Antimicrobial activity and synergism of Sami-Hyanglyun-Hwan with ciprofloxacin against methicillin-resistant Staphylococcus aureus
2015-12-23JangGiChoiJiYoungChoiSuHyunMunOkHwaKangPreetiBharajDongWonShinMyongSooChongDongYeulKwon
Jang-Gi Choi, Ji-Young Choi, Su-Hyun Mun, Ok-Hwa Kang, Preeti Bharaj, Dong-Won Shin, Myong-Soo Chong, Dong-Yeul Kwon*
1Department of Oriental Pharmacy, College of Pharmacy, Wonkwang University, WonkwangOriental Medicines Research Institute, Jeonbuk 570-749, Korea
2Center of Excellence in Infectious Disease Research, Department of Biomedical Sciences, Paul L. Foster School of Medicine, Texas Tech University Health Sciences Center, ElPaso,TX 79905, USA
3Department of Third Medicine, Professional Graduate School of Oriental Medicine, Wonkwang University, Iksan, Jeonbuk 570-749, Republic of Korea
4BK21 Plus Team, Professional Graduate School of Oriental Medicine, Wonkwang University, Iksan, Jeonbuk 570-749, Republic of Korea
5Department of Oriental Medicine Resources, Sunchon National University, Jeonnam 540-742, Republic of Korea
Antimicrobial activity and synergism of Sami-Hyanglyun-Hwan with ciprofloxacin against methicillin-resistant Staphylococcus aureus
Jang-Gi Choi1,2△, Ji-Young Choi3△, Su-Hyun Mun4, Ok-Hwa Kang1, Preeti Bharaj2, Dong-Won Shin5, Myong-Soo Chong3, Dong-Yeul Kwon1*
1Department of Oriental Pharmacy, College of Pharmacy, Wonkwang University, WonkwangOriental Medicines Research Institute, Jeonbuk 570-749, Korea
2Center of Excellence in Infectious Disease Research, Department of Biomedical Sciences, Paul L. Foster School of Medicine, Texas Tech University Health Sciences Center, ElPaso,TX 79905, USA
3Department of Third Medicine, Professional Graduate School of Oriental Medicine, Wonkwang University, Iksan, Jeonbuk 570-749, Republic of Korea
4BK21 Plus Team, Professional Graduate School of Oriental Medicine, Wonkwang University, Iksan, Jeonbuk 570-749, Republic of Korea
5Department of Oriental Medicine Resources, Sunchon National University, Jeonnam 540-742, Republic of Korea
ARTICLE INFO
Article history:
Received 15 April 2015
Received in revised form 20 May 2015
Accepted 15 June 2015
Available online 20 July 2015
Sami -Hyanglyun-Hwan
Methicillin-resistant Staphylococcus aureus
Ciprofloxacin
Synergy
Objective: To investigate the antibacterial activity of SHHextracted with either water or ethanol against methicillin-resistant Staphylococcus aureus (MRSA) and combinatory antimicrobial effect with ciprofloxacin (CIP) by time kill assay and checkerboard dilution test. Methods: The antibacterial activity determined by broth dilution method indicated that the antibacterial activity of Sami -Hyanglyun-Hwan (SHH) water extract (SHHW) and SHH ethanol extract (SHHE) ranged from 250 to 2 000 μg/mL and 125 to 1 000 μg/mL against MRSA, respectively. Results: In the checkerboard method, the combinations of SHHE with CIP had a partial synergistic or synergistic effect against MRSA. The time-kill curves showed that a combined SHHE and CIP treatment reduced the bacterial counts dramatically after 24 h. Conclusions: The present study demonstrates the therapeutic ability of SHHE against MRSA infections.
1. Introduction
Staphylococcus aureus (S. aureus) is a commensal of the human skin, gastrointestinal tract and nares. It causes skin and soft tissue infections, invasive disease, sepsis, and endocarditis[1]. The treatment of S. aureus infections has been evolved by the antibioticresistant strains, called methicillin-resistant S. aureus (MRSA), that have increased resistance feature against infectious diseases therapeutics[2]. MRSA is a human pathogen and a cause of hospitalacquired and community-acquired infections. It is a major global health concern resulting in nearly 20 000 deaths in the United States alone[3]. MRSA isolates are resistant to all possible penicillin and other β-lactam[4]. Antibiotic resistance in MRSA has resulted in limited treatment options. Thus, there is a critical need for the discovery of new antibiotics in development against MRSA and treatment strategies to circumvent this growing public health concern.
Studies have demonstrated that combination drug therapy is a more effective alternative to slow down or stop development of drug resistance against bacteria[5] and is recommended treatment for bacterial infections such as MRSA[6].
Sami -Hyanglyun-Hwan (SHH), is a traditional Korean medicine (TKM) prescription that has been used for several hundred years by the Korean community. This classical botanical formulation consists of four Korean herbs that include the Coptidis rhizoma, Rhei rhizoma, Aucklandiae radix and Arecae semen. Korean herbs are potential sources of useful medicinal plants. Recently, research in herbs prescribed in TKM has attracted great attention as many of themhave been shown to exhibit numerous biological activities including anti-virus[7], anti-inflammatory effects[8,9] and anti-cancer[10,11]. Ciprofloxacin (CIP) a well-known broad range antibiotic working against both gram positive and gram negative bacteria and widely used for common pathogen causing infections[8,9]. In an effort to discover novel antibacterial agents or antiviral formulations from TKM, SHH, one of the most frequently used Korean prescriptions, was investigated for its in vitro antibacterial activity. We herein report also, the promising anti-MRSA synergy of CIP combined with SHH extracted with ethanol.
2. Materials and methods
2.1. Plant materials
Coptidis rhizoma, Rhei rhizoma, Aucklandiae radix and Arecae semen were purchased from the Oriental drug store DaehakHanyakkuk (Iksan, Korea), and authenticated by Dr. D.Y. Kwon. All voucher specimens were deposited in the Laboratory of Herbalogy, College of Pharmacy, Wonkwang University, Iksan, Korea.
2.2. Preparation of the SHH water or ethanol extracts
A total of 500 mL of de-ionized distilled water (dd water) or 70% EtOH were added to 26 g of SHH prescription, which 15 g of Coptidis rhizoma, 6 g of Rhei rhizoma, 3 g of Aucklandiae radix and 2 g of Arecae semen was heated until the preparation boiled. After 2 h the decoction was then percolated to obtain filtrate, and drugs were re-boiled with fresh 500 mL of dd water or 70% EtOH. After two more rounds of percolation and filtration, collected filtrates were then poured together, concentrated under reduced pressure, lyophilized stored at 20 ℃ until use. The yield of the SHH water extract was 17.4% (4.53 g) and EtOH extract was 14.2%.
2.3. Bacterial strains and culture medium
Among the 8 strains of S. aureus used in this study, 2 clinical MRSA isolates were obtained from 2 different patients at Wonkwang University Hospital and 2 strains were commercially purchased S. aureus ATCC 33591 (methicillin-resistant strain) and S. aureus ATCC 25923 [methicillin-susceptible strain (MSSA)] (American Type Culture Collection, Manassas, VA). The remain 4 strains were obtained from Culture Collection of Antimicrobial Resistant Microbes. All bacteria were stored as 30% glycerol stocks and frozen at 70 ℃ until use. The bacterial strains were suspended in Mueller-Hinton broth (MHB) and incubated at 37 ℃ for 24 h.
2.4. Antimicrobial reagents
MHB and Mueller-Hinton agar (MHA) (Difco Laboratories, Baltimore, MD, USA. Ampicillin, oxacillin, CIP, erythromycin, and solvents were purchased from Sigma Aldrich (St. Louis, USA).
2.5. Antimicrobial Resistance Testing
Detection of the mecA gene in MRSA strains was performed by polymerase chain reaction (PCR) amplification (Table 1). Prior to DNA extraction, bacteria stock cultures were subcultured twice on to MHA plates. For rapid extraction, one to five bacterial colonies were suspended in 300 μLof cell lysis buffer and heated at 100 ℃for 20 min. After centrifugation at 12 000 rpm for 10 min, 2 μL of the supernatant was used for the DNA extraction. PCR reactions were performed using a MRSA Primer Mix Kit (Genotek, Daejeon, Republic of Korea). The PCR amplification was consisted of 30 cycles (94 ℃, 60 s; 55 ℃, 60 s; 72 ℃, 60 s). The final PCR products were separated on a 2% agarose gel.
2.6. Disc diffusion
The paper disc diffusion method was used to determine antibacterial activity[10]. Sterile paper discs (6 mm; Toyo RoshiKaihsa, Japan) were loaded with 20 μL of SHHW or SHHE (varying concentrations: 50, 100, and 200 μg) dissolved in 10% dimethyl sulfoxide (DMSO, Sigma, USA), and were left to dry for 12 h at 37 ℃ under sterile conditions. The bacterial suspensions were diluted to match the 0.5 McFarland standard scale (approximately 1.5×108CFU/mL), and were further diluted to obtain the final inoculum. The MHA was poured into petri dishes and inoculated with 100 μL of the suspension containing 1×105CFU of bacteria. The inhibition zone diameter around each of the discs was measured and recorded at the end of the incubation period. Ampicillin was included as positive control and 10% DMSO served as negative controls.
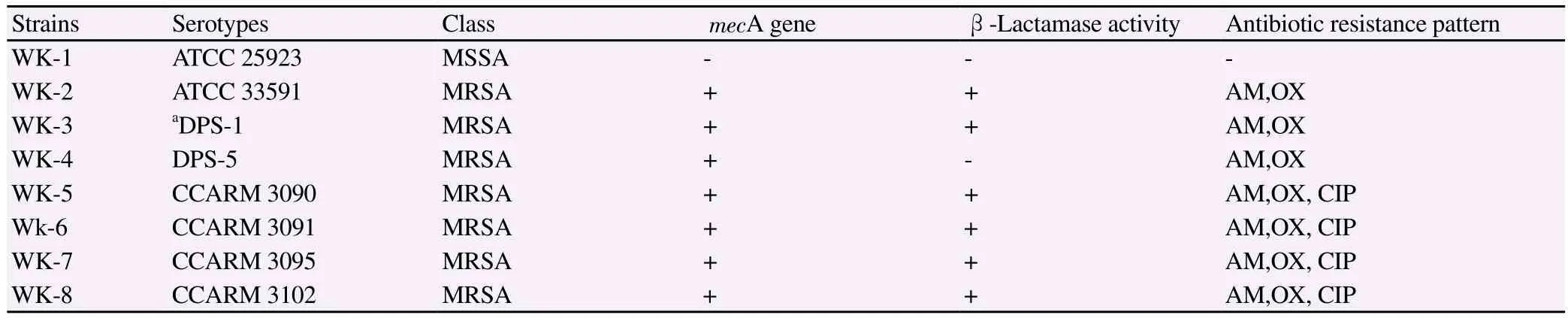
Table 1 Determination of the mecA gene of the S. aureus strains used in the experiment.
2.7. Determination of the minimum inhibitory concentrations (MICs)
The MIC determinations were performed using the broth microdilution method described by the Clinical and Laboratory Standard Institute guidelines[11]. Serial 2-fold dilutions of SHHW or SHHE in MHB were prepared in sterile 96-well microplates and microtubes. The MRSA inocula were adjusted to the 0.5 McFarland standard [approximately colony-forming units (CFU)/mL] in MHB. The final inocula were adjusted to CFU/spot. The MIC was defined as the lowest concentration of SHHE that permits microorganism growth after prior incubation at 37 ℃ for 24 h.
2.8. Checkerboard dilution test
The checkerboard method was used to identify the interactions between SHHE and antibiotics[12]. The antimicrobial assays were performed with SHHE in combination with CIP. Serial dilutions of SHHE with these antibiotics were mixed in cation- supplemented MHB. The inocula were prepared from colonies that had been grown on MHA overnight. The final bacterial concentration after inoculation was CFU/spot. The MIC, determined after incubation at 37 ℃ for 24 hr, was defined as the lowest concentration of drug, alone or in combination with other agents that visibly inhibited the growth of bacteria. Each experiment was performed 3 times. The in vitro interaction between the drugs was quantified by determining the fractional inhibitory concentration (FIC). The FIC index (FICI) was calculated with the following formula: FICI = FICA+ FICB= [A]/MICA+ [B]/MICB, where [A] and [B] are the concentrations of drug A and B, respectively, and MICA/FICAand MICB/FICBare the MIC/FIC of drug A and B, respectively. The FICI was interpreted as follows: 0.5, synergy; >0.5-0.75, partial synergy; >0.75-1, additive effect; >1-4, no effect; and >4, antagonism. Finally, the different values of synergy between the 2 agents were calculated[12].
2.9. Time-kill assay
Time-kill curves were used to determine the synergy effects of the 2 antimicrobial agents on bacterial growth in 96-well plates at 5 different time points (0, 4, 8, 16, and 24 h)[13]. Bacterial cultures diluted with fresh MHB to approximately CFU/mL, and the diluted cultures were incubated at 37 ℃ for 24 h. Aliquots (0.1 mL) of the culture were taken at 0, 4, 8, 16, and 24 h of incubation, and serial 10-fold dilutions were prepared in saline as needed. The numbers of viable cells were determined on a drug-free MHA plate after incubation for 24 h. Colony counts were performed on plates, and 30–300 colonies were enumerated. The lower limit of sensitivity of the colony counts was 100 CFU/mL. The antimicrobial agents used were considered bactericidal at the lowest concentration that reduced the original inoculum by 3 log10 CFU/mL (99.9%) for each of the indicated times. However, they were designated bacteriostatic if the inoculum was reduced by only 0–3 log10 CFU/mL. To confirm the results, the time-kill assays for each experiment were performed at least thrice; the data are represented as mean data ± standard deviation[13].
3. Results
3.1. Anti-bacterial activity of SHHW and SHHE against MRSA
The antimicrobial efficacy of SHHW and SHHE against the MRSA strains was evaluated by the disc diffusion method via determination of the surrounding inhibition zones, as well as by evaluating the MIC using the broth micro dilution method. Both the extracts of SHH extracted either by water or ethanol showed antimicrobial activity against MSSA strains. However, the SHHW extract appeared to work only at the highest concentration tested against one strain of MRSA (200 μg for WK-8, Table 2) showing disc diffusion zone of 7 mm. The SHHE showed antimicrobial activity against all the MRSA strains at 200 μg concentration as determined by disc diffusion method. There was a dose dependent activity of SHHE against the strains of MRSA/MSSA as shown in Table 2. Thus, SHHE was able to restrict bacterial growth successfully in different MRSA strains resistant to ampicillin, oxacillin or ciprofloxacin. Similarly, the MICs needed for antimicrobial activity against all the MRSA strains varied considerably between the two extracts of SHH. For SHHW, the MICs determined using the broth dilution method showed that at least 2-4 fold higher concentration was needed to reach MIC as compared to SHHE. The MIC for SHHW ranged from 1 000-2 000 μg/mL, as compared to a significantly lower concentration needed for SHHE ranging from 125-1 000 μg/ mL. Thus, we demonstrated that SHHE extract had more strong antibacterial activity against MRSA as compared to SHHW (Table 3).
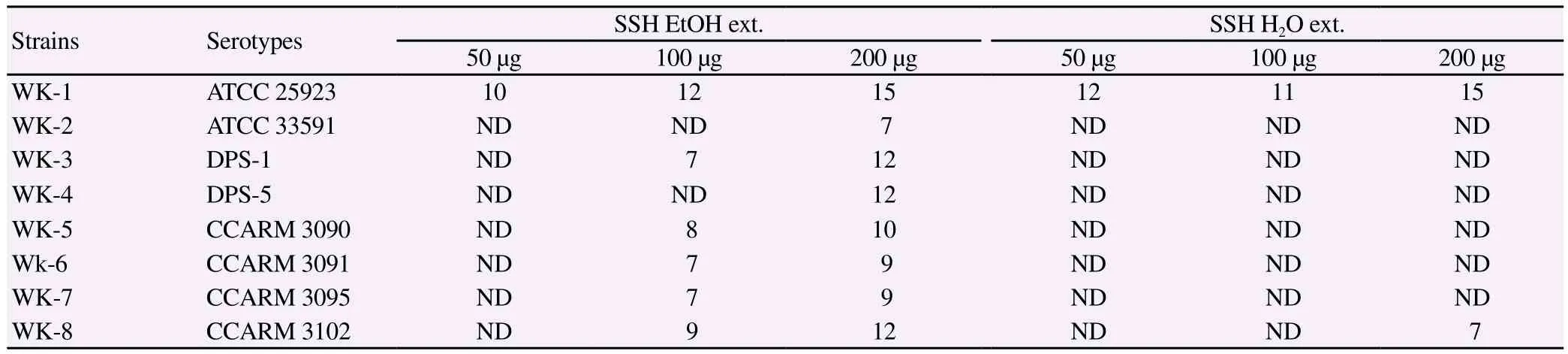
Table 2 Antimicrobial activity (as the inhibition zone diameter) of SSH against 8 methicillin-resistant S. aureus (mm).

Table 3 MIC of the 8 methicillin-resistant S. aureus (μg/mL).
3.2. Synergistic testing
The synergistic effects of SHHE with CIP were tested on four MRSA strains by using a checkerboard dilution assay. The effect of SHHE alone or SHHE combined with CIP was tested towards antimicrobial activity. The MIC results showed that combinatorial effects of SHHE with CIP had 2-32 fold reduction in concentration as those needed by SHHE alone. The antibacterial effects of SHHE and CIP alone or SHHE combined with CIP are shown in Table 4. The antibacterial activity of SHHE markedly reduced the MICs of CIP against S. aureus strains. Thus, a significantly lower dose of SHHE would be required to achieve the same antimicrobial activity with CIP as compared to the extract alone. These results demonstrated that the combination of SHHE with CIP could be used to suppress MRSA growth.
3.3.Time-kill curve assay
The synergistic effects of SHHE with CIP on MRSA were confirmed with a time-kill curve assay on two strains WK-5 and WK-8. Figure 1 shows that, within a 24 h incubation period, either SHHE alone (1/2 MIC) or CIP alone (1/2MIC) inhibition MRSA growth by just 3-5 log. However, when used together, the combination of SHHE and CIP caused rapid inhibition in a timedependent process during an observation period of 24 h showing an eight log difference in bacterial growth. Thus, a synergist effect was observed when both SHHE and CIP were used together. Similar results were seen with strain WK-8. As shown in Figure 2, the combination of SHHE and CIP almost completely inhibited the growth of MRSA after 24 h. the difference between SHHE or CIP alone was 4-6 log decrease in bacterial growth, however with both combined, there was 11 fold (~90%) reduction showing the promising synergist aspect of both used together. Thus, SHHE extracts isolated in our laboratory along with commercially available CIP should be used together to achieve almost complete control of MRSA strains that are almost impossible to control due to their inherent nature.
4. Discussion
In this study, we have demonstrated that SHHE was found to inhibit against both MSSA and MRSA. Although it could inhibit the growth of both bacteria, the ethanol extracts of SHH demonstrated higher antimicrobial activity against MRSA than the water extract of SHH. Consequently, SHHE may be used potent antibacterial agents to be used in combating drug-resistant S. aureus strains.
MRSA began as a acquire resistance to most of antibiotics have significantly increased the global mortality caused by multidrugresistant bacterial infection[14,15]. Ciprofloxacin is the antibiotic of selected for treating skin and soft tissue infections in areas where bacteria such as MRSA and Salmonella are prevalent[16,17].Recently, many reports have shown that infections due to strains of MRSA with a high-level resistance to fluoroquinolones are particularly worrying. The emergence of complete resistance to CIP in MRSA would severely limit the choice of antimicrobial therapies for treating infection[17-20].
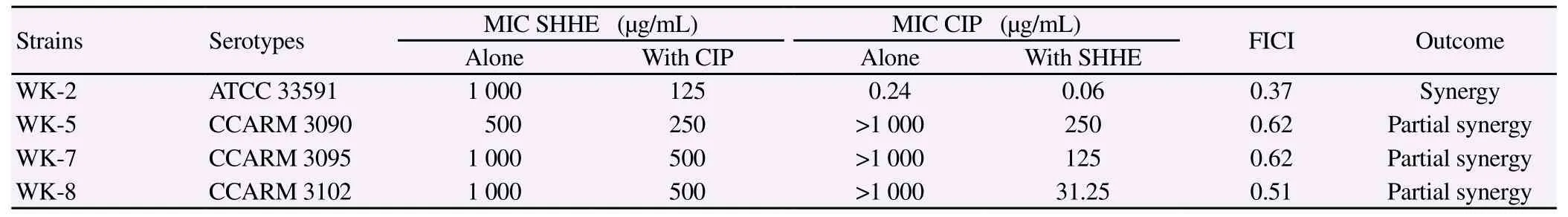
Table 4 Result of the combined effect of SHHE and CIP against methicillin-resistant S. aureus.
To overcome the emerging problem of multi-drug resistant bacterial strains, various studies investigating combinations of plant extracts with antibiotics against MRSA have been reported[21-23]. The use of two antibiotics in combination is slow in the process drug resistance and to restore the activity of drugs that are no longer treatment. Combination therapy is the most commonly recommended empirical treatment for bacterial infections and for preventing the emergence of resistant mutants for bacteria[6]. In this study, we confirm that SHHE has antimicrobial activity and combining sub-MIC concentrations of SHHE with CIP significantly improved the activity of the antibiotic by a checkerboard dilution assay. In addition, the combination of 1/2MIC SHHE + 1/2MIC CIP completely inhibited the growth of MRSA (WK-8) after 24 h by time-kill curve assay. Thus, SHHE was shown to have an effect on the CIP activity, plus the in vitro activity of SHHE against MRSA and its synergistic interactions with CIP were demonstrated for the first time. Therefore, SHHE has the potential to restore the effectiveness of CIP against resistant MRSA, and could be useful in developing valuable clinical treatments.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
This research was supported by Wonkwang University in 2014.
[1] Lowy FD. Staphylococcus aureus infections. N Engl J Med 1998; 339(8): 520-532.
[2] Singh L, Winking H, Jones KW, Gropp A. Restriction fragment polymorphism in the sex-determining region of the Y chromosomal DNA of European wild mice. Mol Gen Genet 1988; 212(3): 440-449.
[3] Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 2007; 298(15): 1763-1771.
[4] Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis 2011; 52(3): 285-292.
[5] McConeghy KW, Bleasdale SC, Rodvold KA. The empirical combination of vancomycin and a beta-lactam for Staphylococcal bacteremia. Clin Infect Dis 2013; 57(12): 1760-1765.
[6] Drago L, De Vecchi E, Nicola L, Gismondo MR. In vitro evaluation of antibiotics' combinations for empirical therapy of suspected methicillin resistant Staphylococcus aureus severe respiratory infections. BMC Infect Dis 2007; 7: 111.
[7] Cheng HY, Lin LT, Huang HH, Yang CM, Lin CC. Yin Chen Hao Tang, a Chinese prescription, inhibits both herpes simplex virus type-1 and type-2 infections in vitro. Antiviral Res 2008; 77(1): 14-19.
[8] Ball P. Quinolone generations: natural history or natural selection? J Antimicrob Chemother 2000; 46 Suppl T1: 17-24.
[9] Oliphant CM, Green GM. Quinolones: a comprehensive review. Am Fam Physician 2002; 65(3): 455-464.
[10] Ali NA, Julich WD, Kusnick C, Lindequist U. Screening of Yemeni medicinal plants for antibacterial and cytotoxic activities. J Ethnopharmacol 2001; 74(2): 173-179.
[11] Choi JG, Mun SH, Chahar HS, Bharaj P, Kang OH, Kim SG, et al. Methyl gallate from Galla rhois successfully controls clinical isolates of Salmonella infection in both in vitro and in vivo systems. PLoS ONE 2014; 9(7): e102697.
[12] Mun SH, Kang OH, Joung DK, Kim SB, Seo YS, Choi JG, et al. Combination therapy of sophoraflavanone B against MRSA: In vitro synergy testing. Evid Based Complement Alternat Med 2013; 2013: 823794.
[13] Choi JG, Kang OH, Brice OO, Lee YS, Chae HS, Oh YC, et al. Antibacterial activity of Ecklonia cava against methicillin-resistant Staphylococcus aureus and Salmonella spp. Foodborne Pathog Dis 2010; 7(4): 435-441.
[14] Lipsky BA, Tabak YP, Johannes RS, Vo L, Hyde L, Weigelt JA. Skin and soft tissue infections in hospitalised patients with diabetes: culture isolates and risk factors associated with mortality, length of stay and cost. Diabetologia 2010; 53(5): 914-923.
[15] Tacconelli E, De Angelis G, Cataldo MA, Pozzi E, Cauda R. Does antibiotic exposure increase the risk of methicillin-resistant Staphylococcus aureus (MRSA) isolation? A systematic review and metaanalysis. J Antimicrob Chemother 2008; 61(1): 26-38.
[16] Drusano GL, Standiford HC, Plaisance K, Forrest A, Leslie J, Caldwell J. Absolute oral bioavailability of ciprofloxacin. Antimicrob Agents Chemother 1986; 30(3): 444-446.
[17] Falagas ME, Matthaiou DK, Vardakas KZ. Fluoroquinolones vs betalactams for empirical treatment of immunocompetent patients with skin and soft tissue infections: a meta-analysis of randomized controlled trials. Mayo Clin Proc 2006; 81(12): 1553-1566.
[18] Heidelbaugh JJ, Holmstrom H. The perils of prescribing fluoroquinolones. J Fam Pract 2013; 62(4): 191-197.
[19] Karageorgopoulos DE, Giannopoulou KP, Grammatikos AP, Dimopoulos G, Falagas ME. Fluoroquinolones compared with beta-lactam antibiotics for the treatment of acute bacterial sinusitis: a meta-analysis of randomized controlled trials. CMAJ 2008; 178(7): 845-854.
[20] Centers for Disease C, Prevention. Update to CDC's sexually transmitted diseases treatment guidelines, 2006: fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb Mortal Wkly Rep 2007; 56(14): 332-336.
[21] Choi JG, Kang OH, Lee YS, Oh YC, Chae HS, Jang HJ, et al. In vitro activity of methyl gallate isolated from galla rhois alone and in combination with ciprofloxacin against clinical isolates of salmonella. J Microbiol Biotechnol 2008; 18(11): 1848-1852.
[22] Mun SH, Kang OH, Joung DK, Kim SB, Choi JG, Shin DW, et al. Anti-MRSA activity of carvone with gentamicin. Exp Ther Med 2014; 7(4): 891-896.
[23] Hossain MA, Park JY, Kim JY, Suh JW, Park SC. Synergistic effect and antiquorum sensing activity of Nymphaea tetragona (water lily) extract. Biomed Res Int 2014; 2014: 562173.
△These two authors shared co-first authorship.
*Corresponding author: Dong-Yeul Kwon, PhD, Department of Oriental Pharmacy, College of Pharmacy, Wonkwang University, Iksan, Jeonbuk, 570-749, South Korea. Tel: +82-63-850-6802
Fax: 82-63-850-6802
E-mail: sssimi@wku.ac.kr
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Andrographolide effect on both Plasmodium falciparum infected and non infected RBCs membranes
- Immunogenicity and efficacy of recombinant 78 kDa antigen of Leishmania donovani formulated in various adjuvants against murine visceral leishmaniasis
- Oral administration of Sauce llorón extract to growing lambs to control gastrointestinal nematodes and Moniezia spp.
- Hepatoprotective and proapoptotic effect of Ecballium elaterium on CCl4-induced hepatotoxicity in rats
- Evaluation of protective effect of cactus pear seed oil (Opuntia ficusincida L. MILL.) against alloxan-induced diabetes in mice
- Role of Aedes aegypti and Aedes albopictus during the 2011 dengue fever epidemics in Hanoi, Vietnam
