Prehibernation Energy Storage in Heilongjiang Brown Frogs (Rana amurensis) from Five Populations in North China
2015-12-13WeiCHENTianpeiGUANLinaRENDujuanHEYingWANGandXinLU
Wei CHEN, Tianpei GUAN, Lina REN, Dujuan HE, Ying WANGand Xin LU
1Ecological Security and Protection Key Laboratory of Sichuan Province, Mianyang Normal University, Mianyang, 621000, China
2College of Life Science and Biotechnology, Mianyang Normal University, Mianyang, 621000, China
3Department of Zoology, College of Life Sciences, Wuhan University, Wuhan, 430072, China
Prehibernation Energy Storage in Heilongjiang Brown Frogs (Rana amurensis) from Five Populations in North China
Wei CHEN1*, Tianpei GUAN1, Lina REN2, Dujuan HE2, Ying WANG2and Xin LU3
1Ecological Security and Protection Key Laboratory of Sichuan Province, Mianyang Normal University, Mianyang, 621000, China
2College of Life Science and Biotechnology, Mianyang Normal University, Mianyang, 621000, China
3Department of Zoology, College of Life Sciences, Wuhan University, Wuhan, 430072, China
Energy storage is an important component in the life history of species that directly infl uences survival and reproduction. The energetic demands of amphibian reproduction can differ between the sexes, with environmental conditions, reproductive pattern or process of the species, and depending upon the timing of breeding, and the reproductive season for a species. Surprisingly, comparative studies of pre-hibernation energy storage for anuran populations from different latitudes are relatively few in Asia, especially in China. Here we investigated the patterns of pre-hibernation energy storage of Heilongjiang brown frogs Rana amurensis, based on fi ve populations along a fi nely latitudinal gradient in north China (40.7-43.7°N). We found that pre-hibernation energy storage of the frogs did not show a clear latitudinal cline, but differed strongly between the sexes, with males depositing more energy reserves into the muscle and liver, whereas females accumulate more energy in the gonads. The sexual differences in energy storage may result from differential timing of energy allocation for reproduction.
energy storage, pre-hibernation, Rana amurensis, sexual differences
1. Introduction
Energy storage is crucial for species survival during winter that they live in temperate and cold zone and plays an important role in shaping the life-history strategy for a species living in temperate and cold zone (Roff, 2002; Wells, 2007; Jönsson et al., 2009). Animals must trade off their energy allocation for tissue maintenance, growth and reproduction as energy resources available may be limited in nature. In general, however, the amount of body energy storage can influence the survival time and the strategy of energy investment into fi tness traits (e.g. egg number or the ability to secure more mates), so energy storage is viewed to strongly influence individual fitness in amphibians (Komoroski et al., 1998; Lu et al., 2008).
Anurans deposit energy in the form of triglycerides and glycogen and in different organs [e.g., liver, abdominal fat bodies (Fitzpatrick, 1976), gonadal tissues (Villecco et al., 1999), and muscle tissue (Donohoe et al., 1998)], and these stores are used for overwintering (Boutilier, 2001; Pope and Matthews, 2002), gamete production (Girish and Saidapur, 2000), and breeding activities (Pope and Matthews, 2002; Jackson and Ultsch, 2010). Because these activities are tightly linked to environmental conditions and resource availability, environmental factors can influence energy storage of species and the relationship between environments and energy storage has been a pivotal point in our understanding of life-history strategy (Elmberg, 1991; Wells, 2007; Jönsson et al., 2009; Chen et al., 2011, 2013).
The reproductive behavior of anurans can be divided into two basic patterns which apply to temperate species of anurans: income breeding or capital breeding (Wells, 2007). Income breeders feed after emergence from winter dormancy and before breeding, whereas capital breeders begin feeding only after breeding (Drent and Daan, 1980;Jönsson, 1997; Jönsson et al., 2009). In anuran income breeders, males and females invest main energy into breeding after overwintering, however, in the anurans with capital breeding strategies, males invest energy into breeding after overwintering, whereas females finish investing the main energy component into egg growth prior to overwintering (Lu et al., 2008; Jönsson et al., 2009; Chen et al., 2011). Thus, sex differences in the timing of energy allocation to breeding are common in the anurans species with capital breeding strategies, and the differences results in life history variation across populations and species (Jönsson et al., 2009; Chen et al., 2011, 2013).
Heilongjiang brown frogs Rana amurensis are distributed in north China, Mongolia and Russia (Kuzmin, 1999; Fei et al., 2010). Individuals of this species overwinter under the water in shallow ponds, and after emerging the following spring breed prior to feeding (Solomonova et al., 2011). Little is known about prehibernation energy storage of this capital breeder (Solomonova et al., 2011), with females larger in body size than males (Chen and Lu, 2011) and larger females producing more eggs (Solomonova et al., 2011). We investigated the prehibernation energy storage of five populations of this species in north China distributed along a finely latitudinal gradient. Our aims were to explore whether there were sex differences in the patterns of body energy storage and investigate whether there was a latitudinal cline of energy storage along a finely latitudinal gradient.
2. Materials and Methods
In total, 89 Rana amurensis (43 males and 46 females) were opportunistically captured by hand in the water from fi ve overwintering sites (Lindong, Gongzhuling, Yitong, Siping , Meihekou and Tonghua) along a latitudinal gradient in north China (40.7-43.7°N, Table 1), when the mean diurnal temperature was around 10°C at each site. Captured frogs were maintained in pond water for 24 hours at 15°C in lab to allow full hydration before being euthanized with an overdose of MS-222 (hydration status can infl uence animal body mass; Ladyman and Bradshaw, 2003). We measured snout-vent length of the frogs with fl attened bodies (SVL; to 0.1 mm), and then dissected out and weighed the liver, fat bodies, gonads and remaining carcass (0.001 g with an electronic balance) after placing the organs on water-absorbing paper for fi ve minutes (Lu, 2004).
Although we were interested in the sexual effects on energy storage, we incorporated altitude, latitude and their interaction into our statistical models to help explain more variance in energy storage among populations. Generalized linear mixed models (GLMMs) were performed to investigate the relationship sex and organ weight as well as between latitude and organ weight, using population as a random variable, sex, SVL, latitude and altitude as covariates, and organ weight as the dependent variable. One-way ANOVAs then were used to investigate whether males and females differ in their pattern of energy storage, using relative organ weight (predicted from GLMMs) as the dependent variable. We log-transformed all variables prior to analysis to match the requirements of GLMMs and ANOVAs. All statistical tests were performed with SPSS software (Version 20.0). All probabilities were two tailed and summary statistics are presented as mean ± standard deviation (SD). All fi eld and laboratory work was performed under permissionfrom the Wildlife Protection Law of China.
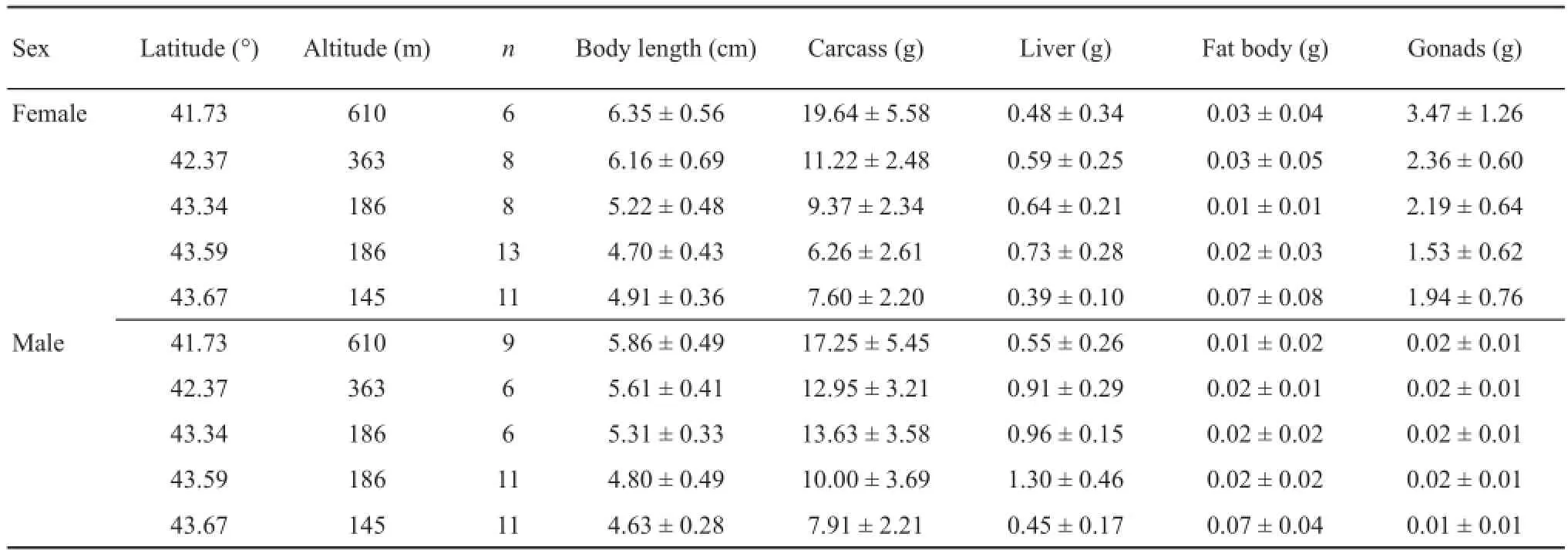
Table 1 Organ mass for female and male Rana amurensis from fi ve populations in north China, showing the latitude, altitude, sample size (n) and organ mass (mean ± standard deviation).
3. Results
We observed significant differences between the sexes with respect to relative weight of the carcass (GLMM: F1,82.001= 25.444, P < 0.001), liver (F1,82.001= 47.077, P <0.001) and gonads (F1,83= 314.608, P < 0.001), but not for the fat bodies (F1,82= 0.147, P = 0.703; Figure 1). Generally, males had signifi cantly heavier carcasses (Oneway ANOVA: F1,87= 5.723, P = 0.019) and livers (F1,87= 18.912, P < 0.001) than females, whereas females had signifi cantly heavier gonads than males (F1,87= 497.673, P < 0.001; Figure 1; Table 1).
We found that energy storage was associated with body size, with larger individuals of both sexes having significantly higher energy storage than smaller individuals for the carcass (GLMM: F1,82.276= 90.057, P< 0.001), liver (F1,40.952= 40.952, P < 0.001), and gonads (F1,83= 37.6, P < 0.001), but not for the fat bodies (F1,82.191= 1.72, P = 0.193; Table 2).
Latitude and altitude were not signifi cantly associated with the energy storage in any of the organs that we measured due to the narrow geographic bounds (latitude: carcass: F1,1.024= 1.462, P = 0.436; liver: F1,1.054= 7.990, P = 0.206; fat bodies: F1,1.016= 0.011, P = 0.934; gonads: F1,83= 2.451, P = 0.121; altitude: carcass: F1,0.918= 0.258, P = 0.707; liver: F1,0.822= 17.498, P = 0.19; fat bodies: F1,0.944= 0.972, P = 0.512; gonads: F1,83= 3.476, P = 0.066; for all interactions between latitude and altitude, P> 0.07, Table 2).
4. Discussion
Patterns of energy storage in anurans can be constrained by environmental conditions and differ between the sexes, which often differ in the timing of energy allocation towards reproduction (Jönsson et al., 2009; Chen et al., 2011; Chen et al., 2013). In colder environments, anurans are expected to accumulate more energy prior to the onset of winter (Irwin and Lee, 2003; Lu et al., 2008; Chen et al., 2011, 2013). In our study, however, we did not detect a clear latitudinal pattern of energy storage in either male or female R. amurensis due to limited geographic bounds. Instead, we found clear sex differences in pre-hibernation energy storage patterns, where males allocate moreenergy to the carcass and females allocate more energy into the gonads.
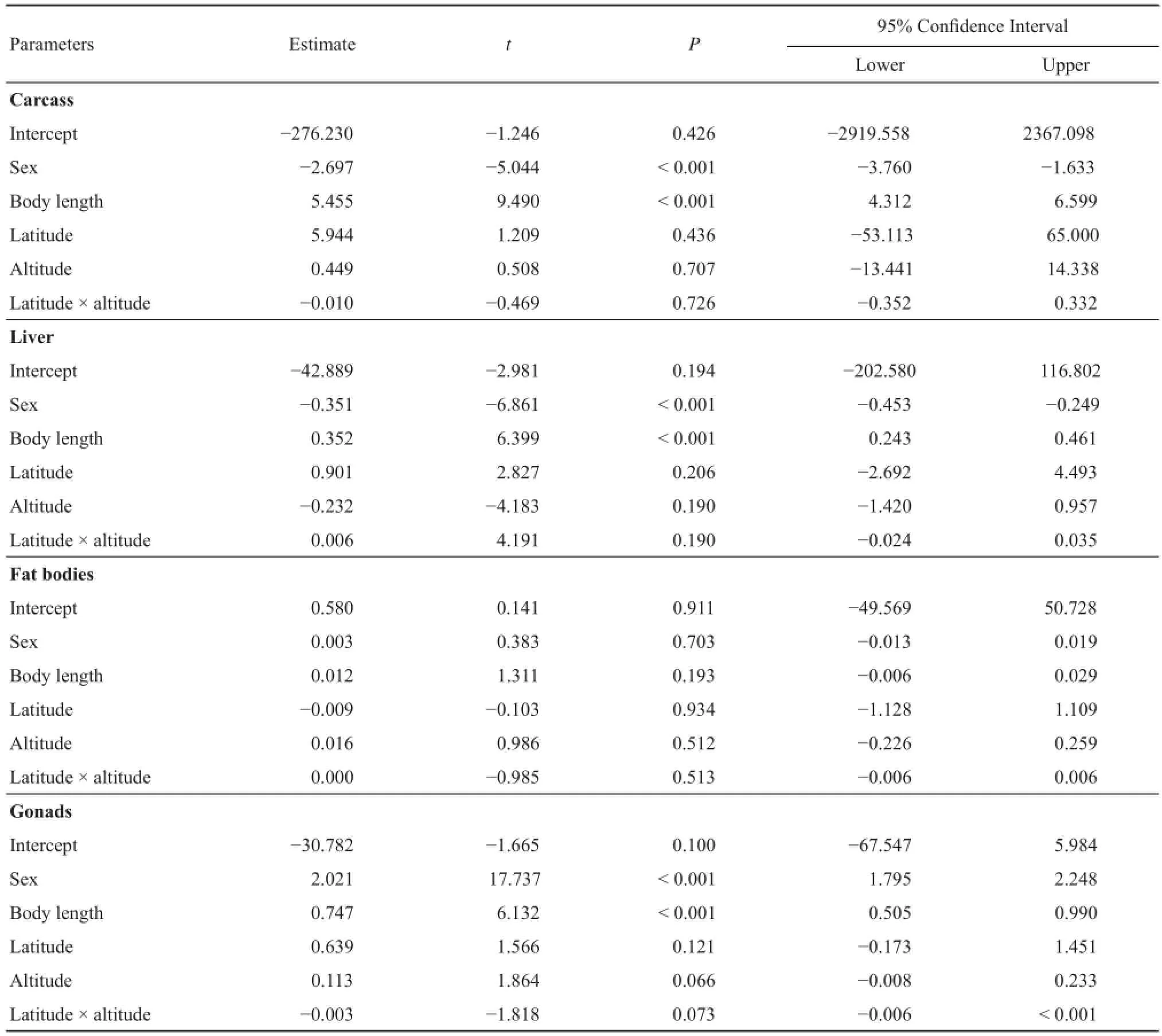
Table 2 Parameters estimated from a generalized linear mixed model analyses comparing organ mass among five Rana amurensis populations in north China.
Reproductive success of female anurans is determined by their ability to produce eggs, and thus females often deposit a high proportion of energy into the gonads to produce more high-energy eggs (Wells, 2007). By contrast, the reproductive success of male frogs is infl uenced by their ability to locate and secure potential mates, and thus males use their energy reserves for breeding activities (such as mate attraction through calling) rather than investing into cheaper spermatogenesis (Halliday and Verrell, 1988; Jørgensen, 1992). This sex difference in reproductive investment leads to differences in the timing of energy allocation, as has been shown for other frogs (R. temporaria: Jönsson et al., 2009; R. chensinensis: Chen et al., 2011; R. kukunoris: Chen et al., 2013). For anurans with capital breeding strategies, females fi nish investing energy in autumn before winter dormancy, whereas males do so during the breeding period of the following spring (Jørgensen, 1981).
In R. amurensis, females significantly increase their gonad weight before winter, finishing most follicular growth before hibernation (Chen, pers. observ.). Typically, somatic tissues have a direct relationship to gonadal growth (Delgado et al., 1990; Girish and Saidapur, 2000; Chen et al., 2011), so the energy in somatic tissues has been invested into gonadal growth and converted into gonads. However, in males the energy in somatic tissues remains until it is invested into breeding activities the next spring, e.g. mate attraction or competition (Pope andMatthews, 2002; Jackson and Ultsch, 2010). Accordingly, female R. amurensis had lighter carcasses but heavier gonads when compared to males. This sexual pattern of pre-hibernation energy storage is in accordance with the study results from R. temporaria (Jönsson et al., 2009) and R. chensinensis (Chen et al., 2011).
The differences in most physiological data results partly from variation in body size of the animals being studied, because the intensity of a physiological process is usually higher in large individuals than in small ones (Packard and Boardman, 1999). In anurans, a common pattern of energy storage is that larger males and females have higher energy storage (Wells, 2007). In R. amurensis, larger males and females also accumulate more energy. This body-size-dependent pattern of pre-hibernation energy storage is in accordance with results from other anurans (e.g. R. temporaria: Jönsson et al., 2009; R. chensinensis: Chen et al., 2011; R. kukunoris: Chen et al., 2013). A possible reason is that larger individuals have enhanced feeding abilities and/or have optimized foraging strategies (Wells, 2007; Chen et al., 2011).
Frogs living in colder environments are expected to store more energy in order to survive prolonged and colder winters (Lu, 2004; Jönsson et al., 2009; Chen et al., 2011; Chen et al., 2013). For example, the liver plays an important role in energy maintenance requirements during winter dormancy (Pasanen and Koskela, 1974; Díaz-Páez and Ortiz, 2001; Irwin and Lee, 2003; Lu et al., 2008), especially in the aquatic environment which has low oxygen or can be hypoxic (Jackson and Ultsch, 2010). Evidence shows that during overwintering the liver glycogen content was reduced by 51% in males and 56% in females of ranid frogs (Tattersall and Ultsch, 2008). The weight of the liver thus will increase with increasing latitude and altitude before winter to satisfy metabolic requirements during longer and colder winter hibernation (Pasanen and Koskela, 1974; Irwin and Lee, 2003; Lu et al., 2008; Chen et al., 2011). In contrast to previous studies (R. temporaria: Jönsson et al., 2009; R. chensinensis: Chen et al., 2011; R. sylvatica: Costanzo et al., 2013), the energy storage of R. amurensis did not increase with increasing latitudes. This could be explained by the fact that the latitudinal range was too limited to detect a clear latitudinal trend, and the environmental differences along the limited latitudinal range that we investigated did not impose strong enough selection to alter energy storage. Further studies should be performed to investigate whether there is a clear latitudinal pattern of energy storage in R. amurensis.
Generally, the males of R. amurensis deposit more energy into the carcass and liver, but the females accumulated more energy into the gonads. This sexual difference in energy storage may result from differential timing of energy allocation for reproduction, which is keeping with capital breeding pattern of frogs. However, we did not detect a clear latitudinal cline of energy storage, which is mainly because of limited latitudinal range. If a comparison of the species from a wider latitudinal gradient is conducted and/or of lager sample size, we might gain more clear results.
Acknowledgements We thank Dr. David PIKE for his comments on the early draft of the manuscript. Financial support was provided by the Scientific Research Foundation of Mianyang Normal University (No. QD2012A13), the Key Foundation of the Sichuan Provincial Department of Education (No.15ZA0298) and Innovation Team Project of Education Department of Sichuan Province (No. 13TD0015).
References
Boutilier R. G. 2001. Mechanisms of metabolic defense against hypoxia in hibernating frogs. Resp Physiol, 128: 365-377
Chen W., Lu X. 2011. Age and body size of Rana amurensis from northeastern China. Curr Zool, 57: 781-784
Chen W., Wang X. Y., Fan X. G. 2013. Do anurans living in higher altitudes have higher prehibernation energy storage? Investigations from a high-altitude frog. Herpetol J, 23: 45-49
Chen W., Zhang L. X., Lu X. 2011. Higher pre-hibernation energy storage in anurans from cold environments: A case study on temperate frog Rana chensinensis along broad latitudinal and altitudinal gradients. Ann Zool Fenn, 48: 214-220
Costanzo J. P., Amaral M. C. F., Rosendale A. J., Lee R. E. J. 2013. Hibernation physiology, freezing adaptation and extreme freeze tolerance in a northern population of the wood frog. J Exp Biol, 216: 3461-3473
Delgado M. J., Gutierrez P., Alonso-Bedate M. 1990. Annual ovarian cycle and plasma levels of 17 betaestradiol in the frog Rana perezi. Physiol Zool, 63: 373-387
Díaz-Paéz H., Ortiz J. C. 2001. Description of the reproductive cycle of Pleurodema thaul Anura, Leptodactylidae. Amphibia-Reptilia, 22: 431-445
Donohoe P. H., West T. G., Boutilier R. G. 1998. Respiratory, metabolic, and acid-base correlates of aerobic metabolic rate reduction in overwintering frogs. Am J Physiol, 274: 704-710
Drent R. H., Daan S. 1980. The prudent parent: energetic adjustments in avian breeding. Ardea, 68: 225-252
Elmberg J. 1991. Ovarian cyclicity and fecundity in boreal common frogs Rana temporaria L. along a climatic gradient. Funct Ecol, 5: 340-350
Fei L., Ye C. Y., Jiang J. P. 2010. Colored Atlas of Chinese Amphibians. Chengdu, China: Sichuan Publishing House of Science and Technology
Fitzpatrick L. C. 1976. Life history patterns and utilization oflipids for energy in amphibians. Am Zool, 16: 725-732
Girish S., Saidapur S. K. 2000. Interrelationship between food availability, fat body, and ovarian cycles in the frog, Rana tigrina, with a discussion of the role of fat body in anuran reproduction. J Exp Zool, 286: 487-493
Hemelaar A. S. M. 1988. Age, growth and other population characteristics of Bufo bufo from different latitudes and altitudes. J Herpetol, 22: 369-388
Irwin J. T., Lee J. R. E. 2003. Geographic variation in energy storage and physiological responses to freezing in the gray tree frogs Hyla versicolor and H. chrysoscelis. J Exp Biol, 206: 2859-2867
Jackson D. C., Ultsch G. R. 2010. Physiology of hibernation under the ice by turtles and frogs. J Exp Zool Part A, 313: 311-327
Jönsson I., Herczeg G., O’Hara R., Söderman F., ter Schure A., Larsson P., Merilä J. 2009. Sexual patterns of prebreeding energy reserves in the common frog Rana temporaria along a latitudinal gradient. Ecography, 32: 831-839
Jönsson K. I. 1997. Capital and income breeding as alternative tactics of resource use in reproduction. Oikos, 78: 57-66
Jørgensen C. B. 1981. Ovarian cycle in a temperate frog, Rana temporaria, with special reference to factors determining number and size of egg. J Zool, 195: 449-458
Jørgensen C. B. 1992. Growth and reproduction. In: Feder M. E. and Burggren W. W. (Eds.), Environmental physiology of the amphibians. University of Chicago Press, 439-466
Komoroski M. J., Nagle R. D., Congdon J. D. 1998. Relationships of lipids to ovum size in amphibians. Physiol Zool, 71: 633-641
Kuzmin S. L. 1999. The Amphibians of the Former Soviet Union. Moscow: Pensoft, Sofi a.
Lu X. 2004. Annual cycle of nutritional organ mass in a temperatezone anuran, Rana chensinensis, from northern China. Herpetol J, 14: 9-12
Lu X., Li B., Li Y., Ma X. Y., Fellers G. M. 2008. Pre-hibernation energy reserves in a temperate anuran, Rana chensinensis, along a relatively fi ne elevational gradient. Herpetol J, 18: 97-102
Packard G. C., Boardman T. J. 1999. The use of percentages and size-specifi c indices to normalize physiological data for variation in body size: wasted time, wasted effort? Comp Biochem Phy A, 122: 37-44
Pasanen S., Koskela P. 1974. Seasonal and age variation in the metabolism of the common frog, Rana temporaria L. in northern Finland. Comp Biochem Phy A, 47: 635-654
Pope K. L., Matthews K. R. 2002. Influence of anuran prey on the condition and distribution of Rana muscosa in the Sierra Nevada. Herpetologica, 58: 354-363
Roff D. A. 2002. The evolution of life histories. Sunderland, MA: Sinauer Associates.
Solomonova T. N., Sedalishchev V. T., Odnokurtsev V. A. 2011. The Siberian tree frog (Rana amurensis Bulenger, 1886) in Yakutia. Contemp Probl Ecol, 4: 69-73
Tattersall G. J., Ultsch G. R. 2008. Physiological ecology of aquatic overwintering in ranid frogs. Biol Rev, 83: 119-140
Villecco E. I., Aybar M. J., Sánchez R. A. N., Sánchez S. S. 1999. Comparative study of vitellogenesis in the anuran amphibians Ceratophrys cranwelli (Leptodactilidae) and Bufo arenarum (Bufonidae). Zygote, 7: 11-19
Wells K. D. 2007. The ecology and behavior of amphibians. Chicago: University of Chicago Press
Dr. Wei CHEN, from Ecological Security and Protection Key Laboratory of Sichuan Province, Mianyang Normal University, with his research focusing on the ecology and adaptive evolution of amphibian.
E-mail: wchen1949@gmail.com
1 July 2014 Accepted: 25 December 2014
杂志排行
Asian Herpetological Research的其它文章
- First Records of Megophrys daweimontis Rao and Yang, 1997 and Amolops vitreus (Bain, Stuart and Orlov, 2006) (Anura: Megophryidae, Ranidae) from Vietnam
- Development and Evaluation of a Loop-mediated Isothermal Amplif cation (LAMP) Assay for Rapid Detection of Chinese Giant Salamander Ranavirus
- Antipredator Behavioral Responses of Native and Exotic Tadpoles to Novel Predator
- Diet and Prey Selection of the Invasive American Bullfrog (Lithobates catesbeianus) in Southwestern China
- Preliminary Report on the Anurans of Mount Hilong-hilong, Agusan Del Norte, Eastern Mindanao, Philippines
- A New Species of Odorrana Inhabiting Complete Darkness in a Karst Cave in Guangxi, China
