Evaluation of protective effect of cactus pear seed oil (Opuntia ficusincida L. MILL.) against alloxan-induced diabetes in mice
2015-12-23BerraaouanAliZiyyatAbderrahimMekhfiHassaneLegssyerAbdelkhaleqAzizMohammedBnouhamMohamed
Berraaouan Ali, Ziyyat Abderrahim, Mekhfi Hassane, Legssyer Abdelkhaleq, Aziz Mohammed, Bnouham Mohamed
Laboratory of Physiology and Ethnopharmacology-URAC40, Mohammed First University, Faculty of Sciences, Oujda, Morocco
Evaluation of protective effect of cactus pear seed oil (Opuntia ficusincida L. MILL.) against alloxan-induced diabetes in mice
Berraaouan Ali, Ziyyat Abderrahim, Mekhfi Hassane, Legssyer Abdelkhaleq, Aziz Mohammed, Bnouham Mohamed*
Laboratory of Physiology and Ethnopharmacology-URAC40, Mohammed First University, Faculty of Sciences, Oujda, Morocco
ARTICLE INFO
Article history:
Received 15 April 2015
Received in revised form 20 May 2015
Accepted 15 June 2015
Available online 20 July 2015
Antioxidant effect
Alloxan prevention
Cactus pear seed oil
Diabetes mellitus
Opuntia ficus-indica
Oxidative stress
Objective: To evaluate the in vitro antioxidant power of cactus pear seed oil [Opuntia ficusincida L. MILL. (CPSO)] and its protective effect against chemically induced diabetes mellitus in mice. Methods: The in vitro antioxidant effect of CPSO was evaluated using 2,2-diphenyl-1-picrylhydrazyl (DPPH) scavenging assay. The preventive effect was conducted on Swiss albino mice treated with CPSO (2 mL/kg, per os), before and after a single intraperitoneal alloxan administration (100 mg/kg). Survival rate, body weight and fasting blood glucose were measured and histopathological analysis of pancreas was performed to evaluate alloxaninduced tissue injuries. Results: CPSO exhibited an antioxidant effect in DPPH scavenging assay. Moreover, the administration of CPSO (2 mL/kg) significantly attenuated alloxaninduced death and hyperglycemia (P<0.001) in treated mice. Morphometric study of pancreas revealed that CPSO significantly protected islets of langerhans against alloxan induced-tissue alterations. Conclusions: Based on theses results, CPSO can prevent alloxan-induced-diabetes by quenching free radicals produced by alloxan and inhibiting tissue injuries in pancreatic β-cells.
1. Introduction
According to International Diabetes Federation, 382 million people worldwide have diabetes mellitus(DM)[1]. This disease results from the interaction of genetic predisposition, behavioral and environmental risk factors. Then, the growing DM prevalence requires efficient preventive interventions[2].
It has been shown that intolerable amounts of cellular free radicals, known as redox imbalance, are harmful and could be fatal for the host cells[3]. Although the genetic basis of DM, there is solid evidence that redox imbalance is a determinant factor leading to the development of this chronic metabolic disease[4,5]. Therefore, it is well known that healthy diet and regular physical activity are beneficial to subjects at high risk of diabetes.
Nutritional status characterized by good supply of antioxidants, might be helpful to prevent DM induced by oxidative stress. In this context, many vegetable oils, containing polyunsaturated fatty acids (PUFAs) and antioxidants (tocopherols, polyphenols and carotenoids, etc.), are reported to be antidiabetic by preventing diabetes complications[6,7], and even more, by inhibiting the disease development[8-11].
Cactus pear seed oil (CPSO), extracted from (Opuntia ficus-indica L. Mill.) seeds, contains high amounts of PUFAs and antioxidant compounds that can be useful in DM management[12-16]. In a previous work, we demonstrated that CPSO improves postprandial hyperglycemia in normal and streptozotocin-induced DM in rats by a partial inhibition of the intestinal glucose absorption[17]. Though, there is no study reporting preventive effect of CPSO againstalloxan (Allx) induced-diabetes in mice.
The aim of this work is to evaluate the antioxidant activity and the preventive effect of CPSO against DM by its oral intake before and during Allx diabetogenic phase. The effect of CPSO was compared to D-α-tocopherol acetate-enriched cooking oil (TCO).
2. Materials and methods
2.1. Plant materiel
CPSO was freely provided by Argan Oil Company, Casablanca, Morocco. Opuntia ficus-indica L. Mill. seeds have been separated from fresh fruit collected in summer period (June-August). Separated seeds have been dried at room temperature and then cold-pressed by use of oil extraction machine. The extraction was conducted without use of solvent system. Extracted CPSO was bottled in 40 mL glass bottle and stored in dark at 6 ℃ until use.
2.2. 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay
The DPPH scavenging assay was performed according to the method described by Liu et al[18] with some modifications. One milliliter of ethanolic solution of DPPH (0.001%; w/v) (Sigma Aldrich) was added to 1.5 mL of final concentrations of CPSO in ethanol (6.00 mg/mL, 4.00 mg/mL, 1.00 mg/mL, 0.40 mg/mL and 0.10 mg/mL) prepared from a stock solution of 10 mg/mL. The mixture was shaken by vortex and the absorbance was immediately determined by spectrophotometer (Spectronic® 20 GENESYS®, Spectronic Instruments USA) at 517 nm. The absorbance was monitored at 30 min intervals during 90 min of incubation at room temperature in dark. Ascorbic acid, a stable synthetic antioxidant, was used as standard reference. All assays were done in triplicates. The scavenging activity of the samples was calculated according to the following formula:
DPPH Scavenging percentage (%S)=100×[(AB-AS)/AB]
Where ABand ASare the absorbance of control and tested samples, respectively, at the measurement time.
2.3. Effect of cactus pear seed oil on Allx-induced DM
2.3.1. Animals
Swiss albino mice (19 weeks old) were used for this experiment. The animals were supplied by the animal house of the Faculty of Sciences, Mohammed First University, Oujda, Morocco. The animals were housed in polycarbonate cages in environmental conditions and fed standard diet and water ad libitum under 12 h light/12 h dark (light period 7:00 AM- 7:00 PM). All animals were cared for in compliance with the Guide for the Care and Use of Laboratory Animals, published by the US National Institutes of Health[19].
2.3.2. Diabetes induction
Diabetes was induced in mice by a single intraperitoneal (ip.) dose (100 mg/kg of body weight) of Allx monohydrate (Allx monohydrate 98%, ACROS Organics). The diabetogenic dose of Allx was freshly prepared in phosphate-citrate buffer (pH=4.5) and injected to overnight fasted mice.
2.3.3. Experimental design
To evaluate the effect of CPSO treatment on the incidence of Allxinduced DM, mice were randomly divided into four groups: control group, positive control group, CPSO treated group and TCO (20% D-α-tocopherol acetate in cooking oil, v/v) treated group.
On the beginning day (day 0), treated animals received oral administration of CPSO (2 mL/kg of body weight and TCO (2 mL/kg of body weight) to their respective groups, followed by a single dose injection of Allx at 1 h of time interval. The treatment was sustained for a week after Allx administration (Figure 1). Control group and positive control group received oral administration of distilled water (10 mL/kg of body weight) followed by an intraperitoneal injection of phosphate-citrate buffer and Allx respectively (Figure 1).On the termination day (day 7), animals were euthanized and the splenic part of pancreas was quickly removed and immediately deposed in 10% buffered formalin to perform histopathological study.
Blood was withdrawn from the mice tail vein, from overnight fasting mice, and glycaemia was measured at the start and at the end of study by electronic glucometer (Optium xceed, Abbott Diabetes Care Ltd., United Kingdom).
2.3.4. Histopathological study of pancreas
The splenic part of pancreas from each mouse was fixed in 10% buffered formalin for 17 h and processed via the paraffin wax embedding method (dehydration, clearing and embedding). The paraffin embedded-sections were cut at 7-micron thickness using microtome (Leitz 1512, Germany), and then stained by hematoxylin and eosin standard method. Stained sections of pancreas were qualitatively (morphological) analyzed on Olympus microscope (Olympus CH Microscope, Japan).
The quantitative (morphometric) analysis was carried out on photomicrographs by use of ImageJ Software[20]. Islets density was determined at 100× magnification; though, the islet diameter, islet area and islet’s cell number were determined at 400× magnification.
2.4. Statistical analysis
The collected data were analyzed by GraphPad Prism 6 for Mac (version 6.0f, Trial) and expressed as means ± standard error of mean (SEM). Glycaemia data were analyzed using Two-way ANOVA analysis of variance, followed by Bonferroni post-hoc test. The other data were analyzed by one-way ANOVA, followed by Bonferroni post-hoc test. The difference was considered statically significant when P<0.05.
3. Results
3.1. DPPH scavenging assay
CPSO antioxidant activity was evaluated over a range of concentrations and the results of DPPH scavenging effect were plotted in Figure 2. CPSO exhibited a significant antioxidant activity with an IC50value of 0.96 mg/mL. However, the maximal efficiency (at 90 min) of CPSO on DPPH scavenging [(86.20 ± 0.13)%, 6 mg/mL] was slower than that of ascorbic acid [(97.12 ± 0.57)%], 1.6 mg/mL). Additionally, the antioxidant ability of CPSO increased proportionally to its concentration in the milieu.
3.2. Effect of CPSO administration on Allx diabetes induction
3.2.1. Effect on survival rate
Treatment with CPSO enhanced the survival rate after Allx (100 mg/kg) injection (77.77%) compared to the group treated with Allx only (40%). In the same way, TCO administration prevented mortality due to Allx administration compared to Allx group (Figure 3).
3.2.2. Effect on blood glucose level
The effect of CPSO administration on blood glucose level of Allx-treated mice is presented in Figure 4. Allx injection induced a significant (P<0.001) increase in fasting blood glucose of Allx group compared to normal control group [(4.41 ± 0.19) mmol/L]. The administration of CPSO (2 mL/kg) with alloxan in mice decreased significantly the incidence Allx-induced hyperglycemia in CPSO-treated group [(4.37 ± 0.38) mmol/L, P<0.001] compared to the grouptreated with only Allx [(13.13 ± 0.95) mmol/L]. Treatment with TCO (2 mL/kg) attenuated significantly (68%, P<0.001) the Allx-induced hyperglycemia compared to Allx group.
These results suggest that CPSO treatment significantly attenuated the development of alloxan-induced DM in swiss albino mice.
3.2.3. Efffect on body weight
The body weight variation is shown in Table 1. Allx induced a significant decrease in body weight in Allx group [-(6.84 ± 3.20)%, P<0.001] compared to control group [+(8.27 ± 2.17)%]. Though, CPSO intake with Allx inhibited significantly the body weight loss in treated group [+(8.780 ± 2.88)%, P<0,001] compared to the group treated with only alloxan.
3.2.4. Histopathological study of pancreas
Light micrographs of hematoxylin and eosin stained sections of pancreas are grouped in Figure 5. It is notable that Allx induced a degeneration of islets of langerhans in aminals treated with only Allx. Administration CPSO and TCO with Allx attenuated the destructive effect of Allx on pancreatic islets.
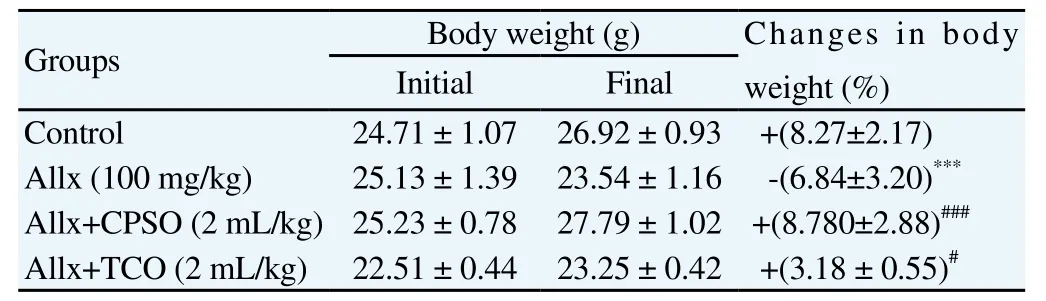
Table 1 Changes in in body weight before and after Allx intraperitoneal administration in CPSO and TCO treated mice groups.
The results of the morphometric analysis of pancreas are presented in Table 2. Allx administration induced a significant reduction in the numerical density of islets (number of islets/mm2of pancreas) (P<0.001), islet diameter (P<0.01), islet area (P<0.01) and numerical number of cells per islet (P<0.01) compared to normal control group. Whereas, the oral administration of CPSO prevented significantly the Allx-induced pancreas injuries by preserving islet diameter [(267.10 ± 23.47) μm], islet area [(0.041 6 ± 0.009 1) mm2] and insular number of cells [(380.80 ± 54.23) cell/islet] in normalstate (no significant difference) compared to normal mice. However, it was a significant reduction in islet density [(1.79 ± 0.20) islet/mm2, P<0.01] compared to normal control group.

Table 2 Effect of CPSO and TCO administration on histomorphometric parameters of hematoxylin and eosin stained pancreas of Allx treated mice.
4. Discussion
This study was undertaken, mainly, to assess the protective effect of cactus pear seed oil against Allx-induced DM in mice.
Allx is an unstable compound, which presents a molecular shape analogy with glucose. Though, Allx by it self is not toxic, but once it is infiltrated to the pancreatic β-cells trough the GLUT2 transporter[21], Allx is reduced to dialuric acid in the presence of different cellular reducing agents. The presence of both Allx and its reduction product leads to the establishment of redox cycle for generation of superoxide radicals (O·). Thereafter, a dismutation of superoxide radicals induces a production of hydrogen peroxide (H2O2), which reacts with ferrous (Fe2+) to produce hydroxyl radical (OH·), a high oxidative agent[22]. The intolerable rise in oxidative agents provokes necrosis of pancreatic β-cells known to be vulnerable to redox imbalance[23]. This suggests that Allx diabetogenicity is a free radical mediated process. Furthermore, Allx toxicity is related to animal death due to hypoglycemic fatal convulsions[24].
This study shows that oral administration of CPSO prevented the diabetogenic effect of Allx; this is possibly due to the presence of antioxidant compounds, which act by inhibiting Allx-induced free radicals production or/and more probably by quenching them if they are produced. This hypothesis is supported by DPPH scavenging ability of CPSO confirmed in this paper.
A chemical composition of cactus pear seed oil is characterized by the presence of fat-soluble vitamins (tocopherols, β-Carotene and vitamin K1)[13]. Recent study demonstrated that D- α-tocopherolenriched diet prevents against Allx-induced diabetes in mice[25]. Though, γ-tocopherol is a major vitamin E in CPSO. It has been reported that γ-tocopherol have an antioxidant and antiinflammatory activities[26]. These findings were supported by Tomasch et al. wich demonstrated that γ-tocopherol rich oil intake improves plasmatic antioxidant capacity and decreases low-density lipoproteins in healthy male volunteers[27]. While γ-tocopherol is less powerful antioxidant than -tocopherol, it is more efficient in macromolecules protecting against oxidation[28].
Moreover, phenolic compounds could also be involved in the protecting action of CPSO againt Allx cytotoxicity. It has been shown that there are more than 20 phenolic compounds in cactus pear seeds and this content is highly correlated to their antioxidant activity[16]. It is well known that phenolic compounds have high antioxidant efficiency and they are effective in care of degenerative diseases[29,30].
Furthermore, reactive oxygen species are involved in free calcium (Ca2+) rise in β-cells cytosol[31]. This abnormal state induces supraphysiological insulin exocytosis, which leads to hypoglycemic fatal convulsions in mice. Free radicals quenching ability of CPSO could be involved in the improvement of survival rate in mice treated by CPSO with Allx.
On the other hand, the lipophilic fraction of CPSO contains 83% of unsaturated fatty acids (UFAs) represented by 56% of linoleic acid (ω-6) and 20% of oleic acid (ω-9)[32]. It was confirmed that ω-6 rich oil prevented Allx-induced diabetes in rats by preserving redox homeostasis in β-cells and by increasing cytoprotective antioxidant compounds such as vitamin E, superoxide dismutase and glutathione reductase[10]. In another study, it has been demonstrated that pretreatment with γ-linolenic acid and arachidonic acid, a metabolites of linoleic acid, prevented the incidence of Allx induced diabetes in rats by 100%[33]. However, some studies reported that ω -6 fatty acids are pro-inflammatory components, but it seems that there are many contradictions between findings.
Lifestyle and diet could be effective in diabetes prevention[34]. Also, healthy diet as source of antioxidants and good fats, is more recommended to manage the metabolic equilibrium in both healthy and diabetic people[7].
In spite of its actual expensive price, CPSO valorization might be helpful in the oil’s price lowering and then more consumers could use it.
In conclusion, CPSO prevented Allx-induced diabetes in mice; this effect might be due to the synergism of their antioxidant compounds in quenching free radicals and the capacity of their UFAs to enhance the antioxidant status in pancreatic β-cells. Further studies could be accomplished to assess the antidiabetic effect in other DM models.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgements
This work was supported by grants from CNRST, Morocco (Project URAC-40) and from Belgium (Program 3, CUD Project). Authors are also thankful to Badraoui Mustapha and Ramdaoui Karim for their technical support and animal breeding.
[1] International Diabetes Federation. IDF diabetes atlas. Brussels: International Diabetes Federation; 2013.
[2] Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet 2011; 378(9785): 31-40.
[3] Anderson KM, Seed T, Ou D, Harris JE. Free radicals and reactive oxygen species in programmed cell death. Med Hypotheses 1999; 52(5): 451-463.
[4] Oberley LW. Free radicals and diabetes. Free Radic Biol Med 1988; 5(2): 113-124.
[5] Droge W. Free radicals in the physiological control of cell function. Physiol Rev 2002; 82(1): 47-95.
[6] Fang YZ, Yang S, Wu GY. Free radicals, antioxidants, and nutrition. Nutrition 2002; 18(10): 872-879.
[7] Pitocco D, Martini F, Zaccardi F, Ghirlanda G. Chapter 24 - antioxidants, healthy diet, and diabetes: Oxidative stress and nutrition in diabetes. In: Bagchi D, Sreejayan N (eds.) Nutritional and therapeutic interventions for diabetes and metabolic syndrome. San Diego: Academic Press; 2012, p. 299-313.
[8] Berraaouan A, Abid S, Bnouham M. Antidiabetic oils. Curr Diabetes Rev 2013; 9(6): 499-505.
[9] Bellahcen S, Mekhfi H, Ziyyat A, Legssyer A, Hakkou A, Aziz M, et al. Prevention of chemically induced diabetes mellitus in experimental animals by virgin argan oil. Phytother Res 2012; 26(2): 180-185.
[10] Krishna Mohan I, Das UN. Prevention of chemically induced diabetes mellitus in experimental animals by polyunsaturated fatty acids. Nutrition 2001; 17(2): 126-151.
[11] El-Missiry MA, El Gindy AM. Amelioration of alloxan induced diabetes mellitus and oxidative stress in rats by oil of Eruca sativa seeds. Ann Nutr Metab 2000; 44(3): 97-100.
[12] Sawaya WN, Khan P. Chemical characterization of prickly pear seed oil, Opuntia ficus-indica. J Food Sci 1982; 47(6): 2060-2061.
[13] Ramadan MF, Mörsel JT. Oil cactus pear (Opuntia ficus-indica L.). Food Chem 2003; 82(3): 339-345.
[14] Medina EMD, Rodriguez EMR, Romero CD. Chemical characterization of Opuntia dillenii and Opuntia ficus-indica fruits. Food Chem 2007; 103(1): 38-45.
[15] Matthäus B, Özcan MM. Habitat effects on yield, fatty acid composition and tocopherol contents of prickly pear (Opuntia ficus-indica L.) seed oils. Sci Hortic-Amsterdam 2011; 131(1): 95-98.
[16] Chougui N, Tamendjari A, Hamidj W, Hallal S, Barras A, Richard T, et al. Oil composition and characterisation of phenolic compounds of Opuntia ficus-indica seeds. Food Chem 2013; 139(1-4): 796-803.
[17] Berraaouan A, Ziyyat A, Mekhfi H, Legssyer A, Sindic M, Aziz M, et al. Evaluation of antidiabetic properties of cactus pear seed oil in rats. Pharm Biol 2014; 52(10): 1286-1290.
[18] Liu W, Fu YJ, Zu YG, Tong MH, Wu N, Liu XL, et al. Supercritical carbon dioxide extraction of seed oil from Opuntia dillenii Haw. and its antioxidant activity. Food Chem 2009; 114(1): 334-339.
[19] National Research Council. Guide for the care and use of laboratory animals. Washington, D.C.: The National Academies Press; 2011.
[20] Schneider CA, Rasband WS, Eliceiri KW. NIH image to ImageJ: 25 years of image analysis. Nat Methods 2012; 9(7): 671-675.
[21] Elsner M, Tiedge M, Guldbakke B, Munday R, Lenzen S. Importance of the GLUT2 glucose transporter for pancreatic beta cell toxicity of alloxan. Diabetologia 2002; 45(11): 1542-1549.
[22] Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res 2001; 50(6): 537-546.
[23] Lenzen S. Oxidative stress: the vulnerable beta-cell. Biochem Soc Trans 2008; 36(Pt 3): 343-347.
[24] Waisbren BA. Alloxan diabetes in mice. Proc Soc Exp Biol Med 1948; 67(2): 154-156.
[25] Kamimura W, Doi W, Takemoto K, Ishihara K, Wang DH, Sugiyama H, et al. Effect of vitamin E on alloxan-induced mouse diabetes. Clin Biochem 2013; 46(9): 795-798.
[26] Wagner KH, Kamal-Eldin A, Elmadfa I. Gamma-tocopherol – an underestimated vitamin? Ann Nutr Metab 2004; 48(3): 169-188.
[27] Tomasch R, Wagner KH, Elmadfa I. Antioxidative power of plant oils in humans: the influence of alpha- and gamma-tocopherol. Ann Nutr Metab 2001; 45(3): 110-115.
[28] Morrissey PA, Kiely M. Vitamin E | Physiology and health effects. In: Benjamin C. (ed.) Encyclopedia of human nutrition (2nd ed). Oxford: Elsevier; 2005, p. 389-398.
[29] Villano D, Fernandez-Pachon MS, Moya ML, Troncoso AM, Garcia-Parrilla MC. Radical scavenging ability of polyphenolic compounds towards DPPH free radical. Talanta 2007; 71(1): 230-235.
[30] Scalbert A, Johnson IT, Saltmarsh M. Polyphenols: antioxidants and beyond. Am J Clin Nutr 2005; 81(1 Suppl): 215S-217S.
[31] Kim HR, Rho HW, Park BH, Park JW, Kim JS, Kim UH, et al. Role of Ca2+in alloxan-induced pancreatic beta-cell damage. Biochim Biophys Acta 1994; 1227(1-2): 87-91.
[32] Tlili N, Bargougui A, Elfalleh W, Triki S, Nasri N. Phenolic compounds, protein, lipid content and fatty acids compositions of cactus seeds. J Med Plants Res 2011; 5(18): 4519-4524.
[33] Suresh Y, Das UN. Protective action of arachidonic acid against alloxaninduced cytotoxicity and diabetes mellitus. Prostaglandins Leukot Essent Fatty Acids 2001; 64(1): 37-52.
[34] Dasari SR, Oza-Frank R, Venkat Narayan KM. Diabetes mellitus prevention. In: Kris H. (ed.) International encyclopedia of public health. Oxford: Academic Press; 2008, p. 146-152.
*Corresponding author: Bnouham Mohamed, Laboratory of Physiology and Ethnopharmacology-URAC40, Mohammed First University, Faculty of Sciences, Boulevard Mohammed VI, Oujda 60000, Maroc.
Tel: +212-6676-27496
Fax: +212-5365-00603
E-mail: mbnouham@yahoo.fr
Foundation project: This work was supported by grants from CNRST, Morocco (Project URAC-40) and from Belgium (Program 3, CUD Project).
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Andrographolide effect on both Plasmodium falciparum infected and non infected RBCs membranes
- Immunogenicity and efficacy of recombinant 78 kDa antigen of Leishmania donovani formulated in various adjuvants against murine visceral leishmaniasis
- Oral administration of Sauce llorón extract to growing lambs to control gastrointestinal nematodes and Moniezia spp.
- Hepatoprotective and proapoptotic effect of Ecballium elaterium on CCl4-induced hepatotoxicity in rats
- Antimicrobial activity and synergism of Sami-Hyanglyun-Hwan with ciprofloxacin against methicillin-resistant Staphylococcus aureus
- Role of Aedes aegypti and Aedes albopictus during the 2011 dengue fever epidemics in Hanoi, Vietnam
