Behavior of Benzene Decomposition by Using Pulse Modulated Power Supply
2015-12-20JIANGHuadong姜华东MATianpeng马天鹏ZHONGFangchuan钟方川
JIANG Hua-dong (姜华东),MA Tian-peng (马天鹏),ZHONG Fang-chuan (钟方川)*
1 College of Science,Donghua University,Shanghai 201620,China
2 College of Environmental Science and Engineering,Donghua University,Shanghai 201620,China
Introduction
Volatile organic compounds(VOCs)treatment is becoming a growing concern issue around the world for its harmful effect to the environment and human being such as ozone layer erosion,photochemical smog,carcinogenic effects[1-2].With the development of modern industry,more and more amounts of VOCs are generated during the industrial production.There are many conventional methods for VOCs reduction including adsorption,catalytic oxidation and thermal incineration.These techniques have drawbacks such as large energy cost,second pollution or low capacity for different initial concentrations.It needs to explore effective and clean solutions to decompose the VOCs.
Non-thermal plasma (NTP) methods present potential advantages in decomposition of dilute VOCs for its easy operation,moderate energy cost and non-selectivity for the abatement species.In NTP,there are high energy electrons,radicals,UV and other substance which have synergistic effect on VOCs.NTP decomposition of VOCs has been studied for two decades.Many types of plasma reactors have been investigated like corona discharge[3],dielectric barrier discharge(DBD)[4-5],and ferroelectric pellet packed-bed reactor[6-7].All the efforts have been done to make the NTP methods more energy-efficient and more environmental friendly.
DBD plasma is a common used NTP to treat VOCs and other pollutants.Usually the DBD plasma is generated by using continuous alternating current (AC)voltage from power-line frequency to several hundreds kHz[8].By using continuous sinusoidal voltage,a large amount of energy is wasted to heat the neutral gas molecules and the discharge chamber instead of accelerating electrons which is the key to decompose VOCs.Besides wasting the energy,raising temperature of the chamber is also harmful to reactor itself.Using nanosecond pulse power supply is one efficient way to improve the energy yield[9].The drawback of this method is the need of special nanosecond pulse power supply which is expensive and large in size.
In order to improve the energy efficiency of NTP decomposition of VOCs in easy way,pulse modulated sinusoidal voltage power was chosen to generate DBD plasma in this study.The behavior of benzene abatement was investigated.
1 Experimental Arrangement
1.1 Experimental setup
Figure 1 shows the schematic of the experimental setup.The DBD reactor is a coaxial cylinder tube.The inner electrode is a copper rod with 3 mm diameter which is enclosed by a quartz tube as dielectric barrier.The outer electrode is a copper mesh covering on a quartz tube with inner diameter of 19 mm.The thickness of both quartz tubes is 1 mm.The discharge gap and length are 7 mm and 15 cm,respectively.During experiments,the outer electrode is grounded while the inner electrode is connected to high voltage.
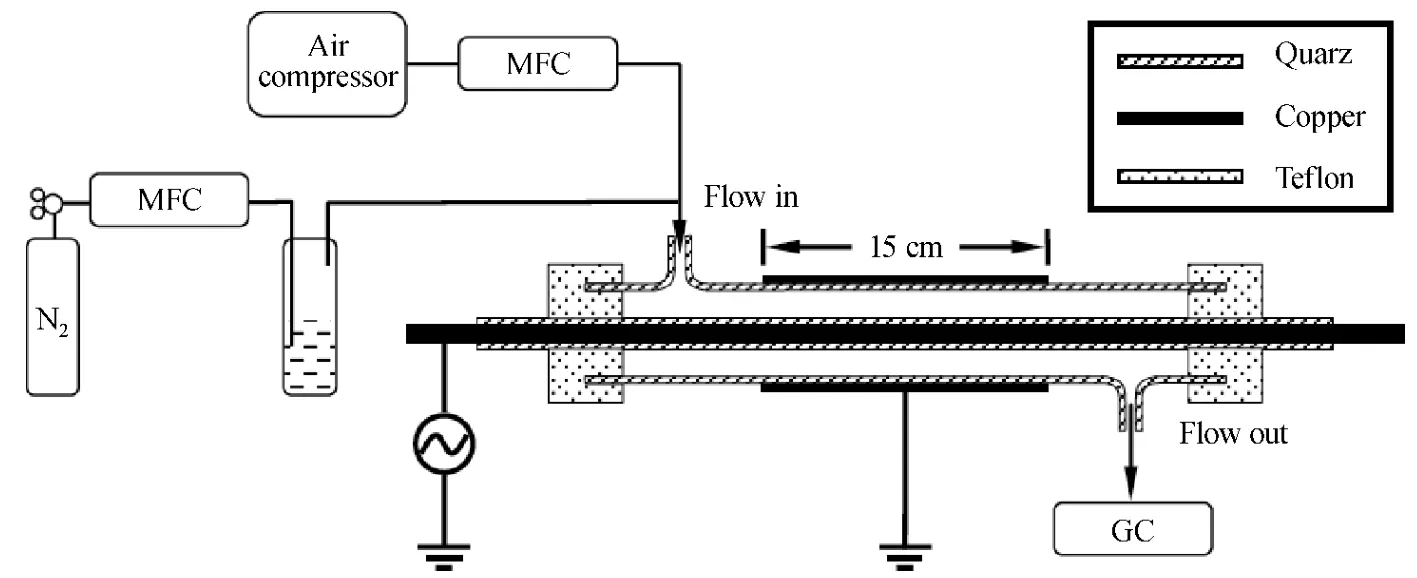
Fig.1 Experimental setup
Benzene was evaporated by bubbling with N2gas and diluted by a stream of compressed air.The reason to use air is to simulate practical VOCs waste streams in real industrial situation.In experiments,the flow rate of air was controlled at 0.9 L/min by the mass flow controller (MFC)after the air compressor,and the flow rate of nitrogen containing benzene was controlled within the range of 2-12 mL/min by the other MFC to obtain the desired initial concentration of benzene,leading to a total flow rate (Q)of around 0.9 L/min.The gas components were detected by gas-chromatography (GC)(Fuli 9790)at the outlet.
1.2 Power supply
A pulse modulated power supply was used to generate plasma in the experiment.As shown in Fig.2,the output of the voltage is a continuous sine wave voltage modulated by a rectangle pulse.The sine wave frequency is around 10 kHz,while the frequency and the duty cycle of the modulation pulse can be changed from 50-1 000 Hz and 10%-90%,respectively.

Fig.2 Signals of high voltage pulse modulated power supply (a)voltage signal with no modulation;(b)voltage signal with modulation;(c)current signal
1.3 Electrical analysis and gas analysis
Specific input energy (SIE),also called injected energy density (IED),is an important parameter commonly used to investigate the VOCs decomposition behavior.Generally,removal rate is directly related to SIE.SIE (ε,kJ/L)is defined by:

where Q is the gas flow rate and P is the input electrical power calculated from:
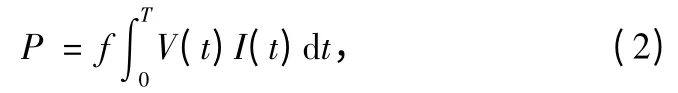
where V (t)and I (t)are pulsed voltage and current of discharge,T is the pulse duration,and f is the modulation pulse frequency.
Gas sample was detected by a GC.Benzene was detected by flame ionization detector (FID)(Column:SE-54,FID temperature 140℃,oven temperature 120℃), and the oxycarbide (CO and CO2) were measured by thermal conductivity detector (TCD) (Column: TDX-01, TCD temperature 120℃,oven temperature 120℃).The removal efficiency of benzene (η,%),carbon balance (δ,%)and CO2selectivity (SCO2,%)are defined as follows.
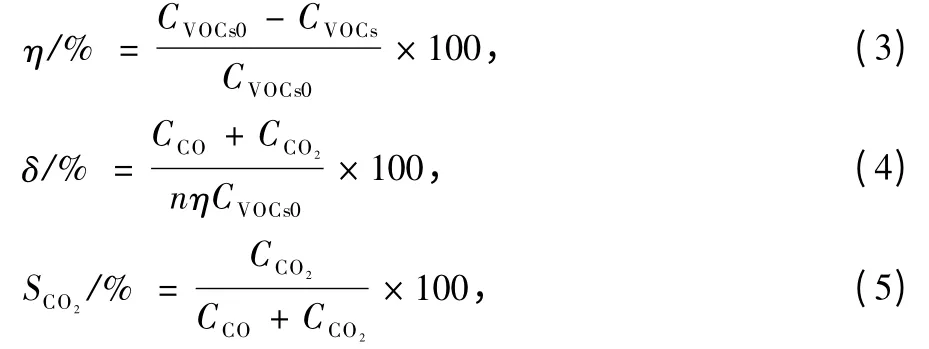
where n is the number of carbon atom in VOCs,CVOCs0and CVOCsindicate the inlet and outlet concentration of VOCs,respectively.
The energy yield (ξ,g/(kW·h))gives the insights of energy cost in VOCs decomposition.It can be calculated with the following equation:

where m is the relative molar mass of benzene.
2 Results and Discussion
2.1 Performance comparison of benzene decomposition with modulated and nonmodulated power supply
Figure 3 is the variation of removal efficiency with the change of the pulse duty cycle for four different discharging voltages when the benzene initial concentration is fixed at 750 ppm.As the duty cycle increases,the behavior of output voltage will be more similar to that of non-modulated continuous mode.The maximum duty cycle of the modulated power is 90%,the behavior of which can be regarded as that of the nonmodulated power output.From Fig.3,it is indicated that the benzene removal efficiency increases with increasing of the duty cycle and the discharge voltage.For example,for both 40%duty cycle,90% benzene was removed at 36 kV and 60%benzene was removed at 32 kV.
Figure 4 is the energy yield of benzene decomposition based on the removal efficiency in Fig.3.Unlike the monotonic increase of removal efficiency with duty cycle,the energy yield shows the parabolic-like curves,firstly increasing and then decreasing with the duty cycle.There is an optimal duty cycle to achieve the highest energy yield for a given discharge voltage.For peak-to-peak (p-p)voltages of 30,32,34 and 36 kV,the optimal duty cycle is 70%,40%,35% and 20%,respectively.The higher p-p voltage is applied,the higher maximum energy yield will be achieved.The maximum energy yield of 24 g/(kW·h)was achieved for p-p voltage of 36 kV at 20% duty cycle,while 20 g/(kW·h)was obtained for p-p voltage of 32 kV at its optimal duty cycle of 40%.These results indicate that the energy yield is improved by using pulse modulated AC power.
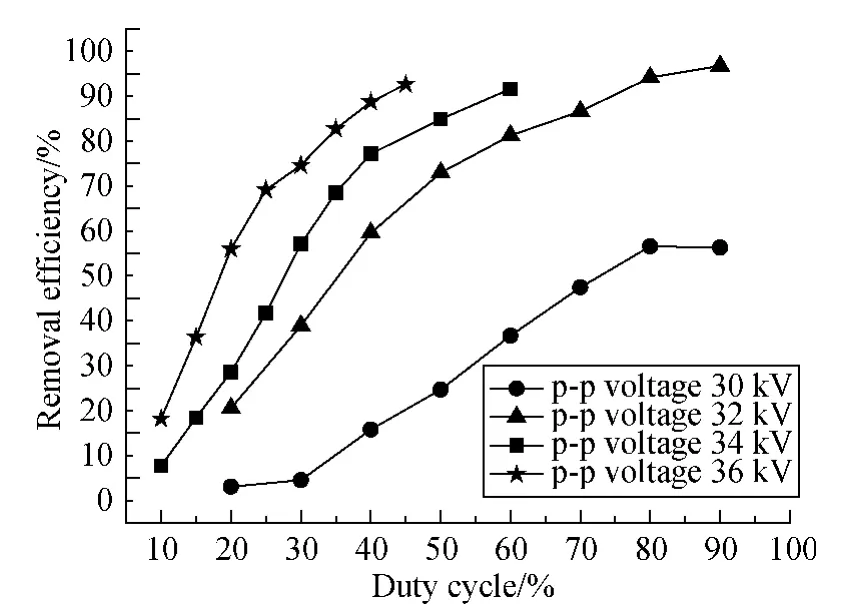
Fig.3 Removal efficiency of benzene versus duty cycle (750 ppm benzene;Q =0.9 L/min;f=150 Hz)
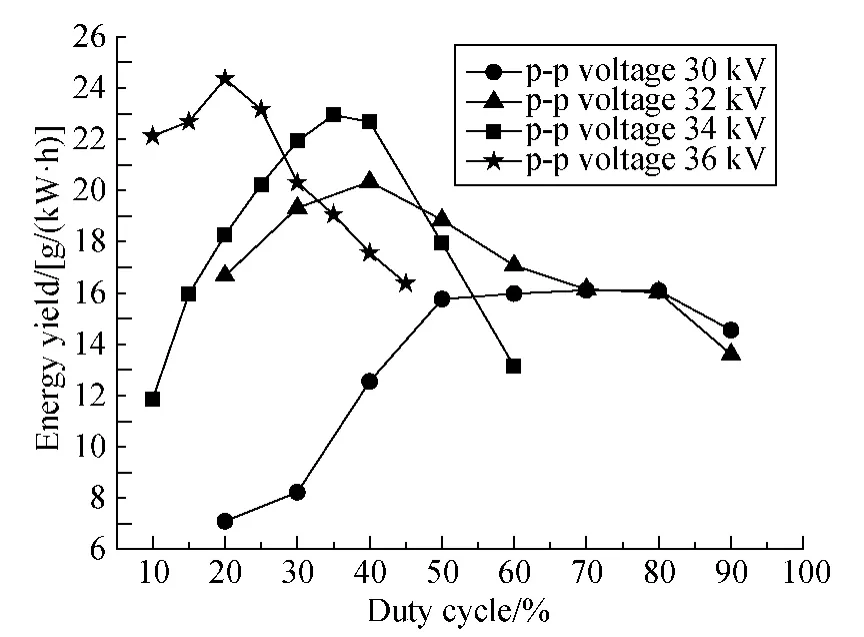
Fig.4 Energy yield of benzene decomposition versus duty cycle(750 ppm benzene;Q =0.9 L/min;f=150 Hz)
The reason for the increasing energy yield with the modulated power is that radicals and ions produced in the discharge period can still exist during the non-discharge period,which lasts for around 5 ms for 150 Hz modulation frequency and 20% duty cycle.For example the life time of O—H is around 100 μs[10],and theand ozone can last over seconds[11],as also the metastable.These residual radicals and ions lead to benzene decomposition without energy input into the reactor.The low energy yield at both lower and higher duty cycles are caused by the low removal efficiency and excessive power input,respectively.Moreover,more radicals will be produced at high voltage amplitude rather than at low voltage amplitude,which will allow longer proper non-discharging time,or could say lower proper duty cycle,to properly consume remained ions and radicals for maximum energy yield.That is the reason why modulated power shows more advantage at 36 kV.
Figure 5 is the temperature of the outer quartz tube wall which is measured by thermocouple for the duty cycle of 20%and 90%,respectively.Temperature of DBD reactor using modulated power is around 60℃,140℃lower than that of the non-modulated power.On the one hand,the use of modulated power decreases the power input and thus the ohm heating effect decreases.On the other hand,the reactor can be cooled during the non-discharging period.
In conclusion,pulse modulated power has potential in increasing the energy yield of VOCs decomposition by NTP.Besides,the low temperature also has benefits to the reactor itself.More experiments should be carried out to explore the characteristics of VOCs treatment by pulse modulated power.In the following sections,the influence of initial concentration on benzene decomposition and carbon balance and CO2selectivity are investigated in more detail.
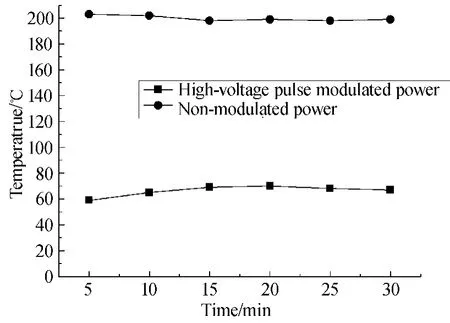
Fig.5 Temperature of reactor chamber in modulated and non-modulated power
2.2 Characteristics of benzene decomposition by pulse modulated power
2.2.1 Effect of initial concentration on benzene removal efficiency
Figure 6 is the relation of the benzene removal efficiency and the SIE under different initial concentrations.In experiments,the modulation pulse with 20% duty cycle and 150 Hz frequency were used.SIE is controlled by changing the p-p voltage.It can be seen that the benzene removal efficiency increases with increasing input energy density.It also clearly shows that with increasing of the initial concentration,the benzene removal efficiency will be decreased,as reported in most references[6,8].At 310 ppm concentration,benzene decomposition efficiency reached above 80% at energy density of 200 J/L and tended to 98% with increase of the SIE,much higher than that of 1 200-ppm benzene,which increased from 52% to 88% for the same SIE variation.
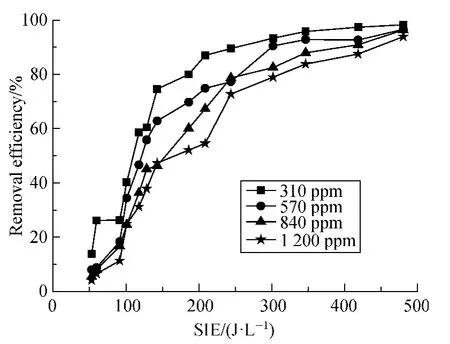
Fig.6 Removal efficiency of benzene (Q =0.9 L/min;f=150 Hz;20% duty cycle)
2.2.2 Effect of initial concentration on energy yield of benzene decomposition
Figure 7 is the energy yield according to the benzene removal efficiency in Fig.6.It can be seen that all the different initial concentrations of benzene reach the best energy yield when SIE is around 180 J/L.It is also indicated that the higher initial benzene concentration,the better the decomposition energy yield.The largest difference in energy yield was about 35 g/(kW·h)between 310 ppm and 1 200 ppm,which appeared from SIE 180 J/L.This difference was almost unchanged with the SIE increasing.
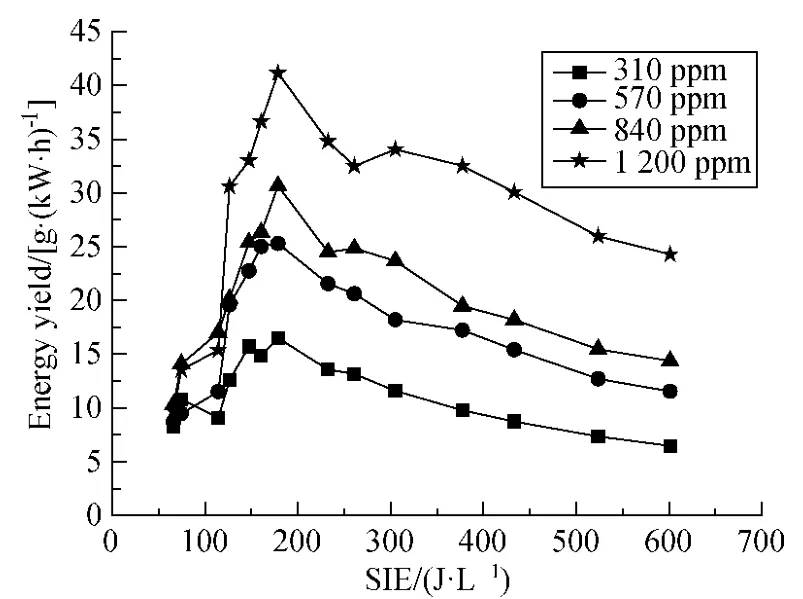
Fig.7 Energy yield of benzene decomposition (Q = 0.9 L/min;f=150 Hz;20% duty cycle)
It should be noted that the SIE region for maximum energy yield does not depend on the benzene concentration.All the maximum energy yields occurred at SIE range from 100 to 200 J/L.This is an interesting point because the concentration of waste gas emitted from factory can hardly be controlled.It would be convenient for fixing power input into the equipment to get the maximum energy yield without concerning the concentration of waste gases.
2.3 Carbon balance and CO2 selectivity of benzene decomposition
2.3.1 Carbon balance
Carbon balance is an important parameter of VOCs decomposition which indicates the distribution of oxidation products of VOCs.It is ideal case that all the byproducts can be oxidized into CO2.Carbon concentration plot,as shown in Fig.8,was used to investigate the carbon balance of benzene decomposition at different concentrations as many research groups did[13-14].In Fig.8,X and Y axes represent the concentration of carbon atoms from removed benzene and the concentration of produced COx,respectively.The diagonal in the figure indicates the carbon balance of 100%,which means that all the removed benzene has been converted into oxycarbide with no organic compounds formed.Dot lines in the figure indicate the carbon balance value of 50% and the dash lines drawn along the experimental points show the change of carbon balance along with SIE or reacted carbon concentration clearly.
It is shown from Fig.8 that higher carbon balance can be achieved for lower concentration of benzene.Both dash lines were close to the straight line at 310 ppm,and the carbon balance reached about 80%.For 570 ppm benzene,the concentration of oxycarbide dropped nearly close to the dot line,and 55% carbon balance was obtained.When the initial benzene concentration increased to 840 and 1 200 ppm,the carbon balance decreased to 50%.
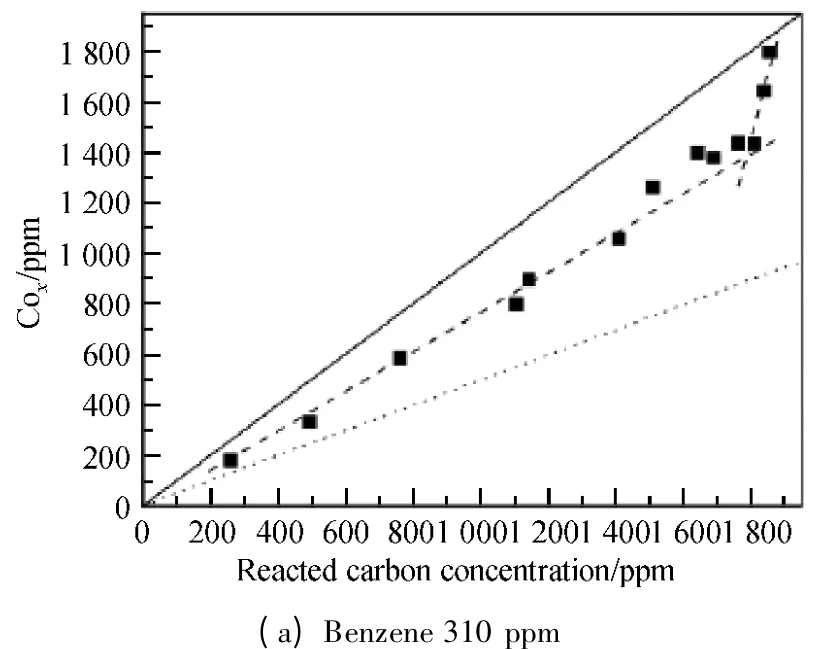
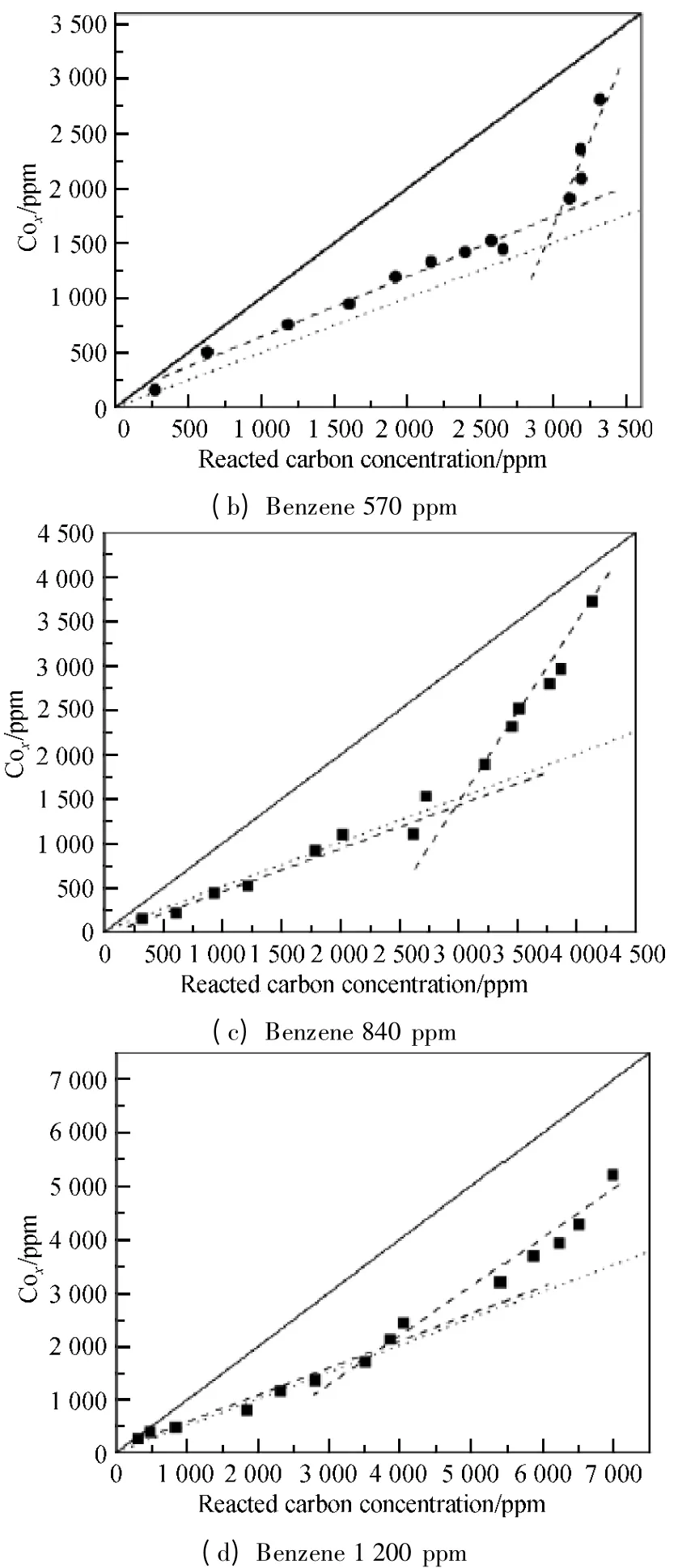
Fig.8 Carbon balance of benzene decomposition (Q =0.9 L/min;f=150 Hz;20% duty cycle)
It is worth emphasizing that there are two slopes for the dash lines for each investigated initial benzene concentration.The slope of the dash line for the larger reacted carbon concentration (higher SIE)is steeper than that for the lower reacted carbon concentration.This means better carbon balance under higher SIE.The distinct change of carbon balance at low and high SIE is due to the concentration limits of high energy electrons produced by plasma.When the SIE is high enough,VOCs' degradability will be saturated.Excessive high energy electrons react with other substances like secondary products,benzene fragments,etc.,which can produce more reactive species and molecular groups to increase the oxycarbide concentration.More importantly,the polymer which sticks on the DBD reactor wall during benzene decomposition would be bombed off by high energy electrons and the ions smashing on the walls and turned into CO,CO2,H2O,etc.So the amount of oxycarbide would increase dramatically and cause the steep increasing of carbon balance at high SIE.In addition,for higher concentration of benzene,a large part of energy would be consumed for benzene decomposition instead of for oxycarbide production.This is the reason why the changing of carbon balance at low and high SIE becomes smaller when the initial concentration is increased.It can be found that the angle of two dash lines in Fig.8 (d)is much smaller than that in Fig.8 (a).
2.3.2 CO2selectivity
CO2selectivity is another criterion for evaluating benzene decomposition performance,and 100% is the ideal value.Figure 9 is the relationship of CO2selectivity and SIE for different initial benzene concentrations.From Fig.9,it is found that better CO2selectivity can be obtained for lower initial concentration of benzene.At low SIE region,it is found that CO2selectivity dramatically decreased from 95% to 76% for 310-ppm benzene and from 86% to 56% for 1 200-ppm benzene with the increase of SIE.By further increasing the SIE,SCO2becomes stable at 76%,70%,65% and 56% for initial benzene concentrations of 310 ppm,570 ppm,840 ppm and 1 200 ppm,respectively.For each initial concentration,the CO2selectivity has only about 8% improvement with increasing SIE from 125 J/L to 500 J/L.
The ionization rate is low and the energy of electrons and ions is small at low SIE,so the benzene decomposition rate is low.Unlike the decomposition rate,the CO2selectivity is high in Fig.9.Similar phenomenon was reported in Refs[12,15].This implies that the benzene fragments produced by plasma in low SIE are more likely to be converted into CO2.One possible reason maybe owing to the formation of formic acid (HCOOH)which was reported can be largest in low SIE[16].Decompositon of HCOOH preferably produces CO2and induces the high CO2selectivity in low SIE.With increasing SIE,more and more reactions which could produce CO due to the diversity of intermediate products during benzene decomposition.The proportion of CO and CO2increased and CO2selectivity decreased until the rate of CO2and CO reactions was stable.Then CO2selectivity slowly increases for higher oxidizability at higher SIE.After 125 J/L,the proportion of CO and CO2becomes stable with a little increasing trend in CO2selectivity.Considering the high carbon balance values in last section,it is surprisingly found that although high SIE is inclined to affect the amount of oxycarbide effectively,the selectivity of CO2is improved by only 8% when SIE increased from 125 J/L to 500 J/L.
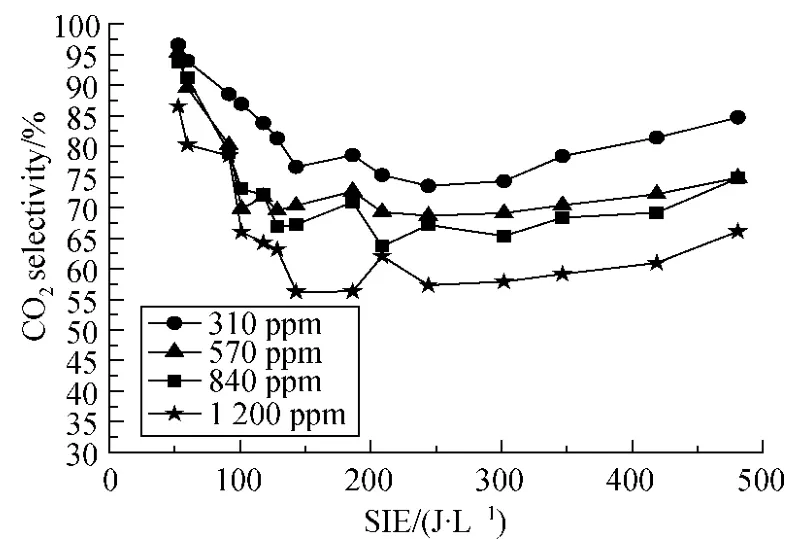
Fig.9 CO2 selectivity of benzene (Q =0.9 L/min;f=150 Hz;20% duty cycle)
Combining the catalyst could be the promising way to improve the carbon balance and CO2selectivity.More experiments should be exerted to further understand and optimize the performance of NTP method by pulse modulated power.
3 Conclusions
A normal sinusoidal AC power with pulse modulation is used to generate DBD plasma for benzene destruction.The characteristic of benzene decomposition in the pulse modulation plasma are studied.The main conclusions are as follows.
(1)The pulse modulation can improve the energy yield and reduce the heating of the chamber wall.For instance,for the 310 ppm benzene at 36 kV p-p voltage,the energy yield reached up to 24 g/(kW·h),two times of the energy yield in continue mode.
(2)There is an optimal duty cycle for achieving the maximum energy yield for a certain discharge voltage.For the 30,32,34,and 36 kV p-p discharging voltages,the optimal duty cycle is 70%,40%,35% and 20%,respectively.
(3)Low initial VOCs concentration is more suitable for decomposition by plasma.Higher removal efficiency,carbon balance and CO2selectivity will be obtained at low initial concentration.
(4)Energy yield is largely improved by using pulse modulated DBD plasmas and increase with increasing initial concentration.Energy yield achieved maximum around 180 J/L SIE for all initial concentrations.
(5)The two slopes in carbon concentration plot imply that the carbon balance becomes much better with the increasing of the SIE after benzene degradability is saturated.
[1]Vandenbroucke A M,Morent R,Geyter N D,et al.Nonthermal Plasmas for Non-catalytic and Catalytic VOC Abatement[J].Journal of Hazardous Materials,2011,195(15):30-54.
[2]Than Quoc A H,Pham Huu T,Le Van T,et al.Application of Atmospheric Non Thermal Plasma-Catalysis Hybrid System for Airpollution Control:Toluene Removal[J].Catalysis Today,2011,176(1):474-477.
[3]Marotta E,Schiorlin M,Rea M,et al.Products and Mechanisms of the Oxidation of Organic Compounds in Atmospheric Air Plasmas[J].Journal of Physics D—Applied Physics,2010,43(12):124011.
[4]Ye Z L,Zhang Y L,Li P,et al.Feasibility of Destruction of Gaseous Benzene with Dielectric Barrier Discharge[J].Journal of Hazardous Materials,2008,156(1/2/3):356-364.
[5]Li J,Han S T,Bai S P,et al.Effect of Pt/g-Al2O3Catalyst on Nonthermal Plasma Decomposition of Benzene and Byproducts[J].Environmental Engineering Science,2011,28(6):395-403.
[6]Sugasawa M,Terasawa T,Futamura S.Additive Effect of Water on the Decomposition of VOCs in Nonthermal Plasma[J].IEEE Transactions on Industry Applications,2010,46(5):1692-1698.
[7]Zhu T,Wan Y D,Li H R,et al.VOCs Decomposition via Modified Ferroelectric Packed Bed Dielectric Barrier Discharge Plasma[J].IEEE Transactions on Plasma Science,2011,39(8):1695-1700.
[8]Zhu S L,Hu T,Zhong F C.Degradation of Toluene by Using Pulse Modulated Dielectric Barrier Discharge and Catalytic Techniques[J].Environment Engineering,2013,31(3):66-70.(in Chinese)
[9]Wang H C,Li D,Wu Y,et al.Removal of Four Kinds of Volatile Organic Compounds Mixture in Air Using Silent Discharge Reactor Driven by Bipolar Pulsed Power[J].Journal of Electrostatics,2009,67(4):547-553.
[10]Holzer F,Roland U,Kopinke F D.Combination of Non-thermal Plasma and Heterogeneous Catalysis for Oxidation of Volatile Organic Compounds[J].Applied Catalysis B:Environmental,2002,38(3):163-181.
[11]Atsushi O J,Shao F Q,Zou D B.Numerical Simulation of Negative Oxygen Ion Generation and Temporal Evolution in Atmospheric Plasma[J].Acta Physics Sinica,2011,60(11):110209.
[12]Hyun H K,Seung M O,Atsushi O,et al.Decomposition of Gas-Phase Benzene Using Plasma-Driven Catalyst (PDC)Reactor Packed with Ag/TiO2Catalyst[J].Applied Catalysis B:Environmental,2005,56(3):213-220.
[13]Zhang Y,Li D,Wang H C.Removal of Volatile Organic Compounds (VOCs) Mixture by Multi-Pin-Mesh Corona Discharge Combined with Pulsed High-Voltage[J].Plasma Science and Technology,2010,12(6):702-707.
[14]Futamura S,Sugasawa M.Additive Effect on Energy Efficiency and Byproduct Distribution in VOC Decomposition with Nonthermal Plasma [J].IEEE Transactions on Industry Applications,2008,44(1):40-45.
[15]Ogata A,Yamanouchi K,Mizuno K,et al.Oxidation of Dilute Benzene in an Alumina Hybrid Plasma Reactor at Atmospheric Pressure[J].Plasma Chemistry and Plasma Processing,1999,19(3):383-394.
[16]Hyun H K,Atsushi O,Shigeru F.Atmospheric Plasma-Driven Catalysis for the Low Temperature Decomposition of Dilute Aromatic Compounds[J].Physics D—Applied Physics,2005,38(8):1292-1300.
猜你喜欢
杂志排行
Journal of Donghua University(English Edition)的其它文章
- Group Performance Evaluation in Universities with Entropy Method
- Synthesis and Application of Polyurethane Modified Organic Silicone Wet Rubbing Fastness Improver
- Inactivation of Giardia Intestinalis by Peroxone Process (H2 O2 /O3)and Its Disinfection Mechanisms
- Optical Measurements to Reveal Roles of Slightly Crosslinked Poly(dimethyldiallylammonium chloride)s in Fixing Anionic Dyes on Cotton Fabric
- Portfolio Choice under the Mean-Variance Model with Parameter Uncertainty
- Experimental Investigation on Constrained Abrasive Fluid Polishing for Optical Glass
