In vitro micropropagation and flowering in Ipomoea sepiaria Roxb. An important ethanomedicinal plant
2015-12-22MeenaCheruvathurJyothiAbrahamDennisThomas
Meena K Cheruvathur, Jyothi Abraham, T Dennis Thomas
Research and development center, Bharathiar University, Maruthamalai Road, Coimbatore, PIN-641046, Tamil Nadu,Postgraduate and Research Department of Botany, St. Thomas College, Pala, India.
Arunapuram (P.O), PIN- 686 574, Kottayam (Dt.), Kerala, India
In vitro micropropagation and flowering in Ipomoea sepiaria Roxb. An important ethanomedicinal plant
Meena K Cheruvathur, Jyothi Abraham, T Dennis Thomas*
Research and development center, Bharathiar University, Maruthamalai Road, Coimbatore, PIN-641046, Tamil Nadu,Postgraduate and Research Department of Botany, St. Thomas College, Pala, India.
Arunapuram (P.O), PIN- 686 574, Kottayam (Dt.), Kerala, India
ARTICLE INFO
Article history:
Received 28 May 2014
Received in revised form 25 December 2014
Accepted 25 December 2014
Available online 20 March 2015
Ipomoea sepiaria
Micropropagation
Nodal cuttings
In vitro flowering
Abscisic acid
Objective: To standardize a protocol for the micropropagation and in vitro flowering of Ipomoea sepiaria(I. sepiaria), an important ethanomedicinal plant. Methods: The nodal cuttings were cultured on Murashige and Skoog (MS) medium supplemented with various concentrations of 6-benzyladenine (BA) or Kinetin (Kn; 1.0-4.0 mg/L) alone or in combination with α-naphthaleneacetic acid (NAA; 0.2-1.0 mg/L) for shoot proliferation. For rooting ½ MS medium supplemented with indole-3-butyric acid (IBA) or NAA (0.5-3.0 mg/L) was used. When the 45-day-old in vitro derived nodal cuttings were subcultured on MS medium supplemented with 3.0 mg/L BA and 0.5 mg/L NAA and various concentrations of abscisic acid (ABA; 0.5-3.0 mg/L), in vitro flowering was observed. Results: The highest shoot induction response in terms of percent cultures responding and number of shoots per explant was observed on 3.0 mg/L BA and 0.5 mg/L NAA. On this medium 100% cultures responded with an average number of 3.2 shoots per explant. The optimum rooting was observed on 2.0 mg/L IBA. Here 100% shoots rooted with an average number of 5.1 roots per shoot. The optimum in vitro flowering response (38%) was observed on 2.0 mg/L ABA. Conclusion: The present protocol is an efficient method for the rapid multiplication, flowering and conservation of this medicinal plant.
1. Introduction
Ipomoea sepiaria(I. sepiaria) is an ethnomedicinal plant belonging to Convolvulaceae family. It is one among the“Dasapushpa” (ten flowers) of aurvedic medicine[1]. It is distributed in streams and hedges near rivers of Indo-Malayan regions of Asia. The whole plant is medicinally useful. The powdered plant is used as a shampoo powder and stimulates hair growth. The plant is having cooling and rejuvenating effect[2]. It is useful in vitiated conditions of pitta, burning sensation, psychic disorders, strangury, hyperdipsia and general debility[3]. In traditional practices it is mainly used in the treatment of women sterility and pediatric diseases[4]. I. sepiaria is used in the treatment of ulcers and considered as a good antidote to arsenic[2] and also reported to have antiviral properties[5]. The powdered leaves and extracts provided good protection for black gram seeds against the pulse beetle, Callosobruchus maculatus by reducing insect oviposition and F1 adult emergence[6]. Significant aphidicidal activity of the hot and cold water extracts of this plant were tested against the bean aphid, Aphis craccivora[7].
Because of its ethnic and medicinal properties, the demand of the plant is increasing day by day. A reliable and efficient micropropagation protocol in this plant can result in superior differentiation, shoot development and entire plant regeneration which is essential for the propagation of selected traits within a specific genotype[8]. In addition to this, micropropagation is an important tool for the recovery and conservation of germplasm[9]. Other advantages of this technique involve rapid propagation rate, space exploitation, the enhancement of sanitary conditions of plants and the facilitation of international germplasm exchange. Plant tissue culture techniques play an important role in obtaining genetically uniform massive clones.
One of the most fascinating events in the lifecycle of angiosperms is the shift from vegetative phase to reproductive phase. This complicated process is often influenced by several aspects especially a combination of exogenous and endogenous factors. Virtually all thesefactors interact in various complex and unpredictable ways[10-14]. Important factors which influence the in vitro flowering include nature of plant growth regulators, light, carbon source and pH of the medium[15]. Similarly internal factors like position of the explant on the intact plant, genotype and genes expressed during flowering[16]. In vitro approach has proven to be a very useful strategy for the investigation of flowering physiology. It also helps to isolate potential flowering sites and use such tissues to test the effects of various parameters on flowering. There are no previous reports on micropropagation in this plant. Hence the present work is an attempt to develop an effective protocol for micropropagation of this plant using nodal explants and induction of in vitro flowering from shoots obtained from nodal segments.
2. Materials and methods
2.1. Plant material and in vitro culture
Single node cuttings of I. sepiaria were collected from the Botanical garden of the Institute and were washed with soap solution for 5 minutes, rinsed 3 times in distilled water, immersed in 70% ethanol for 1 minute, washed 3 times in sterile distilled water. Finally disinfected with freshly prepared 0.1% (w/v) mercuric chloride (HgCl2) for 3 minutes. The explants were again washed 3 times in sterilized distilled water to remove the traces of sterilant. After giving a final trimming at the cut ends, the nodal cuttings were cultured on Murashige and Skoog (MS) medium[17] supplemented with various concentration of 6-benzyladenine (BA) or kinetin (Kn; 1.0-4.0 mg/L) alone or in combination with α-naphthaleneacetic acid (NAA; 0.2-1.0 mg/L).
麻江县位于贵州省黔东南州西部,地处云贵高原向湘桂丘陵盆地倾斜过渡地带的沅江水系清水江上游,地貌类型包括岩溶地貌和侵蚀地貌,属亚热带季风湿润气候区。全县土地总面积1 222.2km2,其中岩溶出露面积1 005.12km2,占土地总面积的84.24%。据2005年调查数据显示,麻江县现有石漠化面积298.51km2,占全县土地面积的24.42%,占岩溶面积的29.70%。此外还有345.85km2的土地存在潜在石漠化趋势。该县石漠化程度在黔东南州16个县(市)中居第二位,严重的石漠化已成为全县生态文明和小康社会建设的一大制约。
The percent response, average number of shoots per explant and average length of shoot were recorded after 45 days of culture. Elongated and well developed shoots above 2 cm height were excised and transferred to rooting medium containing 1/2 MS supplemented with different concentrations of indole-3-butyric acid (IBA; 0.5-3.0 mg/L). Plantlets having more than two roots were taken out from the culture tubes, washed thoroughly with tap water to remove agar and transferred to paper cups containing soil and sand (1:1) and covered with a plastic bag to maintain high humidity. The young plants were kept under 30%-40% natural light, sprayed with water twice a day. The acclimatized plants were transferred to their natural habitat and the survival rate was assessed after 3 months.
2.2. In vitro flowering
For in vitro flowering, nodal segments from in vitro formed shoots were isolated after 5 days and subcultured on MS medium supplemented with various concentrations of (0.2-1.5 mg/L) abscisic acid (ABA). Various parameters like percent response, average number of flowers and days to flowering were recorded after 50 days after culture.
2.3. Culture conditions
The pH of the medium was adjusted to 5.8 prior to adding 0.8% w/v agar before being sterilized by autoclaving at 120 ℃and 104 kPa for 15 min. After the explant transfer, the test tubes were maintained at (25 ± 2) ℃ under 16-h photoperiod provided by cool white fluorescent tubes. Each treatment consisted of at least 12 cultures and all experiments repeated three times. Analysis of variance and Duncan’s multiple range test were used for comparison among treatment means[18].
3. Results
3.1 Multiple shoot initiation and elongation
The nodal segments cultured on MS medium supplemented with various concentrations of BA or Kn (1.0-4.0 mg/L) alone or in combination with NAA (0.2-1.0 mg/L) showed bud break and shoot elongation. However, both these responses vary depending on plant growth regulator concentrations and combinations. The optimum percent response (78%) was observed on MS medium supplemented with 3.0 mg/L BA when used individually. However, the number of shoots was invariably one in all concentrations of BA. The average shoot number varies from 1.1 to 1.6 in various BA and Kn concentrations (Table 1, Figure 1A). The addition of NAA with BA improved the response further. 100% cultures responded with an average number of 3.2 shoots per explant on MS medium supplemented with 3.0 mg/L BA and 0.5 mg/L NAA (Figure 1B). There was very poor response when MS basal medium was used.
The bud break occurred usually after one week in most of the cultures. The shoot emerged from the axil of the explant within 2 weeks after culture initiation. The shoot growth was vigorous and healthy shoots were formed within 3-4 weeks after culture. The addition of other cytokinins like Kin or TDZ alone or in combination with auxin resulted in callus formation at the cut end of the explant (data not shown). Therefore only BA and NAA alone was used as plant growth regulators for shoot multiplication in the present investigation.
Full strength MS medium produced callusing of the basal cut end and the roots were emerged from the calli. Hence ½ MS medium was used throughout the experiment for rooting. Shoots measuring a size of about 2.0 cm were excised and cultured on ½ MS medium supplemented with IBA or NAA in the range of 0.5-3.0 mg/L for root induction.Comparatively IBA gave better results than NAA.
Root induction medium containing ½ MS with IBA produce roots in most of the explants within 2 weeks (Figure 5, Table 2). 1.0 mg/L NAA produced optimum response of 67% cultures responded with an average number of 1.8 roots per shoot. However, the highest response was observed on MS medium supplemented with 2.0 mg/L IBA. Here 100% cultures responded with an average number of 5.1 roots per shoot (Figure 1E). The roots were healthy and whitish in colour.
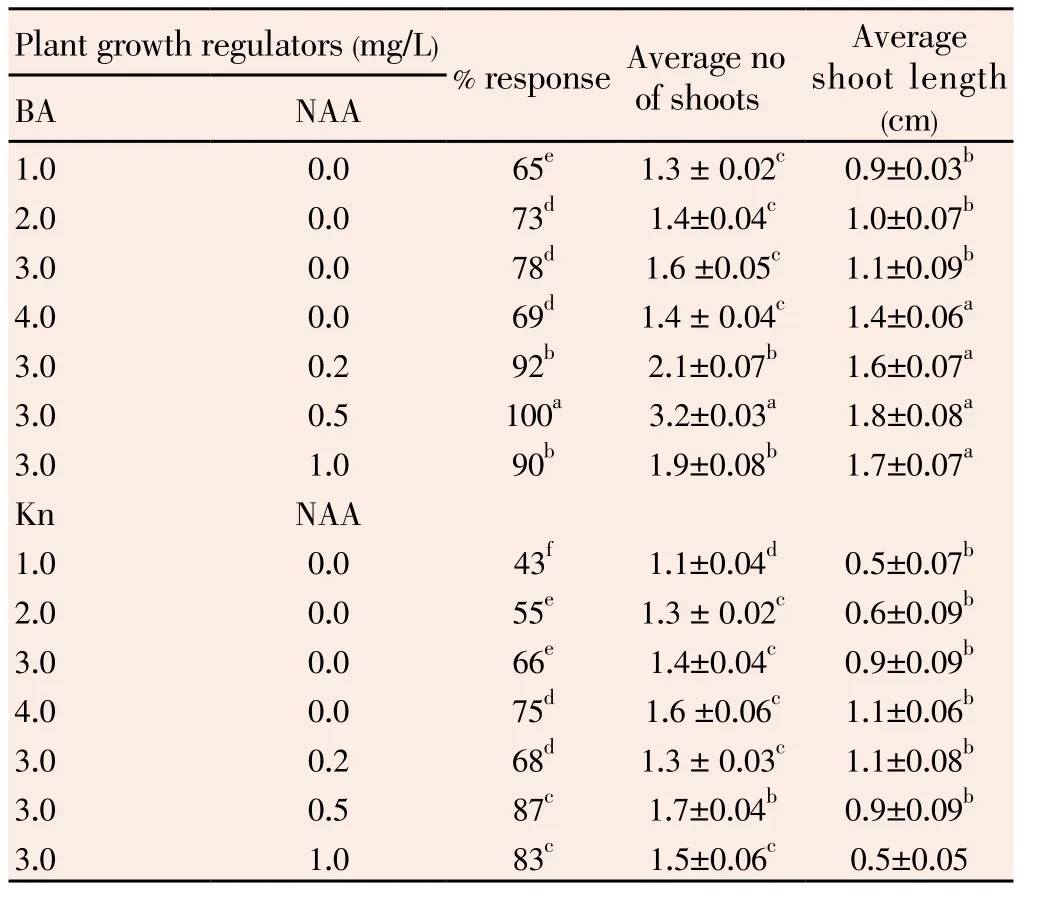
Table 1 Effect of different concentration of BAP, Kn alone or in combination with NAA on shoot proliferation from nodal explants of I. sepiaria.

Table 2 Influence of IBA or NAA on rooting of in vitro formed shoots of I. sepiaria on ½ MS medium after 45 days.
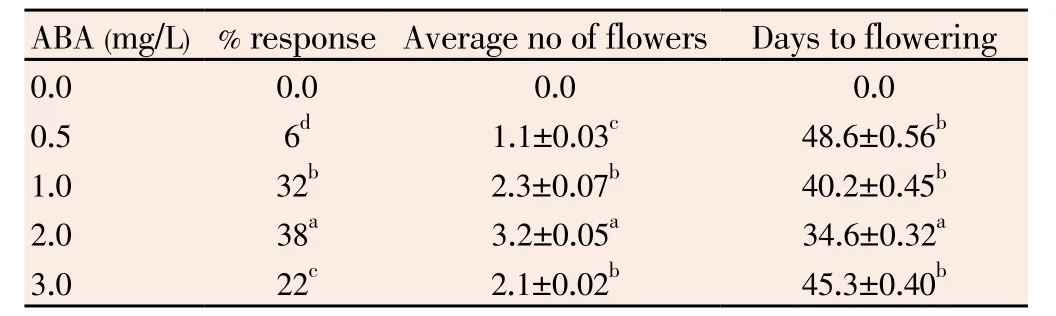
Table 3 Effect of ABA on in vitro flowering in I. sepiaria.
3.3 Hardening
Plantlets with 2 or 3 leaves and well developed roots were taken from the culture tubes and washed in running tap water to remove the traces of agar. The plantlets were subsequently immersed in 1% fungicide (Bavistin) solution and then transplanted to paper cups containing sterilized garden soil and sand (1:1; Figure 1F). The moistened ½ MS medium for a week and gradually transferred into field.
About 80% of the regenerated plants survived following transfer from vermiculite to natural soil and no detectable variation with respect to morphology or growth characteristics was observed. The period of transition during the process of hardening after transfer from the in vitro to the ex vitro condition is considered to be the most crucial step in plant tissue culture. The plant has a heterotrophic mode of nutrition and therefore lack adaptation or exposure to the outside environment, during laboratory to land transfer micropropagated plants are first placed in the hardening chamber with high humidity.
3.4 In vitro flowering
For in vitro flowering specific media combination is necessary. Similarly 45 day old nodal cuttings taken from in vitro cultures were only responded. Such single nodal cuttings measuring a size of about 2.0-3.0 cm were cultured on MS medium supplemented with 3.0 mg/L BA, 0.5 mg/L NAA and various concentrations of abscissic acid (ABA, 0.5-3.0 mg/L) for in vitro flower induction. The optimum flowering was observed on MS medium supplemented with 3.0 mg/L BA, 0.5 mg/L NAA and 2.0 mg/L ABA. On this medium 38% cultures produced flowers with an average number of 3.2 flowers per shoot (Figure 1C, D). Here the first flower bud appeared about 35 days after culture. Flower buds were appeared in the developing shoot only after 40 days. Flowers were emerged from the tip region of the developing shoot. A single bunch consisted of two flower buds (Figure 1D ).
4. Discussion
In the present study maximum multiple shoot induction (3.2 shoots/explant) was observed on MS medium supplemented with 3.0 mg/L BA and 0.5 mg/L NAA. For shoot induction and elongation BA plays a very crucial role. BA induced shoot multiplication has been reported in several systems like Caralluma bhupenderiana[19]. However, the addition of an auxin along with cytokinin considerably increased percent cultures responding as well as shoot number and average shoot length[20,21,22].
For root induction in I. sepiaria, shoots half strength medium is preferred over full strength medium since the latter produced callusing at the basal cut end of the shoots. Lower mineral content is thought to be more efficient for in vitro rooting[23]. Controlled starvation of the plants in culture is often considered as a useful method for root induction in several systems. Our result is in agreement with previous observations by several workers[24-26].
In the present study ABA in combination with BA and NAA were responsible for in vitro flowering. Cytokinins in medium are considered as the main component which induce flowering. Flower induction and development in response to exogenous cytokinins have been observed in a few herbaceous plants[27-29]. According to Chailakhyan and Butenko[30] cyokinin is a conformity with the observations made on the present studies where presence of cytokinin either BAP or kinetin proved essential in the medium for in vitro flowering. However, in the present study we obtained highest in vitro flowering on a medium containing a combination of ABA, BA and NAA. ABA either with an auxin or cytokinin or in combination with auxin and cytokinin induced in vitro flowering in several systems like Panax ginseng[31] Torenia sp.[32], Perilla sp.[33]. The promotive role of ABA on flower induction in vitro could be attributed to their role in water stress management. ABA is considered as a stress hormone often accumulate in floral parts of plants and functions through a set of ABA regulated genes, which in turn lead to accumulation of osmo-protectants like proline[34]. The presence of ABA responsive elements (ABRE) in the genes regulated by dehydration indicated a putative role of ABA in flower induction[35]. In addition, there were indirect evidences that stress induced compounds like abscisic acid (ABA)[32], may influence flowering.
In the present investigation, only 45 day old in vitro grown nodal cuttings produced in vitro flowering. The nodal cuttings subcultured before 45 days did not induce flowering. This is in agreement with earlier studies in some systems where juvenile explants do not flower due to inability to produce flowering factor(s) or the inability of meristems to respond to flowering factors[13,36]. In Rosa sp. Wang et al.[37] noted that the total time from original culture and subculture before flower induction were two very important factors for in vitro flower induction. Many reports are there to support the influence of age of explants in in vitro flowering. Nodal explants of Mulberry incubated in MS medium supplemented with BAP produce in vitro inflorescences only after 45 days of culture and sex expression of this dioecious plant modified by the application of ethrel and silver nitrate[38]. In Ceropegia the flowering occurred only after the incubation for more than 4 weeks on the same media[39].
To our knowledge, this is the first report on micropropagation of I. sepiaria. The protocol described here is reproduciable and could be used for the large scale multiplication and propagation of this important medicinal plant. The micropropagated shoots were rooted and transplanted to soilsuccessfully. Another important observation noticed during our study was the induction of flowering on ABA containing medium. This will give further light to the developmental studies of floral differentiation. The in vitro flowering protocol described here bears immense importance and would further facilitate selective hybridization using pollen from rare stocks and explore the possibility of recombining genetic material via in vitro fertilization.
Acknowledgement
We thank the Principal, St. Thomas College, Palai for providing us with necessary laboratory facilities.
[1] Raman Numboodhiri KR. Athbutha oushada chedikal (In Malayalam). Thrissur, Kerala: H&C publications: 2004, p. 34.
[2] Kirtikar KR, Basu BD. Indian medicinal plants. Bishen Singh, Mahendra Pal Singh, Dehra Dun. 1975, p. 1723.
[3] Prajapati N, Purohit SS, Sharma AS, Kumar T. A hand book of medicinal plants: A complete source book. Agrobios Jodhpur 2007; 5: 291- 292.
[4] Warrier PK, Nambiar VPK, Ramankutty C. Indian medicinal plants. A compendium of 500 species. Orient Longman. Madras 1995; 3: 237.
[5] Bajpai SK, Chandra K. Studies on the antiviral properties of plants with special reference to Zingiber capitatum. Fitoterapia 1990; 61:3-8.
[6] Rahman A, Talukder FA. Bioefficacy of some plant derivatives that protect grain against the pulse beetle, Callosobruchus maculates. J Insect Sci 2006; 3: 1-10.
[7] Das BC, Pankoj KS, Matiur Rahman MD. Aphidicidal activity of some indigenous plant extracts against bean aphid Aphis craccivora Koch (Homoptera: Aphididae). J Pest Sci 2008; 81: 153-159.
[8] Bhojwani SS, Razdan MK. Plant tissue culture: theory and practice, a revised edition. Amsterdam:Elsevier; 1996,p. 1-766.
[9] Delgado-Sanchez P, Saucedo-Ruiz M, Guzman-Maldonado SH, Villordo-Pinesa E, Gonzalez-Chavira M, Fraire-Velazquez S, et al. An organogenic plant regeneration system for common bean (Phaseolus vulgaris L.). Plant Sci 2006; 170: 822-827.
[10] Tran Thanh VM. Regulation of organogenesis from small explants of Nicotiana tabacum L. Planta 1973; 115: 149-159.
[11] Scorza R, Janick J. In vitro flowering of Passiflora suberosa L. J Am Soc Hort Sci 1980; 105: 982-997.
[12] Croes AF, Creemer-Molenaar T, Van den Ende G, Kemp A, Barendse GMW. Tissue age as an endogenous factor controlling in vitro bud formation in explants from the inflorescence of Nicotiana tabacum L. J Exp Bot 1985; 36: 1771-1779.
[13] Lang A. Physiology of flowering. In: Ruthland W. (ed). Encyclopedia of plant physiology. Berlin, Heidelberg: New York;1965,p.1380-1536.
[14] Compton ME, Veilleux RE. Thin cell layer morphogenesis. Hort Rev 1992; 14: 239-264.
[15] Heylen C, Vendrig JC. The influence of different cytokinins and auxins on flower neoformation in thin cell layers of Nicotiana tabacum L. Plant Cell Physiol 1988; 29: 665-671.
[16] Jumin HB, Nito N. In vitro flowering of Fortunella hindsii (Champ.). Plant Cell Rep 1996; 15: 484-488.
[17] Murashige T, Skoog F. A revised medium for rapid growth and bioassay with tobacco tissue culture. Physiol Plant 1962; 15: 473-497.
[18] Duncan DB. Multiple range and multiple F test. Biometrics 1955; 11: 1-42.
[19] Ugraiah A, Sreelatha VR, Krishna Reddy PV, Rajasekhar K, Sandhya Rani S, Karuppusamy S, et al. In vitro shoot multiplication and conservation of Caralluma bhupenderiana Sarkaria - an endangered medicinal plant from South India. Afr J Biotech 2011; 10: 9328-9336.
[20] Jala A. Effects of NAA, BA and sucrose on shoot induction and rapid micropropagation by trimming shoot of Curcuma Longa L. Int Trans J Eng Man App Sci Tech. 2012; 3: 101-109.
[21] Hedayat M, Abdi G, Khosh-Khui M. Regeneration via direct organogenesis from leaf and petiole segments of pyrethrum [Tanacetum cinerariifolium (Trevir.) Schultz-Bip.]. Am-Euras J Agric Environ Sci. 2009; 6: 81-87.
[22] Ndoye M, Diallo I, Gassama YK. In vitro multiplication of the semiarid forest tree. Balanites aegyptiaca (L.) Del. Afr J Biotech 2003; 2: 421-424.
[23] George EF, Sherrington PD. Plant propagation by tissue culture. Edington, England: Exegetics ltd;1984.
[24] Vargheses SK, Inamadar JK, Kalia K, Subramania RB, Nataraj M. Micropropagation of Aegle marmitos (L). Phytomorph 1993; 43: 87-92.
[25] Usha R, Swamy PM. In vitro micropropagation of sweet worm wood (Artemisia annua). J Phytomorph 1998; 48: 149- 154.
[26] Thomas TD. In vitro modification of sex expression in mulberry (Morus alba L.) by ethrel and silver nitrate. Plant Cell Tissue Org Cult 2004; 77: 277-281.
[27] Maheswari SC, Venkataraman M. Induction of flowering in duckweed Wolffia microscopic by a new kinin zeatin. Planta 1966; 70: 304.
[28] Nitsch C. Effect of growth substances on the induction of flowering of a short day plant in vitro. In: Weightman F, Scotterfield F (eds.) Biochemistry and physiology of growth substances. Canada: Runge Press;1968,p. 1385-1397.
[29] Srinivasan C, Mullin MG. Control of flowering in the grape vine (Vitis vinifera L.). Plant Physiol 1978; 61: 127-130.
[30] Chailakhyan MKH, Butenko R. The effect of adenine and kinetin on the differentiation of flower buds in Perilla stem tips. Dokl Akad Nauk SSSR 1959; 129: 293-295.
[31] Lee HS, Lee KW, Yang SG, Liu JR. In vitro flowering of Ginseng (Panax ginseng C. A. Meyer) mzygotic embryos induced by growth regulators. Plant Cell Physiol 1991; 32: 1111-1113.
[32] Tanimoto S, Miyazaki A, Harada H. Regulation by abscisic acid of in vitro flower formation in Torenia stem segments. Plant Cell Physiol 1985; 26: 675-682.
[33] Purse JG. Phloem exudate of Perilla crispa and its effect on flowering of P. crispa shoot explants. J Exp Bot 1984; 35: 227.
[34] Deotale RD, Maske VG, Sorte NV, Chimurkar BS, Yerne AZ. Effect of GA3 and NAA on morpho-physiological parameters of soybean. J Soils Crops 1998; 8: 91-94.
[35] Riechmann JL, Meyerowitz EM. The AP2/EREBP family of plant transcription factors. Biol Chem 1998; 379: 633-646.
[36] Hackett WP. Juvenility, maturation and rejuvenation in woody plants. Hort Rev 1985; 7:109-155.
[37] Wang GY, Yuan MF, Hong Y. In vitro flower induction in roses. In vitro Cell Dev Bio Plant 2002; 38: 513-518.
[38] Thomas TD. Pretreatment in Thidiazuron improves the in vitro shoot induction from leaves in Curculigo orchioides Gaertn., an endangered medicinal plant. Acta Physiol Plant 2007; 29: 455-461. [39] Nair AK, Naik DD. Pandit SS. High- frequency in vitro flowering in six species of Ceropegia. J Plant Biol 2007; 50: 374-377.
*Corresponding author: 1 Research and development center, Bharathiar University, Maruthamalai Road, Coimbatore, PIN-641046, Tamil Nadu, India.
Tel: 91-95481-2537536
Fax: 91-95482-2216313
E-mail: den_thuruthiyil@yahoo.com
猜你喜欢
杂志排行
Asian Pacific Journal of Reproduction的其它文章
- Molecular dysregulation of renal development: Congenital anomalies of the kidney and urinary tract
- Ovine fetal sex determination using circulating cell-free fetal DNA (ccffDNA) and cervical mucous secretions
- A new treatment for premature ejaculation? Case series for a desensitizing masturbation aid
- Reproductive status of Camelus bactrianus during early breeding season in India
- Effect of frequency of collection on seminal characteristics of White Pekin duck
- Evaluation of norgestomet Crestar® on oestrus synchronization and reproductive performance of dairy cows in Algeria
