Effect of frequency of collection on seminal characteristics of White Pekin duck
2015-12-22AKNahakSCGiriDNMohantyPCMishraDashSK
AK Nahak , SC Giri , DN Mohanty , PC Mishra, Dash SK
Department of Animal Reproduction, Gynaecology and Obstetrics, College of Veterinary Science and Animal Husbandry, OUAT, Bhubaneswar-751003
Effect of frequency of collection on seminal characteristics of White Pekin duck
AK Nahak , SC Giri , DN Mohanty , PC Mishra, Dash SK
Department of Animal Reproduction, Gynaecology and Obstetrics, College of Veterinary Science and Animal Husbandry, OUAT, Bhubaneswar-751003
ARTICLE INFO
Article history:
Received 3 July 2014
Received in revised form 10 November 2014
Accepted 12 December 2014
Available online 20 March 2015
White Pekin duck
Objective: To determine an optimum frequency of semen collection, so as to improve the efficacy of artificial insemination techniques and selective breeding programmes in duck farming. Methods: Thirty two ejaculates were collected by abdominal massage method from two groups of drakes, with 10 in each, at one day and two day intervals respectively. Routine seminal attributes such as volume, colour, pH, spermatozoa concentration, number of spermatozoa per ejaculate, individual motility, live sperm percentage, total sperm abnormalities and time taken to reduce Methylene blue (MBRT) were studied for comparison of their values in one day and two days of interval of semen collection. Twenty female laying ducks maintained for examining the fertility percent were regularly inseminated with 0.1 mL of neat pooled semen for fertility test. Results: The test of significance indicated a significant decrease (P<0.05) in the time taken to reduce methylene blue, a highly significant increase in the sperm concentration (P<0.01) and number of spermatozoa per ejaculation (P<0.01) and a non significant improvement in the seminal characteristics with respect to semen volume and mass motility was found in the ejaculates of two day interval than that of one day interval collections. Conclusion: Collection of semen at two days intervals is recommended for enhancing fertility in artificial insemination of duck. The methylene-blue reduction test, along with spermatozoa count and initial motility estimates were reported to be better indicator of semen quality.
1. Introduction
Ducks form about 10% of the total poultry population and contribute 6%-7% of total eggs produced in the country. The growing preference of duck eggs over that of chicken is due to the production of more number of eggs per bird per year and it’s comparatively larger size. To augment the egg production of the country as a whole, priorities are given to encourage duck production especially through integrated agriculture practices. But availability of ducklings as per the demand of farmers is far away which needs to be achieved. Therefore, increased production of fertile eggs from ducks through artificial insemination technique is the need of the hour. AI technique has been widely used in livestock breeding programmes to increase productivity. Breed Improvement Programmes of the native ducks through artificial breeding is also needed for getting varied genotype that can adapt to the existing environment. Besides, Artificial insemination is also practised to alleviate the unsatisfactory fertility problems in ducks resulting from the impaired mating behaviour. Drakes and ganders produced a small ejaculate volume with a low sperm concentration and a low number of live normal spermatozoa[1]. The length of fertile period in birds has been defined by Lake[2] as the interval between artificial insemination and the last fertile egg laid. The length of this interval depends on the sperm storage in the tubules at the utero-vaginal junction where the spermatozoaare released for movement toward the infundibulum for ova fertilization[3,4]. Moreover, the frequency of artificial ejaculation has a great impact on different seminal characteristics. Keeping this in view, the present study was carried out to standardise collection interval and evaluation methods of duck semen for utilisation of artificial insemination technique in duck breeding programmes.
2. Materials and methods
Ten drakes and 20 female laying ducks (30-45 weeks of age) of White Pekin breed were selected for the experiment. The birds were housed in individual cages(1 m×1 m×0.6 m), fed with a layer diet (18% crude protein and 2650 kcal ME/kg), two weeks prior to the date of estimation of semen quality and were maintained under optimum managemental practices. The reproductive characteristics of the male and female ducks were carefully observed. Initial massage was practiced daily for one week without semen collection for acclimatizing them against fear and stress during handling as the semen ejaculation depends upon the reflex action. Semen was collected in the wide mouth glass vial by dorso-abdominal massage method[5] with possible measures to avoid any contamination. The process involved two persons; one for careful handling of the bird and the other person applying abdominal message to collect the semen. The attendant held the drake by grasping its thigh along with soma of the flight feathers, to prevent wind flapping, in a horizontal position at a height convenient to the collector so that the bird rests its neck on the shoulder of the attendant. The collector then by gently massaging collects the semen by stroking the lumbar region of the back towards the tail two or three times with the palm of the hand. The tips of the thumb and forefinger were gently thrust deep into the soft part at the base of the pygostyle (Parson’s nose) in order to get behind the cloacal opening and the visible erected copulatory appendages. Evagination of the phallus (the copulatory appendage) in the cloacae was caused by those stroking movements and the semen was immediately squirted from the base of the swollen ejaculatory papillae. Semen thus expressed was caught into the wide mouth glass vial held on the operator’s free hand.
Routine analysis of different physical and quality parameters were carried out immediately after collection. Ejaculate volume was measured by a tuberculin syringe. The appearance of semen was scored from 1 to 5 by visual examination following the method as described by McDaniel and Craig[6]. The scoring system for mass motility was from 1 to 5[7]. Spermatozoa concentration was measured by Haemocytometer method as described by Jones and Wilson[8]. Number of spermatozoa per ejaculate was calculated by multiplying concentration with semen volume. Eosin-Nigrosin stain[9] was used to count the livability per cent, and Giemsa staining technique[10] used for sperm abnormality. Individual motility studied under microscope by standard procedure described by Zemjanis[11]. Methylene blue reduction time was recorded as per Herman and Madden[12]. Female ducks thus maintained to examine the fertility percent were regularly inseminated with 0.1 mL of pooled semen after being diluted with normal saline solution (NSS) in 1:1 ratio. Eggs collected for seven continuous days were incubated and fertility per cent was recorded by candling the eggs on 10th day of incubation to study the effect of frequency of collection on quality of semen.
3. Results
Semen was collected at the base of the phallus in the abdominal massage method with satisfactory result in the morning hours before feeding. A good amount (2 to 4 mL) of clear and thin fluid was encountered before the actual semen discharge. The Mean ± Standard Error of different seminal attributes obtained at one day and two days of interval of collection are presented in the Table 1.
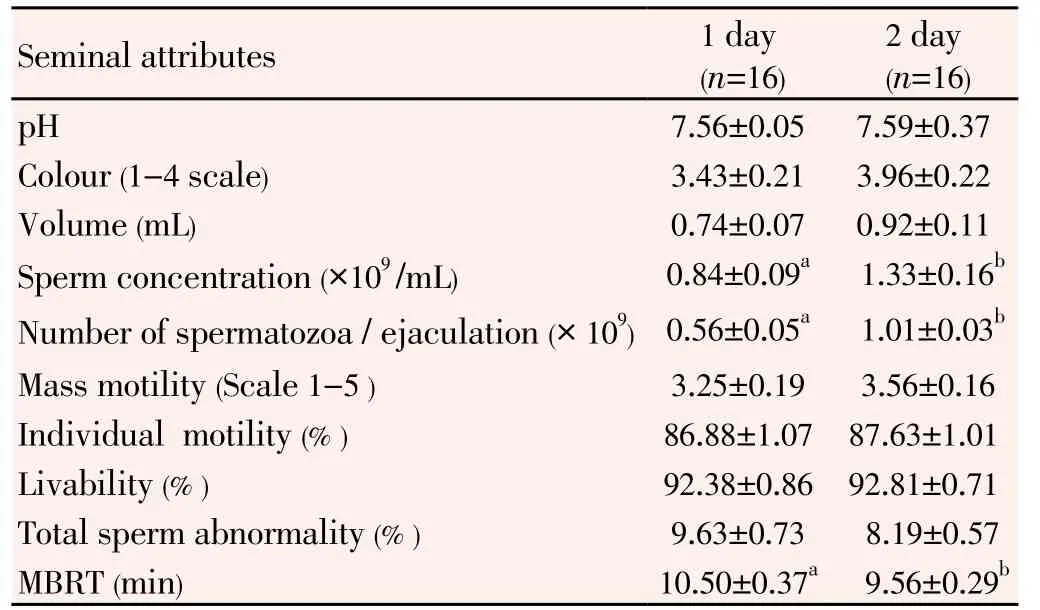
Table 1 Effect of frequency of collection on seminal characteristics of White Pekin drakes.
The pH of the freshly collected duck semen was slightly alkaline ranging between 7.3 and 7.8. The natural colourof semen collected from drakes was mostly creamy white. Mass motility ranged between 2 to 4 in a 0-4 scale. Test of significance revealed no significant difference in pH, colour, volume, motility, liveability, and abnormality percentage between the samples collected at one day and two day intervals of time. Similarly a significant difference (P<0.05) between spermatozoa concentration, number of spermatozoa per ejaculation and time taken to reduce methylene blue dye has been observed in semen samples collected at one day and two days of intervals of collections.
It was also observed that the fertilizability was higher in natural breeding with a mean of 73.35 %, than that obtained after artificial insemination (49.92 %). But, the fertilizability percent of eggs obtained by the two experimental periods as determined by candling method was also higher for two day interval collection period than one day.
4. Discussion
Drakes are unique in their bizarre penises stretching up to 20 cm in size in an anticlockwise spiral manner. The phallus is found coiled along the ventral wall of cloaca when not erect. After initial arousal, the duck phallus extends as sperm travel in the outer layer of penis in a corkscrew path. The transparent fluids were usually secretion from a number of glands available in the genital tract which mainly intended for lavage of the tract.
It was observed that the semen examined after 30 to 60 minutes of collection at room temperature showed a sharp decline in pH which was due to production of increased amount of lactic acid released by fructolysis. The mean pH value of ejaculates so obtained supports the findings of Cyriac et al. [13]. The variation in pH of semen recorded in the present investigation might be due to variation in components of secretion from accessory glands and spermatozoan concentration[14].
The colour of semen samples largely was governed by the concentration of semen. Clear opalescent semen containing a large number of epithelial cells with a few numbers of motile spermatozoa was typical in samples collected at short time interval. Majority of the semen samples from White Pekin drakes were medium thick in consistency and this was in agreement with the findings of Cyriac et al.[13]. The discolouration of semen was mostly due to contamination with excreta and glandular secretions
Though no significant difference in ejaculate volume was reported in the present study, a significant increase (P<0.05) in semen volume has been reported by about 67.56% and 62.16% for the frequency of twice and thrice a week groups as compared to once a week collection group[15].
A significant difference between spermatozoa concentration in semen samples at one day and two day interval of collections in the present report corrobates with the finding of Ghonim et al. [15] who also found a significant decrease of spermatozoa concentration with increase in frequency of semen collection. The higher concentration of semen achieved in two day intervals of collection encountered during the present experiment, is also in agreement with the findings of Allen and Champion[16], who concluded that the wide variations noticed in the sperm concentration between cockerels and period of collections could be attributed to the collection interval, semen volume as well as individual variation between sires.
The reported mean mass motility score of spermatozoa was similar to the findings of Cyriac et al. [13], who recorded an initial motility of 3.38 in White Pekin drakes. The finding of individual motility per cent was also similar to those of Domyati drakes as reported by Ghonim et al. [17]. The fact that the test of significance between the days of interval did not show any significant difference in motility percentage, the percentage of live and abnormal spermatozoa, implies least effect of collection intervals on these parameters.
The present observation of live percent of spermatozoa is comparable to the findings of Ghonim et al.[15] in Domyati drakes but little lower than that found by Cyriac et al.[13]. who reported 96.45% liveability. Total abnormality percent of sperm are found to be lower as compared to that in Domyati drakes[15]. However no effect of frequency of collection on liveability and abnormality of spermatozoa were noticed.
The significantly longer time taken for methylene reduction in the semen samples of one day interval time of collection might be attributed to low concentration and liveability or metabolic activity of the spermatozoa.
The routine seminal parameters could be taken into account during selection of drakes for both natural service and artificial insemination. Moreover, these seminal parameters will not only improve fertility, but also enhance hatchability of eggs in artificial insemination programme in ducks. The technique of insemination to prevent involuntary constriction of vaginal aperture, encountered in many cases, could also have influenced the fertility parameters, which can suitably be prevented. The fertilizability per cent of eggs obtained by the two experimental periods as determinedby candling method was also higher for two day interval collection period than one day, therefore, it was concluded that semen collection at two days interval is probably more justified in case of drakes as far as semen quality is concerned. This finds support results of Ghonim et al.[15], who preferred twice collection per week for good semen and better sperm output in Domyati drakes. Many other options like semen additives, semen extenders and enhanced dose etc., are expected to improve the fertilizability of the eggs obtained after artificial insemination. The methylene-blue reduction test, along with spermatozoa count and initial motility estimates were reported to be better indicator of semen quality.
Conflict of interest statement
We declare that we have no conflict of interest statement.
Acknowledgement
The authors are thankful to Central Avian Research Institute, Regional Centre, Bhubaneswar for providing laboratory, experiment birds, man power and other necessary facilities.
[1] Lukaszewicz E, W Kruszynski. Evaluation of fresh and frozenthawed semen of individual ganders by assessment of spermatozoa motility and morphology. Theriogenology 2003;59: 1674-1640.
[2] Lake PE. Gamete production and the fertile period with particular reference to domesticated birds. Symp Zool Soc Lond 1975;35: 225-244.
[3] Brillard JP. Sperm storage and transport following natural mating and artificial insemination. Poult Sci 1993;72: 923-928.
[4] Brillard JP, Beaumont C, Scheller MF. Physiological responses of hens divergently selected on the number of chicks obtained from a single insemination. J Reprod &Fertil 1998;114: 111-117.
[5] Kammer DM, Moreng RE, Muller HD, Hobbs HW. Turkey semen evaluation for fertility prediction. Poul Sci 1972 51:77-82.
[6] Mc Daniel GR, Craig JV. Behaviour traits, semen measurements and fertility of White Leghorn males. Poul Sci 1959; 38: 1005-1014.
[7] Etches RJ. Artificial insemination. In: Reprod poult. Cambridge. Wallingford: CAB International;1996,p. 234-262.
[8] Jones JE, Wilson HR. Use of an electronic counter for sperm concentration determination in chicken semen. Poult Sci 1967; 46: 532-533.
[9] Saxena MS. Veterinary andrology and artificial insemination. 1st ed. New Delhi: India; 2000,p. 89-94.
[10] Sharma PV. A simplified staining technique for evaluation of acrosomal status of sperm cells. Indian J Anim Reprod 1995;16: 127.
[11] Zemjanis R. Collection and evaluation of semen. In: Diagnotic and therapeutic techniques in animal reproduction. 2nd ed. Baltimore: Williams and Wilkins Co.;1970,p. 139-156.
[12] Herman HA, Madden FW. The artificial insemination of dairy cattle. Columbia: Lucas Bros. Publ.; 1953.
[13] Cyriac S, Joseph L, Peethambaran PA, Narayanankutty K, Karthiayini K. Semen quality characteristics of White Pekin, Kuttanad (Anas platyrhynchos domesticus) and Muscovy (Cairina moschata momelanotus) drakes. Indian J Anim Sci 2013;83(6): 595-599.
[14] Bandyopadhyaya SK, Bhatacharya, Choudhury RR, Basu S. Text book of gynaecology, artificial insemination, obstetrics and assisted reproduction. 2nd ed. India: Kalyani Publishers/Lyall Bk Depot 2007.
[15] Ghonim AIA, Awad AL, El.sawy AM, Fatouh MH, Ibraheim ZA. Effect of frequency of semen collection, dilution rate and insemination dose on semen characteristics and fertility of Domyati ducks. Egyptian Poult Sci 2009; 29: 1023-1045.
[16] Allen CJ, Champion LR. Competitive fertilization in the fowl. Poult Sci 1955;34: 1332-1342.
[17] Ghonim AIA, Awad AL, Elkloub K El. Moustafa M. Effect of feeding different levels of energy and crude protein on semen quality and fertility of Domyati ducks. Egyptian J Poult Sci 2010;30: 583-600.
*Corresponding author: A.K. Nahak, Ph.D. Scholar, Department of ARGO, C.V.Sc &A.H., OUAT, Bhubaneswar-751003, Odisha, India.
E-mail: imaknahak@gmail.com
Semen collection interval
Artificial insemination
杂志排行
Asian Pacific Journal of Reproduction的其它文章
- Molecular dysregulation of renal development: Congenital anomalies of the kidney and urinary tract
- Ovine fetal sex determination using circulating cell-free fetal DNA (ccffDNA) and cervical mucous secretions
- A new treatment for premature ejaculation? Case series for a desensitizing masturbation aid
- Reproductive status of Camelus bactrianus during early breeding season in India
- Evaluation of norgestomet Crestar® on oestrus synchronization and reproductive performance of dairy cows in Algeria
- Metabolic syndrome among infertile women with polycystic ovary syndrome
