
[16] Wilson M, McAuley JM. Toxoplasma. In: Murray PR. Manual of clinical microbiology. 7th edition. American Society for Microbiology; 1999:1374–1382.
[17] Bessong PO, Mathomu LM. Seroprevalence of HTLV1/2, HSV1/2 and Toxoplasma gondii among chronic HIV-1 infected individuals in rural northeastern South Africa. Afr J Microbiol Res 2010; 4: 2587–2591.
[18] Thaller R, Tammaro F, Pentimalli H. Risk factors for toxoplasmosis in pregnant women in central Italy. Infez Med 2011; 19: 241–247.
[19] Bekele T, Kasali O.B. Toxoplasmosis in sheep, goats and cattle in central Ethiopia. Vet Res Commun 1989; 13: 371–375.
[20] Liu Q, Wei F, Gao S, Jiang L, Lian H. Toxoplasma gondii infection in pregnant women in China. Trans R Soc Trop Med Hyg 2009; 103: 162–166.
[21] Sakikawa M, Noda S, Hanaoka M, Nakayama H, Hojo S, Kakinoki S. Anti-Toxoplasma antibody prevalence, primary infection rate, and risk factors in a study of toxoplasmosis in pregnant women in Japan. Clin Vaccine Immunol 2012; 19: 365–367.
[22] Lin YL, Liao YS, Liao LR, Chen FN, Kuo HM, He S. Seroprevalence and sources of Toxoplasma infection among indigenous and immigrant pregnant women in Taiwan. Parasitol Res 2008; 103: 67–74.
[23] Ishaku BAI, Umoh J, Lawal I, Randawa A. Seroprevalence and risk factors for Toxoplasma gondii infection among antenatal women in Zaria, Nigeria. Res J Med & Med Sc 2009; 4: 483–488.
[24] Deji-Agboola OS, Busari OA, Osinupebi OJ Amoo. Seroprevalence of Toxoplasma gondii Antibodies among pregnant women attending antenatal clinic of federal medical center, Lagos, Nigeria. Int J Biol Med Res 2011; 2: 1135–1139.
[25] Mwambe B, Stephen E, Benson R, Anthony N. Sero-prevalence and factors associated with Toxoplasma gondii infection among pregnant women attending antenatal care in Mwanza, Tanzania. Parasit Vec 2013; 6: 222.
[26] Elnahas AGA, Elbashir MI, Eldien ES, Adam I. Toxoplasmosis in pregnant Sudanese women. Saudi Med J 2003; 24:868–870.
[27] Mohamed K, Ahmed A, Intisar E, Rayah L. Prevalence and risk factors for Toxoplasma gondii infection in humans from Khartoum State, Sudan. Int J Public Health Epid 2013; 2: 60–66.
[28] Ertug SOP, Tukmen M, Yuksel H. Seroprevalence and risk factors for Toxoplasma infection among pregnant women in Aydin province, Turkey. BMC Public Health 2005; 5: 66.
[29] Mohamed K, Kodym P, Maly M, Intisar E, Rayah L. Environmental and food habitat risk factors associated with Toxoplasma gondii infection in rural women in Sudan. Int J Curr Microbiol App Sci 2014; 3: 208–222.
[30] Anna T, Sucilathangam G, Velvizhi G. Seroprevalence of Toxoplasma gondii in pregnant women with bad obstetric history. Indian J Res 2013; 2: 11.
[31] Borkakoty BJ, Borthakur AK, Gohain M. Prevalence of Toxoplasma gondii infection amongst pregnant women in Assam, India. Indian J Med Microbiol 2007; 25: 431-432.
[32] Jones JL, Kruszon-Moran D, Wilson M, McQuillan G, Navin T, McAuley JB. Toxoplasma gondii infection in the United States: seroprevalence and risk factors. Am J Epidemiol 2001; 154: 357–365.
[33] Fernanda L, Maria R, Otílio M. Prevalence and risk factors for Toxoplasma gondii infection among pregnant and postpartum women. Med Tropical 2013; 46: 200–207.
*Corresponding author: Endalkachew Nibret, Biology Department, Bahir Dar University, P.O.Box 79, Bahir Dar, Ethiopia.
E-mail: endtg2002@yahoo.com
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Andrographolide effect on both Plasmodium falciparum infected and non infected RBCs membranes
- Immunogenicity and efficacy of recombinant 78 kDa antigen of Leishmania donovani formulated in various adjuvants against murine visceral leishmaniasis
- Oral administration of Sauce llorón extract to growing lambs to control gastrointestinal nematodes and Moniezia spp.
- Hepatoprotective and proapoptotic effect of Ecballium elaterium on CCl4-induced hepatotoxicity in rats
- Evaluation of protective effect of cactus pear seed oil (Opuntia ficusincida L. MILL.) against alloxan-induced diabetes in mice
- Antimicrobial activity and synergism of Sami-Hyanglyun-Hwan with ciprofloxacin against methicillin-resistant Staphylococcus aureus
