Preliminary Report on the Anurans of Mount Hilong-hilong, Agusan Del Norte, Eastern Mindanao, Philippines
2015-12-13JeszianlennPLAZAandMaritesSANGUILANaturalSciencesandMathematicsDivisionArtsandSciencesProgramFatherSaturninoUriosUniversitySanFranciscoStreetButuan8600Philippines
Jeszianlenn L. PLAZA and Marites B. SANGUILANatural Sciences and Mathematics Division, Arts and Sciences Program, Father Saturnino Urios University, San Francisco Street, Butuan 8600, Philippines
Preliminary Report on the Anurans of Mount Hilong-hilong, Agusan Del Norte, Eastern Mindanao, Philippines
Jeszianlenn L. PLAZA and Marites B. SANGUILA*
Natural Sciences and Mathematics Division, Arts and Sciences Program, Father Saturnino Urios University, San Francisco Street, Butuan 8600, Philippines
Mount Hilong-hilong is a key biodiversity area, spanning several municipalities in the provinces of the Caraga Region (Agusan del Norte, Agusan del Sur, Surigao del Norte and Surigao del Sur), northeastern Mindanao Island, southern Philippines. The Hilong-hilong massif remains one of the most signifi cant forested areas in Mindanao, threatened with habitat modification (forest removal, degradation) and other anthropogenic disturbances related to renewable resource extraction. Amphibians are key indicator species for environmental quality and are useful focal taxa for conservation efforts. Relying on historical museum database information and new survey work on Mount Hilonghilong, we provide species accounts and describe microhabitat preferences of the anurans (frogs and toads) present in the area. Twenty-seven species representing seven anuran families were studied in detail at elevations between 700 to 1300 meters above sea level; 16 of these species are Mindanao faunal region endemics. Qualitative overlap in microhabitat use was observed, suggesting that, for the species recorded, intact forest may ensure species persistence to some levels of anthropogenic disturbance. A more extensive herpetofaunal survey is needed to fully estimate the herpetofaunal diversity of Mount Hilong-hilong. Because amphibians represent fi ne-scale indicators of environmental quality and microendemism, we recommend appropriate fi ne-scaled regional strategies geared towards the conservation of amphibians in the Caraga area, northeast Mindanao Island.
environmental indicator species, key biodiversity areas, species accounts, historical records, microhabitat preferences, anthropogenic disturbance
1. Introduction
The Philippine archipelago, composed of more than 7100 islands, is internationally recognized as a global conservation biodiversity hotspot (Catibog-Sinha and Heaney, 2006; Heaney and Mittermeier, 1997), with high levels of vertebrate diversity and endemism (Brown and Diesmos, 2002, 2004, 2008; Esselstyn and Brown, 2009; Esselstyn et al., 2010; Heaney, 1986; Inger, 1954; Oliveros and Moyle, 2010). These high levels of diversity and endemism have been continuously reaffirmed by biogeographical hypothesis testing, based on the Pleistocene Aggregate Island Complex (PAIC) model by Heaney (1985, 1986). Employing this perspective, nine herpetological sub-regions (PAICS) have been recognized as centers of biological diversity and endemism, each supporting unique herpetofaunal communities (Brown and Diesmos, 2002; Brown et al., 2013; Diesmos and Brown, 2011).
Survey efforts on herpetofaunal regions such as Luzon, Mindoro, Palawan, and central Philippine (west Visayan) and its neighboring satellite islands, have been the subject of intensive biodiversity studies over the last 20 years (Brown et al., 2008, 2012, 2013; Diesmos and Brown, 2011; Stuart and Bain, 2008), which provided baseline information on the herpetofauna (Brown et al., 1996; Brown et al., 2000; Causaren, 2009; Diesmos et al., 2003; Ferner et al., 2000; Oliveros et al., 2011), as well as interesting discoveries of new species of frogs (Brown and Gonzales, 2000; Diesmos et al., 2002; Fuiten et al., 2011; Siler et al., 2009), snakes (Brown et al.,1999; Brown et al., 2011: Gaulke et al., 2002; Wallach et al., 2007), geckos (Brown et al., 2008; Roster et al., 2006; Welton et al., 2009), a remarkable discovery of a frugivorous monitor lizard (Welton et al., 2010) and redescriptions, major range extensions, and re-discoveries of other herpetological species (Brown et al., 2000; Diesmos et al., 2008; Siler, 2010; Siler et al., 2011; Welton et al., 2012).
Even with extensive efforts to adequately survey these regions, many remain unexplored, incompletely sampled, or lacking in baseline survey data (Brown et al., 2012, 2013; Diesmos and Brown, 2011). This is particularly apparent on the large island of Mindanao, southern Philippines (Balete et al., 2008; Jones and Kennedy, 2008; Siler et al., 2009; Peterson et al., 2008), where only the significant historical explorations of high elevation habitats have been undertaken, many of which were fi rst provided by Brown and Alcala (1970, 1978, 1980), Rabor and Alcala (1959), and Taylor (1920, 1928). Other recent site-specific survey efforts were only centered on the southern and western regions (Delima et al., 2006, 2007; Nuñeza et al., 2010) leaving forested areas in the eastern Mindanao and much of its remaining portions deficient of baseline survey data (but see Ates and Delima, 2008; Smith, 1993a, 1993b; Taylor, 1975).
The major lack of survey data of globally important biodiverse regions is compounded with threats of intense habitat destruction of forested areas in the archipelago. From 90%-95% of original forest cover, this has been greatly reduced to 8%-10% forest cover (Brown and Diesmos, 2009), where its remaining forest cover could still be found on Mindanao (Remollino, 2004). On the eastern part of the island of Mindanao, Mount Hilonghilong remains one of its last remaining forested areas, and has been recognized as a Key Biodiversity Area (KBA) (Mallari et al., 2001). Supporting endemic, critically endangered and threatened fauna, Mount Hilong-hilong is the type locality of an interesting rhacophorid Philautus poecilus (Brown and Alcala, 1994; Mallari et al., 2001). This isolated mountain has only been historically surveyed for herpetofauna by Brown and Alcala (1970, 1978, 1980) with collections—deposited at the California Academy of Sciences (CAS) and Smithsonian National Museum of Natural History (USNM). Threats to the area include timber extraction, land conversion (e.g. slash and burn shifting agriculture, road construction), encroachment and wildlife hunting (Mallari et al., 2001). Because of the historical biodiversity significance of the mountain, its global conservation importance as a KBA (Mallari et al., 2001), and due to the continuing threat of deforestation, new survey efforts of indicator species are desirable and justifi ed.
Of the significant herpetological diversity in the archipelago (see studies above), an important total of 108 native amphibian species have been recorded and several species still awaits formal descriptions (Brown et al., 2013). Amphibians, particularly anurans, are known to be sensitive to environmental changes (Stuart et al., 2004), and used as indicator species for habitat degradation (Alford and Richards, 1999; Davic and Welsh, 2004; Welsh and Droege, 2001; Welsh and Olivier, 1998). Accounts of high amphibian species diversity and their important function as ecological indicator species, along with continued threats to Mount Hilong-hilong provide a basis for this study, which is focused on the mountain’s amphibian fauna.
Here we provide species accounts of the anurans of Mount Hilong-hilong (Figure 1) using data from new survey effort and museum historical records, with notes on each species, its distribution and conservation status. Our results indicate that the forests of Mount. Hilong-hilong continue to support substantial endemic herpetofaunal diversity both at low and high elevations, suggesting that conservation efforts continue to be a priority in this unique Philippine highly priority area.
2. Materials and Methods
2.1 Methods Field surveys were conducted from 11-16 October 2010. Anurans were collected using random sampling and visual encounter technique. Scan searching was conducted during diurnal (07:00-10:00) and nocturnal (18:00-22:00) searches, employing opportunistic approach such as capture by hand. We utilized standard collection and specimen preservation techniques (Heyer, 1994; Simmons, 2002) and the collected specimens were photographed in life. Notes on microhabitat preference and coloration were taken, and initial identification of specimens followed Inger (1954), Alcala and Brown (1998) and Frost (2010). Representative samples were initially fi xed in 10% formalin in the fi eld, and were later transferred to 70% ethanol at the Father Saturnino Urios University Biology Laboratory.
2.2 Study sites Mount Hilong-hilong is the largest mountain in northeastern Mindanao (2062 meters above sea level) spanning several political boundaries of the CARAGA region, and a conservation priority site in the Eastern Mindanao Biodiversity Corridor. It is located in the Mindanao PAIC—recognized center of biologicaldiversity and endemism (Brown and Diesmos, 2002) and in the eastern Philippine island arc, which biogeographers have hypothesized as a possible route of “island hopping”dispersal into the archipelago (Diamond and Gilpin, 1983; Brown and Guttman, 2002; Brown et al., 2009; Jones and Kennedy, 2008; Oliveros and Moyle, 2010; Roberts et al., 2011). Thus, Mount Hilong-hilong is considered an important site, worthy of studying as part of efforts aimed at understanding the distribution of Mindanao fauna. This mountain is isolated from other Mindanao mountains by substantial stretches of low-lying areas (Hall, 1998), and lies within the geologically-active Philippine Fault Zone, with lithologies characterized largely of sedimentary rocks and major mineral deposits of copper and gold (Yumul et al., 2009). Unfortunately, mining and logging (covered by Timber License Agreement) have taken a large toll on its forests, which provided wildlife habitat, clean water, and acted as buffers to inclement weather conditions.
We surveyed Mount Hilong-hilong at two sites (see below), and identified three-broad qualitative categories of habitat types—arboreal (Figure 2), terrestrial (Figure 3) and aquatic (Figure 4) as microhabitat preferences of the anurans found in the area. We defi ne—arboreal as habitat type where anurans are found in and on vegetation; terrestrial if individuals are observed on ground and among rotting forest fl oor leaves, and aquatic if anurans are found on rocks in river or stream habitats.
Site 1: Philippines, Mindanao Island, Eastern Mindanao, Region-13, Agusan del Norte, Municipality of Remedios T. Romualdez; Barangay San Antonio;
Mount Hilong-hilong; Panarog; 09.0723oN; 125.6654oE;
700-850 meters above sea level.
This site is a secondary forest near agricultural land, characterized with vegetation such as small and short stature trees, ferns and shrubs, with prominent rotting forest floor leaves, and riverine habitat of two-three meters wide-river. The area is found to exhibit arboreal, terrestrial and aquatic habitat types, as we have broadly defi ned.
Site 2: Philippines, Mindanao Island, Eastern Mindanao, Region-13, Agusan del Norte, Municipality of Remedios T. Romualdez; Barangay San Antonio;
Mount Hilong-hilong; Aguruan; 09.0723oN; 125.68995oE;
1250-1300 meters above sea level.
Site 2 is characterized with secondary (small and large trees observed) and montane forests and stream habitat types. This site exhibits arboreal, terrestrial and aquatic habitat types, again, as we have broadly defi ned.
3. Results
We present species accounts for 27 species of anurans belonging to seven families from Mount Hilong-hilong, eastern Mindanao, southern Philippines. Notes on its status and microhabitat preferences, which are broadly categorized as arboreal, aquatic and terrestrial, are presented below.
Bufonidae
Ansonia muelleri (Boulenger, 1887) Ansonia muelleri (Figure 5) was documented from 700-1300 m above sea level and was observed on two microhabitat types, aquatic and terrestrial based on historical records and observations in this study. Specimens we collected were observed exposed on forest ground, on top or among leaf litter in forest trails, and also on or beneath rocky crevices on mountain river systems or on stream banks.
Oriental stream toads of the genus Ansonia have a disjunct distribution, with two species in India and remaining species in Southeast Asia (Frost et al., 2010; Matsui et al., 1998). In the Philippines, two members (Ansonia muelleri and A. mcgregori) of the genus Ansonia co-occur and have wide distributions in the Mindanao faunal region (Alcala and Brown, 1998). Ansonia muelleri has been recorded in Mount Sinaka and Mount Hamiguitan (Ates and Delima, 2008), Mount Malindang (Nuñeza et al., 2010), Mount Pasian (Siler et al., 2009) and across Mindanao island (Sanguila et al., 2011). This species has the IUCN status “Vulnerable”(IUCN, 2013). Because of A. muelleri’s wide distributions across Mindanao island, a comprehensive species delimitation study is needed so that species boundaries of this widespread species complex can be defi ned (Sanguila et al., 2011).
Specimens: CAS 133153-61, 133182-98, 133216-29, 133249, 133283-85, 133344, 133377-80, 133388-90, 133402-06, 133443-55, 133489-93, 133520, 133528-29, 133535-39, 133545-46, 137509-15, 185731, 248315-19; USNM 305558-62, 305564-70; MBS 106, 136, 143-50, 152-55, 157
Ceratobatrachidae
Platymantis corrugatus (Dumeril, 1853) Platymantis corrugatus (Figure 6) was observed in terrestrial microhabitats. Historical data showed that this species were encountered among rotting leaves and moss of forest fl oor in secondary forest and pristine, partly logged and logged dipterocarp forest. This species is widespread in the Philippines.
It has been recorded in Bataan, Zambales, Balbalasangbalbalan Natural Park, Aurora Memorial Natural Park, Cagayan, Isabela, Ilocos Norte, and Kalinga Province of Luzon island (Brown et al., 1996, 2000, 2013, 2013), Panay island of the Visayas, and in Mount Sinaka and Mount Hamiguitan of Mindanao island (Ates and Delima, 2008; Ferner et al., 2000). Platymantis corrugatus is indicated as “Least Concern” (IUCN, 2014).
Specimens: CAS 133572, 133622, 133779, 185732-33
Platymantis dorsalis (Dumeril, 1853) Platymantis dorsalis (Figure 7) uses terrestrial microhabitats. Based on historical records, this species was documented among rotting leaves on forest ground on logged dipterocarp forest. It can also be found on sandy soil substrate (Brown et al., 1996), in detritus of forest fl oor, as well as on tree cavities and low tree ferns (Alcala, 1962; Alcala and Brown, 1998).
As currently understood, this species has a wide distribution throughout the Philippines. However, many cryptic species may soon be recognized in this species group. Further taxonomic studies emphasizing the use of molecular data and advertisement calls are needed to address the taxonomic issues in this group (Mcleod et al., 2011). Platymantis dorsalis has the IUCN status “Least Concern”(IUCN, 2014).
Specimens: CAS 133678-80, 133690
Platymantis guentheri (Boulenger, 1882) Platymantis guentheri (Figure 8) utilizes two microhabitat types, arboreal and terrestrial based on historical records and new observations of this study. It can be found on forest duff and low vegetation (Alcala and Brown, 1998). Specimens were also collected on the forest fl oor, hiding in small crevices, or under buttress roots.
This species is known in the Mindanao faunal region and was documented in Mount Sinaka and Mount Hamiguitan (Ates and Delima, 2008) and Siargao island (Diesmos, A.C., unpublished data). This species is considered “Vulnerable” (IUCN, 2014).
Specimens: CAS 133148-49, 133411, 133644-45, 133651-53, 133202, 133213, 133470, 196378-79, 133257, 133287-88, 133518-19, 133531, 133307, 133313, 133547-48, 133547-48, 146460, 133332-33, 146468-71, 133573, 186122-26, 200177; USNM 305589-93; MBS 098-99, 103-04, 107-09, 112, 122
Platymantis rabori Alcala, Diesmos and Alcala, 1997 Platymantis rabori is found in arboreal microhabitats. Historical records documented this species on leaves, 1-3 meters above the ground. This species is found in the Mindanao faunal region and has been documented in Mount Malindang (Nuñeza et al., 2010). Platymantis rabori is considered “Vulnerable” (IUCN, 2014).
Specimens: CAS 197880
Platymantis sp. An unidentifi ed species of Platymantis was observed in terrestrial microhabitats. The specimen we collected was found on narrow holes on the ground, near tree roots and buttresses, or seen exposed on forest fl oor. Mindanao forest frogs were historically referred to as P. dorsalis, but new evidence suggests the Mindanao population is possibly a separate distinct species. Distributional information for this species, as well as conservation status are not yet available, however, we suspect that this species will prove to be a Mindanao faunal region endemic (personal communication, with Rafe M. Brown).
Specimens: MBS 158
Dicroglossidae
Fejervarya moodiei (Taylor, 1920) Fejervarya moodiei based on historical records utilizes aquatic microhabitats and are usually encountered in ditches, fl ooded rice fi elds, swamps and ponds, and are seen in all forms of standing water throughout the Philippines (Alcala, 1986; Inger, 1954).
Previously considered conspecific with Fejervarya cancrivora, which is widespread in Asia (Frost, 2010), recent genetic data reveal that Philippine populations are distinct (Kurniawan et al., 2010, 2011), and are recognized as F. moodiei following Taylor (1920). F. moodiei appears to be widespread in the Philippines, and is an estuarine specialist, which can be found in a variety of coastal areas including brackish water swamps (Brown et al., 2013). This species is indicated as “Data Defi cient”(IUCN, 2014).
Specimens: CAS 185728-30
Fejervarya vittigera (Wiegmann, 1834) Fejervarya vittigera utilizes the aquatic microhabitats and was documented in rivers or streams based on historical records. This species has been observed in low elevation, highly disturbed areas with standing water, or along small, denuded streams near coastal areas or canals bordering agricultural areas (Brown et al., 2013). This species is widespread in the Philippines and is indicated as “Least Concern” (IUCN, 2014).
Specimens: CAS-SU 16486-87, CAS 133726-29, 146463
Limnonectes diuata (Boettger, 1893) Limnonectes diuata was observed in aquatic microhabitats in or on rocks of mountain streams on dipterocarp forest based on historical records.
Members of the genus Limnonectes are characterized by distinct “fang-like” odontoid processes in their lowerjaw with distributions in South Asia, Southeast Asia, and the Philippines (Evans et al., 2003; Setiadi et al., 2011). In the Philippines, there are 11 recognized species of Limnonectes and six species are known in Mindanao island (Brown and Alcala, 1994; Siler et al., 2009).
Limnonectes diuata is known only from the Mindanao faunal region. This species was documented in Mount Hamiguitan (Ates and Delima, 2008), but it is known to have a limited geographic range, recorded in its type locality, the Diwata Mountains (Siler et al., 2009). It has the IUCN status “Vulnerable” (IUCN, 2014).
Specimens: CAS 133430-34, 133500, 139389-93
Limnonectes leytensis (Brown and Alcala, 1977) Limnonectes leytensis was observed in two microhabitat types, aquatic and terrestrial. Historical records indicate that this species was collected in or on rocks of rivers, near water in river banks, or amongst rotting leaves on forest ground on pristine and partly logged dipterocarp forest.
This species has a wide distribution in the Mindanao faunal region. Limnonectes leytensis previously has been recorded in Mount Sinaka, Mount Pasian, and Mount Malindang (Ates and Delima, 2008; Nuñeza et al., 2010; Siler et al., 2009) and is considered “Least Concern” by the IUCN (2014).
Specimens: CAS 133681-2, 133738-39, 145939
Limnonectes magnus (Stejneger, 1910) Limnonectes magnus (Figure 9) was recorded in both aquatic and terrestrial microhabitats based on historical records and new observations in this study. This species was documented in and on rocks of rivers and streams in dipterocarp forest (Brown and Alcala, 1994). Specimens we collected were observed sitting on rocks, boulders of mountain streams, or hidden among littered leaves on forest ground near stream banks.
This species has a wide distribution and is endemic in the Mindanao faunal region. Limnonectes magnus has the IUCN (2014) status “Near Threatened”. Anecdotal evidence suggests that individual of this species are exploited for human consumption and are prized by local gatherers because of its large size and fair market value (Rowley et al., 2009). However, no actual declines have been documented.
Specimens: CAS 133384-86, 139396, 133429, 133433, 133203-06, 133673-74, 133232-33, 133554, 133792, 186128; USNM 305598-99; MBS 105, 111, 114, 123-25, 128, 137, 159, 162
Occidozyga laevis (Gunther, 1858) Occidozyga laevis utilizes aquatic microhabitats and was documented in a small pond in secondary forest based on historical records. This species also occupies shallow, slow-moving streams, small pools of water in disturbed areas, and even muddy wallows created by water buffalo (Inger, 1954; Taylor, 1920).
Occidozyga laevis has a wide distribution throughout the Philippines and is considered “Least Concern” of the IUCN (2014).
Specimens: CAS 133637-42, 133675-77; USNM 305581-82, 305769-70
Megophryidae
Leptobrachium lumadorum Brown et al., 2009 Leptobrachium lumadorum (Figure 10) uses two microhabitat types, aquatic and terrestrial and was collected on or near rocks in mountain streams, resting on exposed leaf litter on the forest fl oor. A metamorphic and adult male of this species was collected on higher elevation at 1250-1300 meters above sea level.
A recently described species, Leptobrachium lumadorum was once referred to as L. hasselti comprising populations throughout Indochina and Southeast Asian islands (Brown et al., 2009). This species is distributed in the Mindanao faunal region, and is absent in Samar, Leyte and smaller island associated with these larger islands. This study presents new distribution record of L. lumadorum in Mount Hilong-hilong after its description as a separate species restricted in the Mindanao faunal region. Past surveys have failed to detect these species on Dinagat and Siargao island (Brown et al., 2009; Ross and Lazell, 1990). L. lumadorum is indicated as “Not yet assessed” (IUCN, 2014).
Specimens: MBS 113, 156
Megophrys stejnegeri (Taylor, 1920) Megophrys stejnegeri (Figure 11) was observed on both established sites from 700-1300 meters above sea level. It uses terrestrial microhabitats. Individuals of this species were encountered among leaf litter, or exposed on the forest fl oor, near tree roots and standing water pools.
This species is a Mindanao faunal region endemic with records on other montane regions of the island, Mount Sinaka, Mount Hamiguitan (Ates and Delima, 2008), Mount Pasian (Siler et al., 2009), Mount Malindang (Nuñeza et al., 2010), and Siargao island (Diesmos, A. C., unpublished data). Megophrys stejnegeri is indicated as“Vulnerable” (IUCN, 2014).
Specimens: CAS 133391-92, 133636, 133409-10, 133181, 133657, 133465-68, 133474-76, 133250, 133486-88, 133266, 133516-17, 133527, 133302-04, 133310, 133549-51, 133782, 133324, 133337, 133345-52, 133127-32, 200178; USNM 305571-80; MBS 100-01, 110, 115-16, 126, 132-35, 161, 167-68
Microhylidae
Chaperina fusca Mocquard, 1892 Chaperina fusca was observed on two microhabitats, arboreal and terrestrial and was observed among rotting leaves, moss and on ground fern based on historical records.
This species is known to occur in Thailand, Peninsular Malaysia, Borneo, and the Philippines. In the Philippines, Chaperina fusca is distributed on the Mindanao and Palawan faunal region. In Mindanao, this species has been recorded in Mount Malindang and Mount Sinaka (Atesand Delima, 2008; Brown and Diesmos, 2002; Nuñeza et al., 2010) and is in indicated as “Least Concern” (IUCN, 2014).
Specimens: CAS 133623, 133542
Kalophrynus pleurostigma Tschudi, 1838 Kalophrynus pleurostigma (Figure 12) was documented in both aquatic and terrestrial microhabitats. Historical records observed this species in water ponds and among rotting leaves and moss on the forest ground on secondary forest and partly logged and logged dipterocarp forest.
Previously considered widely distributed in Southeast Asia, Kalophrynus pleurostigma is currently known to occur in the Philippines (Ohler and Grosjean, 2005). In the country, it is distributed in the Mindanao faunal region, with records from Mount Sinaka, Mount Lumot, and Taguibo watershed (Ates and Delima, 2008; Brown and Diesmos, 2002; Sanguila et al., unpublished data). This species is indicated as “Least Concern” (IUCN, 2014).
Specimens: CAS 137516-18, 133600-03, 133567-69, 133633, 133771-76
Ranidae
Hylarana grandocula (Taylor, 1920) Hylarana grandocula (Figure 13) utilizes three terrestrial and some arboreal microhabitats. Historical records documented this species on branches and leaves of trees, on rocks, on rotten logs in or along rivers and streams, and among rotting leaves on forest floor near rivers of cultivated areas, secondary and dipterocarp forests.
This species is a member of the Rana signata complex and is known in the Mindanao faunal region (Brown et al., 2002). It has been recorded in Mount Malindang, Mount Sinaka, Mount Hamiguitan and Mount Lumot (Ates and Delima, 2008; Nuñeza et al., 2010). Hylarana grandocula is indicated as “Least Concern” (IUCN, 2014).
Specimens: CAS 133382, 137535-39, 139397-98, 133664-65, 133722, 145938, 133503-04, 133553, 186127; USNM 305600-01
Sanguirana albotuberculata (Inger, 1954) Sanguirana albotuberculata (Figure 14) was documented in some arboreal and stream side microhabitats. Historical records documented this species on or in rocks of mountain streams, rivers on dipterocarp forest.
This species is a Mindanao faunal region endemic and recent expeditions on montane regions of central Mindanao, on Mount Lumot have recorded this species (unpublished data, M. B. Sanguila). Sanguirana albotuberculata is indicated as “Data Defi cient” (IUCN, 2014).
Specimens: CAS 139394-95, 133422-23, 133501, 137533-34
Sanguirana everetti (Inger, 1954) Sanguirana everetti utilizes aquatic and terrestrial microhabitats. Historical records documented this species among leaves near rivers on a dipterocarp forest.
This species occurs widespread in the Philippines and has been recorded in Panay island, Mount Sinaka and Mount Hamiguitan (Ates and Delima, 2008; Brown et al., 2000; Ferner et al., 2000; Nuñeza et al., 2010). Sanguirana everetti is indicated as “Data Deficient”(IUCN, 2014).
Specimens: CAS 133469; USNM 305594-97, 3055600
Staurois natator (Gunther, 1858) Staurois natator uses two microhabitat types, arboreal and aquatic. This species occupies clear, rocky, and swift streams in tropical rainforests (Arifi n et al., 2011; Inger and Stuebing, 1989). We collected and observed this species close to streams, sitting on rocks, boulders, and stream banks, or perched on vegetation on or near stream banks.
Previously considered shared between both the Palawan and Mindanao faunal regions (Inger, 1954), this species is now restricted to the Mindanao PAIC. Recent genetic data suggest that Palawan populations of Staurois natator are distinct (i.e. S. nubilis), restricting S. natator in the Mindanao faunal region (Arifin et al., 2011). Previous Mindanao records of S. natator include collection from Mount Apo (Alcala and Brown, 1998), Mount Sinaka and Mount Hamiguitan (Ates and Delima, 2008), and Mount Malindang (Nuñeza et al., 2010). This species has the IUCN (2014) status “Least Concern”.
Specimens: CAS 133368, 133605-08, 133146-47, 137525-32, 133168-77, 133658-61, 133435-42, 133672, 133214, 133683, 133230, 133464, 133244-48, 133494, 133264-65, 133282, 133323, 133555, 133342, 133366-67; USNM 305602-11
Rhacophoridae
Nyctixalus spinosus (Peters, 1871) Nyctixalus spinosus (Figure 15) utilizes herb-layer arboreal microhabitats. Based on historical records this species have been documented, seen on leaves of shrubs, leaf axils of bird’s nest fern, gabi, among procket ferns and rotting leaves on the forest ground of secondary forest.
This species is a Mindanao faunal region endemic and has been recorded in Mount Sinaka and Mount Hamiguitan (Ates and Delima, 2008; Nuñeza et al., 2010). This species has the IUCN (2014) status “Vulnerable”.
Specimens: CAS 133629-32, 139319, 133561
Philautus acutirostris (Peters, 1876) Philautus acutirostris (Figure 16) occupies two microhabitattypes, arboreal and terrestrial. This species is most often found in arboreal microhabitats suitable to its direct development mode of reproduction (Alcala, 1962; Alcala and Brown, 1998; Brown and Alcala, 1982). Specimens we collected were encountered at higher elevations at 1250- 1300 meters above sea level, observed perched on leaves or clumps of moss.
Philautus acutirostris is distributed in the Mindanao faunal region and has been recorded Mount Apo (Brown, 1998), Mount Sinaka and Mount Hamiguitan (Ates and Delima, 2008), and Mount Malindang (Nuñeza et al., 2010). This species has the IUCN (2014) status“Vulnerable”.
Specimens: CAS 133140, 133394, 133164-67, 133649-50, 133207, 133211-12, 133259, 133262, 133289-90, 133298-00, 133308-12, 133334-36, 133576; USNM 497019-21; MBS 118, 120-21, 138-39, 141, 164-66, 169
Philautus worcesteri (Stejneger, 1905) Philautus worcesteri utilizes two microhabitat types, arboreal and terrestrial. Based on historical records, this species was documented on, among, and in leaf axils, leaves of Pandanus and on bark of rotting log on forest ground on secondary forest and clearing of dipterocarp forest.
This species is distributed in the Mindanao faunal region and has been recorded in Mount Apo (Brown, 1998). It is indicated as “Vulnerable” (IUCN, 2014).
Specimens: CAS 183415, 133237, 133252, 133305-06; USNM 497022
Philautus poecilus Brown and Alcala, 1994 Philautus poecilus utilizes arboreal microhabitats. Based on historical records, this species was collected in bird’s net ferns and leaf axils of Pandanus three to six meters high on mossy forest.
This species is distributed in the Mindanao faunal region, where its type locality is Mount Hilong-hilong. It has been documented in other montane regions of Mindanao, on Mount Malindang and Mount Sinaka (Ates and Delima, 2008; Nuñeza et al., 2010). Philautus poecilus is indicated as “Vulnerable” (IUCN, 2014).
Specimens: CAS 133524-26, 133530-32, 133543-44
Philautus surdus (Peters, 1863) Philautus surdus occupies arboreal and terrestrial microhabitats. Based on historical data, this species was documented in leaf axils of Pandanus approximately three to six meters high and bird’s nest fern approximately eight meters high, on ground fern or on leaf of tree near stream and among rotting leaves on forest floor of logged and pristine dipterocarp and mossy forests.
This species occurs widespread in the Philippines. In Luzon, Philautus surdus has been recorded in Aurora Memorial Natural Park, Cagayan, Isabela and Kalinga provinces (Brown et al., 2012, 2013; Siler et al., 2011). In Mindanao, this species was recorded in Mount Sinaka and Mount Hamiguitan (Ates and Delima, 2008). P. surdus has the IUCN (2014) status “Least Concern”.
Specimens: CAS 133163, 133646-47, 133199-04, 133698-99, 133709-10, 182565, 133521-23, 133533-34, 133791, 133793, 133343; USNM 305622-25, 497024
Polypedates leucomystax (Gravenhorst, 1829) Based on historical data, it appears Polypedates leucomystax occupies both arboreal and terrestrial microhabitats. This species was observed perched on leaves or branches of trees, ground fern, leaves of vines, and among rotting leaves near river bed on cultivated areas, secondary, and logged dipterocarp forest. It is known to build foam nests on surfaces overhanging or near stagnant pools (Brown and Alcala, 1982).
This species is known to occur widespread in the Philippines, however, recent genetic data, reveal that Polypedates leucomystax in the country has two genetic types, which are both found in the Mindanao faunal region (Brown et al., 2010). P. leucomystax is indicated as“Least Concern” (IUCN, 2014).
Specimens: CAS 137520-24, 133626-28, 133643, 133667-71, 133712-25, 133562
Rhacophorus bimaculatus (Peters, 1863) Rhacophorus bimaculatus (Figure 17) utilizes arboreal microhabitats. It has been observed among leaves, on leaves of trees and shrubs and procket ferns and on rocks near river. This species is known to be widespread in the Luzon and Mindanao faunal regions and is indicated “Vulnerable”(IUCN, 2014).
Specimens: CAS 133621, 133393, 133395, 139399, 133178-80, 133655-56, 133427, 133666, 180678-79, 133251, 182564, 133295-97, 133787-88, 133558-60
4. Discussion
Our recent survey effort on Mount Hilong-hilong was limited (2-3 days per site). However, we believe that our data, combined with newly synthesized historical records, contribute substantially, and provide additional information on amphibian species diversity of eastern Mindanao. The species accounts in this paper present a preliminary view of the anurans on Mount Hilong-hilong, are derived from two surveys efforts to different portions of the mountain, and provide signifi cant new distribution records for Mindanao. The collected specimens are important, as our work is the first effort aimed atdocumenting anurans on Mount Hilong-hilong since the historical explorations in the 1950s (survey work of A. C. Alcala, and the late W. C. Brown).
Of the 27 species of anurans presented in this paper, 16 are identified as Mindanao faunal region endemics (Ansonia muelleri, Platymantis guentheri, P. rabori, Limnonectes diuata, L. leytensis, L. magnus, Leptobrachium lumadorum, Megophrys stejnegeri, Kalophrynus pleurostigma, Hylarana grandocula, Sanguirana albotuberculata, Staurois natator, Nyctixalusspinosus, Philautus acutirostris, P. worcesteri, and P. poecilus), fi ve species are widespread in the Philippines except in the Palawan faunal region (Platymantis corrugatus, P. dorsalis, Occidozyga laevis, and Sanguirana everetti), one is limited to the eastern island arc of the Philippines (Rhacophorus bimaculatus), four species are widespread in the Philippines (Fejervarya mooidei, F. vittigera, Philautus surdus and Polypedates leucomystax), one occurs in the Mindanao and Palawan faunal regions (Chaperina fusca), and finally, we report a single specimen of Platymantis sp. for which distributional information is not yet available—however we suspect this species will also prove to be a Mindanao PAIC endemic (personal communication, with R. M. Brown). The presence of high proportions of Mindanao faunal region endemics suggests, that the rich diversity of endemic anurans can be used as biological indicators of overall environmental health for practical conservation purposes, especially in forested areas. However, to fully develop this suite of indicator species for practical purposes, additional taxonomic study will be necessary to clarify species boundaries, particularly among widespread Mindanao PAIC taxa (Brown, 2007). Examples of species that warrant additional taxonomic studies include Leptobrachium lumadorum, Limononectes magnus and L. leytensis, Sanguirana everetti, Megophrys stejnegeri and Staurois. natator, because of their widespread occurrence throughout the region (Diesmos and Brown, 2011).
Anurans are sensitive to environmental conditions, and alteration of their habitats greatly affects species presence at a given site, especially if they are found to occur restricted in forested areas (Stuart et al., 2004). Although preliminary, we present three-broad qualitative categories of microhabitat preferences of the anurans of Mount Hilong-hilong. We defi ne arboreal as habitat type where anurans are found on vegetations (e.g. ground ferns, leaf axils, on leaf of shrubs), terrestrial, if individuals typically are observed on ground and among rotting forest floor leaves, and aquatic, if directly found in water pools in rivers and streams, on rocks in river and stream habitats. We found that majority of the anurans of Mount Hilonghilong utilize variety of microhabitats, and to exhibit overlap in microhabitat preferences (Table 1). Generally, this variability in microhabitat use stems from a species differential use of separate microhabitats for breeding, foraging and refuge sites, or for physiological needs (Duellman and Trueb, 1994). This fi nding confi rms results from other inventories conducted in the country that have also observed overlapping of microhabitat use in frogs (Alcala et al., 1997; Diesmos et al., 2003), suggesting the need of intact, minimally disturbed habitats to ensure species persistence.
Again, because of limited sampling effort, the anurans we documented are mostly forest-restricted species, and we document only one disturbance tolerant species (Occidozyga laevis), which is an historical record. However, it is interesting to note that the fi rst specimen we collected (Megophrys stejnegeri) was found on forest edge at 700 meters above sea level, adjacent to a cultivated land. Also, we were not able to document invasive frog species (e.g. Bufo marinus, Hoplobatrachus rugulosus, Lithobates catesbiana, Hylarana erythraea, Kaloula pulchra) that are observed to have wide distributions in other parts of the country (Diesmos et al., 2006).
We present the IUCN Red List Status of the anurans found in the area (Table 1), and found that a number of the species listed are Mindanao faunal region endemics (Ansonia muelleri, Platymantis guentheri, P. rabori, Limnonectes diuata, Megophrys stejnegeri, Nyctixalus spinosus, Philautus acutirostris, P. poecilus, and P. worcesteri), with conservation status indicated as “Vulnerable.” The currently recognized conservation status of these species could be use as primary information, important for future conservation related researches. This information could also be useful in species-specific surveys targeting data on basic systematics and ecology for any of these species, subsequently useful in practical conservation efforts—such as establishing protected areas.
Our attempt to provide preliminary information on the diversity of anurans of Mount Hilong-hilong provides important data on their natural history, and the presence of a number of Mindanao faunal-region endemics from an isolated mountain in the eastern portion in the large island of Mindanao. We emphasize the urgent need for exhaustive and comprehensive studies on species diversity, natural history and ecological studies, as well as other important vertebrate groups. Providing these invaluable studies in support of conservation efforts will impart improved strategies for high-priority Key Biodiversity Areas of Mindanao.
Acknowledgements We are particularly thankful to R. L. PLAZA, R. B. PLAZA, C. G. LUZON and R. M. LUZON for the generous financial support for the fieldwork. Special thanks are due to G. L. G. LUZON for her effort in the collection of specimens. We thank the Department of Environment and Natural Resources (CARAGA) for administering our collection permit and to Father Saturnino Urios University for logistical support,local government unit of the Municipality of Remedios T. Romualdez, and the indigenous people of Mount Hilonghilong. We thank the Wildlife Conservation Society of the Philippines (WCSP), R. M. BROWN and P. HOSNER for generously sharing photos, and C. D. SILER for providing the map. Lastly, we offer our thanks and compliments to L. G. F. JARANILLA, M. G. T. MEDRANO, A. C. DIESMOS, R. M. BROWN and an anonymous reviewer for comments on earlier drafts of the manuscript.
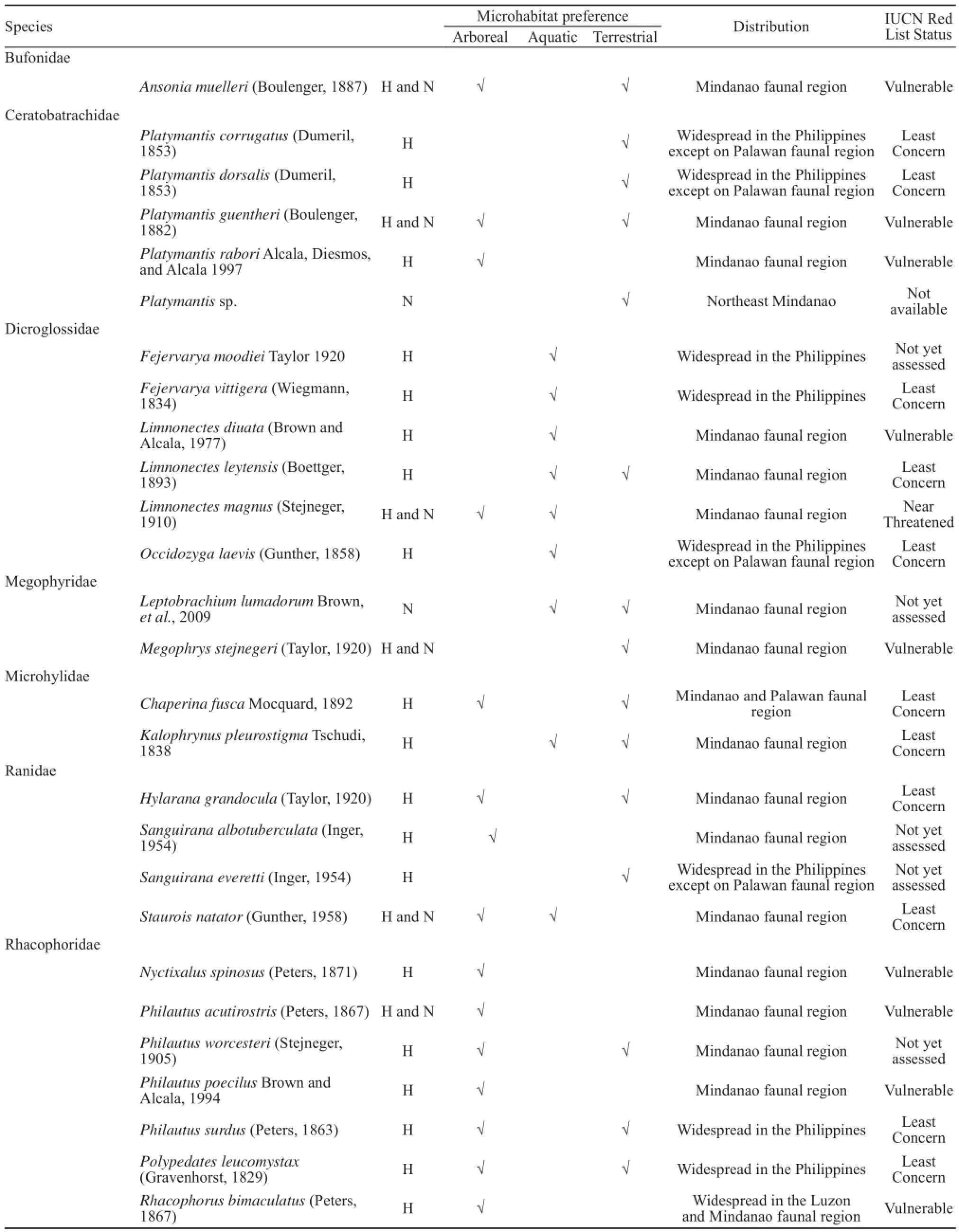
Table 1 Frogs documented from Mt. Hilong-hilong with notes on microhabitat preferences, and distribution. H indicates historical records from natural history museum collections. N indicates new records observed in this study.
References
Arif n U., Iskandar D. T., Bickford D. P., Brown R. M., Meier R., Kutti S. N. 2011. Phylogenetic relationship within the genus Staurois (Amphibia, Ranidae) based on 16S rRNA sequences. Zootaxa, 2733: 39-52
Alcala A. C. 1962. Breeding behavior and early development of frogs and toads of Negros, Philippine Islands. Copeia, 1962: 679-726
Alcala A. C., Brown W. C. 1986. Guide to Philippine flora and fauna, Vol. X, Amphibians and Reptiles. Natural Resources Management Center, Ministry of Environment and Natural Resources and University of the Philippines, Manila. 195 pp
Alcala A. C., Brown W. C. 1998. Philippine amphibians: An illustrated fi eld guide. Makati, Philippines: Bookmark Inc. Press, 113 pp
Alford R. A., Richards S. J. 1999. Global amphibian declines: A problem in applied ecology. Annu Rev Ecol Syst, 30: 133-165
Amphibia Web (http://amphibiaweb.org/)
Ates F. B., Delima E. M. 2008. Assemblage and microhabitat of anurans from Mt. Sinaka, Arakan, Cotabato and Mt. Hamiguitan, Davao Oriental, Mindanao Island, Philippines. JNS, 7(1): 101-107
Balete D. S., Heaney L. R., Rickart E. A., Quidlat R. S., Ibanez J. C. 2008. A new species of Batomys (Muridae: Murinae) from eastern Mindanao Island, Philippines. P Biol Soc of Wash, 121: 411-428
Brown W. C., Alcala A. C. 1970. The zoogeography of the Philippine Islands: A fringing archipelago. Proc California Acad Sci, 38: 105-130
Brown W. C., Alcala, A. C. 1978. Philippine Lizards of the Family Gekkonidae. Dumaguete City, Philippines: Silliman University Press, 146 pp
Brown W. C., Alcala A. C. 1980. Philippine lizards of the Family Scincidae. Dumaguete City, Philippines: Silliman University Press, 264 pp
Brown W. C., Alcala A. C. 1982. Modes of reproduction of Philippine anurans. In Rodin A. G. J., Miyata K. (Eds.), Advances in Herpetology and Evolutionary Biology. Museum of Comparative Biology, Cambridge, MA, USA, 416-428
Brown W. C., Alcala A. C. 1994. Philippine frogs of the family Rhacophoridae. Proc California Acad Sci, 48: 185-220
Brown W. C., Alcala A. C., Brown R. M. 1998. Taxonomic status of Cornufer worcesteri. J Herpetol, 33: 131-133
Brown R. M., Ferner J. W., Sison R. V., Gonzales P. C., Kennedy R. S. 1996. Amphibians and reptiles of the Zambales Mountains of Luzon Island, Republic of the Philippines. Herpetol Nat Hist, 4: 1-22
Brown R. M., Leviton A. E., Sison R. V. 1999. Descriptions of a new species of Pseudorabdion (Serpentes: Colubridae) from Panay Island, Philippines with a revised key to the genus. Asiat Herpetol Res, 8: 7-12
Brown R. M., Mcguire J. A., Diesmos A. C. 2000. Status of some Philippine frogs referred to Rana everetti (Anura: Ranidae), description of a new species and resurrection of R. igorota Taylor 1922. Herpetologica, 56: 81-104
Brown R. M., Diesmos A. C., Alcala A. C. 2002. The state of Philippine herpetology and the challenges for the next decade. Silliman J, 42 (1): 18-87
Brown R. M., Dolino C. N., Alcala E., Diesmos A. C., Alcala A. C. 2002. The advertisement calls of two endangered species of endemic Philippine frogs: Platymantis spelaeus and P. insulatus (Anura; Ranidae). Silliman J, 43: 91-109
Brown R. M., Fernandez R., Rivero C., Buenviaje R., Diesmos A. C. 2002. Mt. Isarog’s herpetological wonders. Haring Ibon, 3: 12-16
Brown R. M., Diesmos A. C., Fernandez R. B. 2003. The rising costs of cabbage and potatoes. Haring Ibon, 9: 8-12
Brown R. M., Diesmos A. C. 2004. Application of lineagebased species concepts to oceanic island frog populations: The effects of differing taxonomic philosophies on the estimation of Philippine biodiversity. Silliman J, 42 (1): 133-162
Brown R. M., Gonzales J. C. 2007. A new forest frog of the genus Platymantis (Amphibia: Anura: Ranidae) from the Bicol Peninsula of Luzon Island, Philippines. Copeia, 2: 251-266
Brown R. M. 2007. Robert Inger’s Systematics and Zoogeography of the Philippines. Introduction (1-24) reprint of Inger R. F. 1954. Systematics and Zoogeography of Philippine Amphibia. Kota Kinabalu: Natural History Publications, 1-17
Brown R. M., Diesmos A. C., Alcala A. C. 2008. Philippine amphibian biodiversity is increasing in leaps and bounds. 82-83. In Stuart S. N., Hoffmann M., Chanson J. S., Cox N. A., Berridge R., Ramani P. and Young B. E. (Eds). Threatened Amphibians of the World. Arlington, Barcelona and Gland: Lynx Ediciones, IUCN—The World Conservation Union and Conservation International, 82-83
Brown R. M., Oliveros C., Siler C. D., Diesmos A. C. 2008. A new Gekko from the Babuyan Islands, Northern Philippines. Herpetologica, 64: 305-320
Brown R. M., Diesmos A. C. 2009. Philippines, Biology. In Gillespie R., Clague R. (Eds). Encyclopedia of Islands. Berkeley, CA: University of California Press, 723-732
Brown R. M., Siler C. D., Diesmos A. C., Alcala A. C. 2009. Philippine frogs of the genus Leptobrachium (Anura: Megophyridae): Phylogeny-based species delimitation, taxonomic review and descriptions of three new species. Herpetol Monogr, 23: 1-44
Brown R. M., Linkem C. W., Siler C. D., Sukumaran J., Esselstyn J. A., Diesmos A. C., Iskandar D. T., Bickford D., Evans B. J., McGuire J. A., Grismer L., Supriatna J., Andayani N. 2010. Phylogeography and historical demography of Polypedates leucomystax in the islands of Indonesia and the Philippines: Evidence for recent human-mediated range expansion? Mol Phylogenet Evol, 57: 598-619
Brown R. M., Diesmos A.C., Oliveros C. 2011. A new fl ap-legged forest gecko (Genus Luperosaurus) from the north-eastern Philippines. J Herpetol, 45: 202-210
Brown R. M., Diesmos A. C., Sanguila M. B., Siler C. D., Diesmos M. L. D., Alcala A. C. 2012. Amphibian conservation in the Philippines. FrogLog. 104: 40-43
Brown R. M., Stuart B. L. 2012. Patterns of biodiversity discovery through time: An historical analysis of amphibian species discoveries in the Southeast Asian mainland and island archipelagos. In Gower D. J., Johnson K. G., Richardson J. E., Rosen L. R., Williams S. T. (Eds). Biotic Evolution and Environmental Change in Southeast Asia. London: Cambridge University Press, 348-389
Brown R. M., Siler C. D., Oliveros C. A., Esselstyn J. A., Diesmos A. C., Hosner P. A., Linkem, C. W., Barley A. J., Oaks J. R., Sanguila M. B., Welton L. J. Blackburn D. S., Moyle R. G., Peterson A. T., Alcala A. C. 2013. Evolutionary Processes of Diversifi cation in a Model Island Archipelago. Annu Rev Ecol Evol Syst, 44: 24.1-24.5
Catibog-Sinha C. S., Heaney L. R. 2006. Philippine Biodiversity: Principles and Practice. Quezon City: Haribon Found Conserv Nat Resour
Causaren R. M. 2009. Preliminary report on the anurans of Mts. Palay-palay Mataas na Gulod Protected Landscape, Luzon Island, Philippines. PJSB, 3: 40-56
Davic R. D., Welsh H. H. Jr. 2004. On the ecological roles of salamanders. Annu Rev Ecol Evol Syst, 35: 405-434
Delima E. M., Ates F. B., Ibañez J. C. 2006. Species composition and microhabitats of frogs within Arakan Valley Conservation Area, Cotabato, Mindanao Island, Philippines. Banwa, 3: 16-30
Delima E. M. M., Diesmos A. C., Ibañez J. C. 2007. The herpetofaunal importance of Mt. Hamiguitan Range, Mindanao Island, Philippines. Banwa, 4: 27-40
Diamond J. M., Gilpin M. E. 1983. Biogeographic umbilici and the origin of Philippine avifauna. Oikos, 41: 307-321
Diesmos, A. C. 1998. The amphibian faunas of Mt. Banahao, Mt. Cristobal and Mt. Makiling, Luzon island, Philippines. M.Sc. Thesis. University of the Philippines
Diesmos A. C., Brown R. M., Alcala A. C., Sison R. V., Afuang L. E., Gee G. V. A. 2002. Philippine amphibians and reptiles. In Ong P. S., Afuang L. E., and Rosell-Ambal R. G. (Eds.) Philippine Biodiversity Conservation Priorities: a Second Iteration of the National Biodiversity Strategy and Action Plan. Department of the Environment and Natural Resources—Protected Areas and Wildlife Bureau, Conservation International Philippines, Biodiversity Conservation Program—University of the Philippines Center for Integrative and Developmental Studies, and Foundation for the Philippine Environment. Quezon City, Philippines, 26-44
Diesmos A. C., Brown R. M., Gee G. A. 2003. Preliminary report on the amphibians and reptiles of Balbalasang-Balbalan National Park in Luzon Island, Philippines. Sylvatrop, 13(1and2): 63-80
Diesmos A. C., Diesmos, M. L., Brown R. M. 2006. Status and distribution of alien invasive frogs in the Philippines. J Environ Sci Manag, 9: 41-53
Diesmos A. C., Brown R. M., Alcala A. C., Sison R. V. 2008. Status and distribution of non-marine turtles of the Philippines. Chelonian Conserv Biol, 7: 157-177
Diesmos A. C., Brown R. M. 2011. Diversity, biogeography and conservation of Philippine amphibians. In Biology and Conservation of Tropical Asian Amphibians. Proceedings of the Conference “Biology of the Amphibians in the Sunda Region, south-east Asia.’’ In Das I., Haas A. and Tuen A. A. (Eds). Institute of Biodiversity and Environmental Conservation, Universiti Malaysia Sarawak, Kota Samarahan, Sarawak, Malaysia, 26-49
Duellman W. E. Trueb L. S. 1994. Biology of amphibians. Baltimore: The John Hopkins University Press. 670 pp
Esselstyn J. A., Brown R. M. 2009. The role of repeated sea-level fluctuations in the generation of shrew (Soricidae: Crocidura) diversity in the Philippine Archipelago. Mol Phylogenet Evol, 53: 171-181
Esselstyn J. A., Oliveros C. H., Moyle R. G., Peterson A. T., Mcguire J. A., Brown R. M. 2010. Integrating phylogenetic and taxonomic evidence illuminates complex biogegraphic patterns along Huxley’s modifi cation of Wallace’s Line. J Biogeogr, 37: 2054-2066
Evans B. J., Brown R. M., Mc Guire J. A., Supriatna J., Andayani N., Diesmos A. C., Iskandar D., Melnic D. J., Cannatella D. C. 2003. Phylogenetics of fanged frogs: Testing biogeographical hypotheses at the interface of the Asian and Australian faunal zones. Syst Biol, 52: 794-819
Ferner J. W., Brown R. M., Sison R. V., Kennedy R. S. 2000. The Amphibians and Reptiles of Panay Island, Philippines. Asiat Herpetol Res, 9: 34-70
Frost Darrel R. 2010. Amphibian Species of the World: An Online Reference. Version 5.4 (8 April, 2010). Electronic Database accessible at http://research.amnh.org/vz/herpetology/amphibia/ American Museum of Natural History, New York, USA.
Fuiten A., Diesmos A. C., Welton L. J., Barley A., Oberheide B., Rico E. L. B., Brown R. M. 2011. New species of stream frog from the mountains of Luzon Island, Philippines. Herpetologica, 67: 89-103
Gaulke M. 2002. A new species of Lycodon from Panay Island, Philippines (Reptilia, Serpentes, Colubridae). Spixiana, 25: 85-92
Hall R. 1998. The plate tectonics of Cenozoic SE Asia and the distribution of land and sea. In Hall R., Holloway J. D. (Eds.), Biogeography and Geological Evolution of SE Asia. Backhuys Publishers, Leiden, Netherlands, 99-131
Heaney L. R. 1985. Zoogeographic evidence for middle and late Pleistocene land bridges to the Philippines. Mod Quat Re, 9: 127-143
Heaney L.R. 1986. Biogeography of small mammals in Southeast Asia estimates of rates of colonization, estimation and speciation. Biol J Linn Soc, 28: 127-165
Heaney L. R., Mittermeier R. A. 1997. Philippines. In Mittermeier R. A., Gil R. P., Mittermeier C. G. (Eds), Megadiversity: Earth’s biologically wealthiest nations, 236-255
Herpwatch Retrieved from http://www.herpwatch.org
Heyer W. R., Donnelly M. A., McDiarmid R. W., Hayek L. A., Foster M. S. 1994. Measuring and monitoring biological diversity: standard methods for amphibians. Washington D. C.: Smithsonian Institution Press, 364 pp
IUCN 2014. IUCN Red List of Threatened Species. Version 2013.2. Retrieved from www.iucnredlist.org
Jones A. W., Kennedy R. S. 2008. Evolution in a tropical archipelago: Comparative phylogeography of Philippine fauna and flora reveals complex patterns of colonization and diversifi cation. Biol J Linn Soc, 95: 620-639
Kurniawan N., Islam M. M., Djong T. H., Igawa T., Daicus M. B., Yong H. S., Wanichanon R., Khan M. M. R., Iskandar D. T., Nishioka M., Sumida M. 2010. Genetic divergence and evolutionary relationship in Fejervarya cancrivora from Indonesia and other Asian countries inferred from allozyme and mtDNA sequence analyses. Zool Sci, 27: 222-233
Kurniawan N., Djong T. H., Islam M. M., Nishizawa T., Belabut D. M., Sen Y. H., Wanichanon R., Yasir I., Sumida M. 2011. Taxonomic status of three types of Fejervarya cancrivora from Indonesia and other Asian countries based on morphological observations and crossing experiments. Zool Sci, 28: 12-24
Mallari N. A. D., Tabaranza B. R., Crosby M. J. 2001. Key conservation sites in the Philippines. A Haribon Foundation and Birdlife International directory of important bird areas. Makati, Philippines: Bookmark Inc.
Matsui M., Nabhitabhata J., Panha S. 1998. A new Ansonia from northern Thailand (Anura, Bufonidae). Herpetologica, 54: 448-454
Matsui M., Tominaga A., Liu W., Khonsue W., Grismer L. L. Diesmos A. C., Das I., Sudin A., Yambun P., Yong H., Brown R. M. 2010. Phylogenetic relationships of Ansonia from Southeast Asia inferred from mitochondrial DNA sequences: Systematic and Biogeographic Implications (Anura: Bufonidae). Mol Phylogenet Evol, 54: 561-570
Mcleod D. S., Siler C. D., Diesmos A. C., Diesmos M. L., Garcia V. S., Arkonceo A. O., Balaquit K. L., Uy C. C., Villaseran M. M., Yarra E. C., Brown R. M. 2011. Amphibians and reptiles of Luzon Island, V: The Herpetofauna of Angat Dam, Bulacan Province, Luzon Island, Philippines. Asian Herpetol Res, 2(4): 177-198
Nuñeza O. M., Ates F. B., Alicante A. A. 2010. Distribution of endemic and threatened herpetofauna in Mt. Malindang, Mindanao, Philippines. Biodivers Conserv, 19: 503-518
Ohler A., Grosjean S. 2005. Color pattern and call variation in Kalophrynus from south-east Asia. Herpetozoa, 18: 99-106
Oliveros C. H., Moyle R. G. 2010. Origin and diversification of Philippine bulbuls. Mol Phylogenet Evol, 54: 822-832
Oliveros C. H., Ota H., Crombie R. I., Brown R. M. 2011. The herpetofauna of the Babuyan Islands, Northern Philippines. Sci Pub Nat His Mus Univ Kansas, 43: 1-20
Peterson A. T., Brooks T., Gamauf A., Gonzales J. C. T., Mallari N. A. D. 2008. The avifauna of Mt. Kitanglad, Bukidnon Province, Mindanao, Philippines. Fieldiana Zool N Ser,14: 1-43
Rabor D. S., Alcala A. C. 1959. Notes on a collection of amphibians from Mindanao Island, Philippines. Silliman J, 2: 93-102
Remollino A. M. 2004. Desertifi cation in the making. Bulatlat. Vol. 4, December 12-18, 2004. Retrieved from http://www.bulatlat. com/news
Roberts T. E., Lanier H. C., Sagris E. J., Olson L. E. 2011. Molecular phylogeny of treeshrews (Mammalia: Scandentia) and the timescale of diversification in Southeast Asia. Mol Phylogenet Evol, 60: 358-372
Ron S., Brown R. M. 2008. Filling the black hole: Challenges in taxonomy to protect amphibians. In Stuart S. N., Hoffmann M., Chanson J. S., Cox N. A., Berridge R., Ramani P., and Young B. E. (Eds.), Threatened Amphibians of the World. Barcelona: Lynx Ediciones; IUCN—The World Conservation Union, Gland, Switzerland; and Conservation International, Arlington Virginia, USA, 133 pp
Ross C. A., Lazell J. D. 1990. Amphibians and reptiles of Dinagat and Siargao Islands, Philippines. Philippine J Sci,119: 257-286
Rowley J., Brown R. M., Bain R., Kusrini M., Inger R., Stuart B., Wogan G., Chan-ard T., Trung C. T., Diesmos A. C., Iskandar D. T., Lau M., Ming L. T., Makchai S., Thy N., Truong N. Q., Phimmachak, S. 2009. Impending conservation crisis for southeast Asian amphibians. Biol Letters, 6: 336-338
Sanguila M. B., Siler C. D., Diesmos A. C., Nuñeza O. M., Brown R. M. 2011. Phylogeography and conservation implications of geographic structure, genetic variation, and potential species boundaries in Philippine slender toads. Mol Phylogenet Evol, 61: 333-350
Setiadi M. I., McGuire J. A., Brown R. M., Zubairi M., Iskandar D. T., Andayani N., Supriatna J., Evans, B. J. 2011. Adaptive radiation and ecological opportunity in Sulawesi and Philippine fanged frog (Limnonectes) communities. Am Nat, 178: 221-240
Siler C. D., Mcvay J., Diesmos A. C., Brown R. M. 2009. A new species of fanged frog (Dicroglossidae: Limnonectes) from Southern Mindanao Island, Philippines. Herpetologica, 65: 105-114
Siler C. D., Diesmos A. C., Alcala A. C., Brown R. M. 2009. A new species of limestone forest frogs, genus Platymantis (Amphibia; Anura; Ceratobatrachidae) from Samar Island, Philippines. Herpetologica, 65: 92-104
Siler C. D. 2010. Reptilia, Squamata, Scincidae, Brachymeleselerae (Taylor, 1917): Rediscovery in Old Balbalan, Cordillera Mountain Range, Luzon Island, Philippines, and Natural history. Checklist, 6: 616-618
Siler C. D., Jones R. M., Welton L. J., Brown R. M. 2011. Redescription of tetradactyl, Philippine slender skinks (genus Brachymeles). Herpetologica, 67: 300-317
Siler C. D., Crombie R. I., Diesmos A. C., Brown R. M. 2011. Redescription of two poorly known loam-swimming skinks, Brachymeles bicolor and Brachymeles pathfinderi (Reptilia: Squamata: Scincidae) from the Philippines. J Herpetol, 45: 355-369
Simmons J. 2002. Herpetological collecting and collections management. SSAR Herp Circ, 31: 1-153
Smith B. E. 1993a. Notes on a collection of squamate reptiles from eastern Mindanao, Philippine Islands, Part I: Lacertilia. Asiat Herpetol Res, 5: 85-95
Smith B. E. 1993b. Notes on a collection of squamate reptiles from eastern Mindanao, Philippine Islands, Part 2: Serpentes. Asiat Herpetol Res, 5: 95-102
Stuart B. L., Platt S. G. 2004. Recent records of turtles and tortoises from Laos, Cambodia, and Vietnam. Asiat Herpetol Res, 10: 129-150
Stuart R., Brown R. M. 2008. Filling the black hole: Challenges in taxonomy to protect amphibians. In Stuart S. N., Hoffmann M., Chanson J. S., Cox, N. A., Berridge R., Ramani P. and Young B. E. (Eds.), Threatened Amphibians of the World. Barcelona: Lynx Ediciones; IUCN—The World Conservation Union, Gland,Switzerland; and Conservation International, Arlington Virginia, USA, 133 pp
Taylor E. H. 1920. Philippine amphibia. Philippine J Sci, 16: 213-359
Taylor E. H. 1928. Amphibians, lizards, and snakes of the Philippines. In Dickerson R. (Ed.), Distribution of Life in the Philippines. Manila: Bureau of Science, Monogr, 21: 322 pp
Taylor E. H. 1975. Philippine adventures: An autobiographical memoir. In Taylor E. H., Leonard A. B., Smith H. M., Pisani G. R. (Eds.), Recollections of an Herpetologist. Lawrence: University of Kansas Museum of Natural History Monogr, 1-105
Wallach V., Brown R. M., Diesmos A. C., Gee G. V. A. 2007. An enigmatic new species of blind snake from Luzon Island, northern Philippines, with a synopsis of the genus Acutotyphlops (Serpentes:Typhlopidae). J Herpetol, 41: 690-702
Welsh H. H. Jr., Olliver L. M. 1998. Stream amphibians as indicators of ecosystem stress: A case study from California’s redwoods. Ecol Appl, 8: 1118-1132
Welsh H. H. Jr., Droege S. 2001. A case for using plethodontid salamanders for monitoring biodiversity and ecosystem integrity of North American forests. Conserv Biol, 15: 558-569
Welton L. J., Siler C. D., Diesmos A. C., Brown R. M. 2009. A new bent-toed gecko (Genus Cyrtodactylus) from southern Palawan Island, Philippines, and clarifi cation of the taxonomic status of C. annulatus. Herpetologica, 65: 323-343
Welton L. J., Siler C. D., Bennett D., Diesmos A. C., Duya M. R., Duya R., Rico E. L., van Weerd M., Brown R. M. 2010. A spectacular new Philippine monitor lizard reveals a hidden biogeographic boundary and a novel fl agship for conservation. Biol Letters, 6: 654-658
Welton L. J., Siler C. D., Diesmos A. C., Diesmos M. L. D., Lagat R., Causaren R., Brown R. M. 2012. Genetic identity, geographic range, and major distribution records for frugivorous monitor lizards of Luzon Island, Philippines. Herpetol Rev, 43: 226-230
Yumul G., Dimalanta C. B., Queaño K., Marquez E. 2009. Philippines, Geology. In Gillespie R., Clague D. (Eds.), Encyclopedia of Islands. Berkeley: University of California Press, 732-738
Dr. Marites B. SANGUILA, from Father Saturnino Urios University, Butuan, Philippines, with her research focusing on systematics and conservation of herpetofauna in Southern Philippines.
E-mail: mbsanguila@urios.edu.ph
1 July 2014 Accepted: 19 December 2014
杂志排行
Asian Herpetological Research的其它文章
- First Records of Megophrys daweimontis Rao and Yang, 1997 and Amolops vitreus (Bain, Stuart and Orlov, 2006) (Anura: Megophryidae, Ranidae) from Vietnam
- Development and Evaluation of a Loop-mediated Isothermal Amplif cation (LAMP) Assay for Rapid Detection of Chinese Giant Salamander Ranavirus
- Antipredator Behavioral Responses of Native and Exotic Tadpoles to Novel Predator
- Prehibernation Energy Storage in Heilongjiang Brown Frogs (Rana amurensis) from Five Populations in North China
- Diet and Prey Selection of the Invasive American Bullfrog (Lithobates catesbeianus) in Southwestern China
- A New Species of Odorrana Inhabiting Complete Darkness in a Karst Cave in Guangxi, China
