Adsorption Kinetics of NH4+by Purple Soils with Different pH Values
2015-12-13DinanZHANGXianjunJIANG
Dinan ZHANG, Xianjun JIANG
College of Resources and Environment, Southwest University, Beibei 400716, China
The nitrogen fertilizer applied into soil is mainly as NH4+-N[1-2].Part of the NH4+is consumed and retained by ammonia volatilization,biological uptake and soil adsorption; but a considerable part of the NH4+is nitrified to NO3-. The NO3-can be leached easily into deep soil, resulting in groundwater contamination[3],and is also likely to enter surface water, resulting in eutrophication[4-5],thereby affecting the quality of water environment[6]. The adsorption of NH4+by soil can suppress nitrogen loss. So discussing on the adsorption and desorption properties of soil for NH4+is an important foundation for understanding the migration, transformation and other processes of nitrogen in the soil,and is also a theoretical basis for reducing nitrogen loss and preventing groundwater contamination. Purple soil is mainly formed in the Sichuan Basin in Southwest China. It is named as regosol by the Food and Agriculture Organization (FAO) of the United Nations,and Entisol by the United States Department of Agriculture (USDA)[7].Purple soil has soft parent material,poor corrosion resistance, shallow soil layer and serious soil erosion, and is easy to be disintegrated by weathering and to lead to non-point source pollution[8].Although the adsorption of NH4+has been well studied, there are rare reports on adsorption of NH4+by purple soil. Xie et al.[9]ever studied the adsorption of NH4+by purple soil,but they did not investigate the effect of pH on adsorption and desorption properties of purple soil for NH4+.In this study,the purple soils with different pH values were collected from Beibei,Chongqing, and their adsorption and desorption properties for NH4+were discussed so as to quantitatively study the adsorption and desorption laws.The study results were further fitted with three kinds of traditional isothermal adsorption equations to determine the adsorption kinetics of NH4+by purple soils with different pH values.
Materials and Methods
Soil sampling
The soil was collected from Beibei District, Chongqing City (106°25′45″E,29°49′18″N),and it was calcareous purple soil developed from Jurassic mudstone. The top 0-20 cm soil was sampled. After dried, the soil was passed through a 1-mm sieve. The basic properties of the soil were as follows: pH 8.0, organic matter content 0.69%,total nitrogen content 0.1%,total phosphorus content 0.13%, total potassium content of 2.9% , rapidly available phosphorus 6.5 mg/kg and rapidly potassium content of 110.4 mg/kg.
The pH value of the purple soil was adjusted to 6.0 and 7.2 respectively by adding HCl.A certain amount(100 g) of dried soil that had been passed through a 1-mm sieve was dissolved in 250 ml of distilled water.The mixture was stirred vigorously with a glass rod for 1-2 min, and then stood for 30 min.Subsequently,the pH value of the soil suspension was measured with a pH electrode. During the measurement,0.1 mol/L of HCl was slowly added,along with the stirring,to adjust the pH value to 6.0 or 7.2.After the adjustment, the soil samples were dried at(40±1)℃,ground,and then passed through a 1-mm sieve.Finally,the accurate pH values of the soil samples were measured.
Ammonium adsorption
Certain amounts (1.5 g) of airdried purple soil sample were placed in centrifuge tubes. Subsequently, certain volumes(30 ml)of NH4Cl solutions with concentrations of 0, 50, 100, 200,400, 700 and 1 000 mg/L respectively(prepared with 0.01 mol/L of NaCl solution) were added to the centrifuge tubes.After shaken at(25±1)℃for 24 h, the tubes were centrifuged at 4 000 r/min for 5 min. The NH4+concentrations in the supernatants were determined. The NH4+adsorption am-ounts were calculated with the difference assay. Based on the calculation results,the adsorption curves of NH4+by purple soils with different pH values were drawn.
Ammonium desorption
The precipitates in the centrifuge tubes obtained above were washed with 30 ml of distilled water two times.A certain volume (30 ml) of 2 mol/L KCl solution was added to each of the centrifuge tubes. The tubes were shaken at (25±1)℃for 2 h and then centrifuged at 4 000 r/min for 5 min.The obtained supernatants were desorption solutions. The desorption amounts of NH4+were calculated.Based on the calculation results, the isothermal desorption curves of NH4+were drawn.
Chemical analysis
The NH4+concentration was determined with the diffusion method[10].
Calculations and kinetic models
Calculations The calculation formula was as follows:
Wherein, Q indicates adsorption amount/desorption amount; C0indicates the original NH4+concentration,mg/L; Ceindicates the NH4+concentration at the adsorption/desorption equilibrium, mg/L; V indicates the volume of added NH4Cl solution,ml;m indicates the mass of weighed soil sample,g.
Kinetic models The kinetic models used in this study included Langmuir isothermal adsorption equation, Freundlich isothermal adsorption equation and Temkin isothermal adsorption equation.
Data analysis
The graphing and the isothermal adsorption equation simulation were performed using Excel.
Results and Analysis
Effects of purple soils with different pH values on isothermal adsorption of NH4+
The isothermal adsorption curves of NH4+by purple soils with different pH values were shown in Fig.1. The adsorption amounts of NH4+by purple soils with different pH values were all increased with the increase of NH4+concentration, and they ranged from 0.659 to 13.5 mg/g. The adsorption amounts of NH4+were also increased with the increase of pH value. Among the purple soils with three different pH values,the purple soil with the pH value of 8.0 showed the strongest adsorption capacity for NH4+with the largest adsorption amount of 13.5 mg/g, followed by the purple soil with the pH value of 7.2 with the largest adsorption amount of 12.8 mg/g, and the purple soil with the pH value of 6.0 showed the weakest adsorption capacity with the largest adsorption amount of 10.3 mg/g.
Effects of purple soils with different pH values on isothermal desorption of NH4+
The isothermal desorption curves of NH4+by purple soils with different pH values were shown in Fig.2. The desorption amounts of NH4+by purple soils with different pH values were also trended to be increased with the increase of NH4+concentration,and they ranged from 0.115 to 7.96 mg/g. With the increase of pH value, the desorption amounts of NH4+by purple soils were reduced.The desorption capacity of purple soil with the pH value of 8.0 for NH4+was weakest with the largest desorption amount of 2.23 mg/g, followed by that with the pH value of 7.2 with the largest desorption amount of 4.62 mg/g, and the desorption capacity of purple soil with the pH value of 6.0 was strongest with the largest desorption amount of 7.96 mg/g.
Isothermal adsorption equation simulation for purple soils with different pH values
The isothermal adsorption equa-tion simulation related parameters for different purple soils were shown in Table 1. As shown in Table 1, the adsorption kinetics of NH4+by purple soils showed better correlation with Freundlich equation (R >0.95), showed relatively good correlation with Temkin equation(0.72≤R≤0.89),but showed poor correlation with Langmuir equation(R<0.74).The correlation between adsorption curve of NH4+by purple soil with pH value of 7.2 and Langmuir equation was only 0.043. Therefore,in this study, the Freundlich equation was selected to describe the adsorption characteristics of NH4+by purple soils with different pH values.
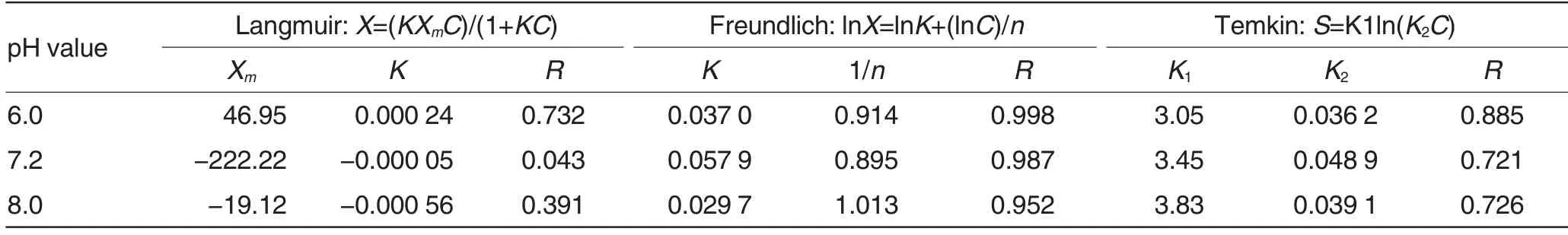
Table 1 Isothermal equation simulation for purple soils with different pH values
Discussion
Effects of purple soils with different pH vales on isothermal adsorption and desorption of NH4+
Fig.1 showed that the adsorption amounts of NH4+by purple soils with three different pH values ranged from 0.659 and 13.5 mg/g,and they were all increased with the increase of NH4+concentration.According to the chemical equilibrium theory, if the other conditions are remained unchanged,the larger the substrate concentration is, the easier the positive reaction is.The equilibrium will be distorted to the direction of production generation.Therefore, the larger the NH4+concentration is, the easier the adsorption is.Moreover, under the condition of high NH4+concentration, the probability of collision between NH4+and adsorbent surface will be increased[11].In addition,adsorbents all have a certain adsorption capacity for water. With the increase in original concentration, more ions enter into the interior of adsorbents until the adsorption saturation[12].Therefore, the adsorption amounts of NH4+by purple soils with three different pH values were all increased with the increase of NH4+concentration.
The largest adsorption amounts of NH4+by purple soils at pH 6.0,7.2 and 8.0 were 10.3, 12.8 and 13.5 mg/g,respectively. So it could be concluded that the adsorption amounts of NH4+by purple soils were also increased with the increase of pH value. This might be because that NH4+and H+have the same adsorption site. The changes in pH value will inevitably lead to the competition for adsorption sites between NH4+and H+. Under the condition of low pH,H+concentration is very high, so the anionic groups on adsorbents surface are prone to select H+.The lower the pH is, the more intense the competition is. So the soil with higher pH value can weaken the competition for adsorption site between NH4+and H+,increasing the adsorption amount of NH4+[13-16]. In addition, the changes in electrostatic potential and charge on adsorbent surface can also influence the effect of pH value on NH4+adsorption. The electrostatic potential on variable charge surface is reduced with the increase of pH value,increasing the negative charge amount on surface[17]. Therefore, the increased pH is conducive to the NH4+adsorption by soils.
As shown in Fig.2, the desorption amounts of NH4+by purple soils with three different pH values ranged from 0.115 and 7.96 mg/g, and they were basically increased with the increase of NH4+concentration, of which the fundamental principle was the same with that of increased adsorption amount. The largest desorption amounts of NH4+by purple soils at pH 6.0, 7.2 and 8.0 were 7.96, 4.62 and 2.23 mg/g, respectively. So it could be concluded that with the increase of pH value, the desorption amounts of NH4+by purple soils were reduced. This is because the adsorption and desorption of NH4+are essentially the adsorption of charges, of which the adsorption and desorption processes belong to diffusion process[18].The adsorption of NH4+by soil is influenced by anions on soil colloids surface. When the pH value of desorbing solution is too low,the H+, instead of NH4+, will bind with anions, resulting in increased NH4+concentration in low-pH solution[12].Thus, the lower the pH value is, the larger the desorption amount is.
Isothermal adsorption equation simulation for purple soils with different pH values
In the researches on adsorption characteristics of NH4+by soil,isothermal adsorption curve is often used,and it is a thermodynamic method.Isothermal adsorption curve refers to the relationship curve between the equilibrium concentration or activity of adsorbent in solution and amount of adsorbate on solid particle surface under the conditions of a constant temperature. Equilibrium adsorption isotherm equation can be used to quantitatively describe the distribution of ions in the solid phase[19]. The isotherm equations include Langmuir,Freundlich and Temkin.
As shown in Table 1, the isothermal adsorption of NH4+by purple soils showed a very good correlation with Freundlich equation (R >0.95). So in this study, the Freundlich equation was selected to describe the adsorption characteristics of NH4+. The adsorption constant K reflects the level of soil adsorption solutes to some extent.When the K value is positive,the reaction can proceed spontaneously at room temperature. The size of the K value reflects the spontaneous degree of adsorption reaction. The larger the K value is, the larger the spontaneous degree is;the larger the K value is,the more stable the products are; the larger the K value is, the stronger the adsorption capacity for solutes is. However, the larger the K value is, theweaker the solutes providing capacity is[20]. The K value of the purple soil at pH 7.2 was largest (0.057 9), followed by that at pH 6.0 (0.037 0),and the K value of the purple soil at pH 8.0 was smallest (0.029 7).So it could be concluded that the adsorption of NH4+by purple soils with three different pH values all could proceed spontaneously at room temperature. The spontaneous degree of purple soil at pH 7.2 was highest, followed by that at pH 6.0, and the spontaneous degree of purple soil at pH 8.0 was lowest.The n in Freundlich equation is used to indicate the size of nonlinearity of adsorption isotherms. When n= 1, the isotherms are liner[21].The closer the n value to 1 is, the better the linearity is[22].Generally, when the value of 1/n ranges from 0.1 and 0.5, the adsorption is considered to be easy, and when the value of 1/n >2, the adsorption is considered to be difficult[23-24]. In this study, the 1/n value of purple soil at pH 8.0 was largest(1.013),followed by that at pH 6.0 (0.914), and the 1/n value of purple soil at pH 7.2 was smallest(0.895).Although the 1/n values of purple soils with three different pH values were all not in the range of 0.1-0.5,they were all less than 2,indicating that the adsorption of NH4+by purple soils was all relatively easy.The 1/n value of purple soil at pH 8.0 was closest to 1, indicating the linearity of adsorption isotherm of purple soil at pH 8.0 was best.The 1/n value of purple soil at pH 6.0 ranked second. But the 1/n value of purple soil at pH 7.2 was most distant from 1,indicating the linearity of adsorption isotherm of purple soil at pH 7.2 was poorest.
Conclusions
In this study, the effects of different pH values on adsorption and desorption capacities of purple soil collected from Beibei District,Chongqing City are investigated. The results indicate that the adsorption amounts of NH4+by purple soils with three different pH values are all increased with the increase of NH4+concentration, and they are all increased with the increase of pH value;the desorption amounts of NH4+by purple soils with three different pH values are all increased with the increase of NH4+concentration, but they are all decreased with the increase of pH value;the correlation between the isothermal adsorption of NH4+by purple soils and the Freundlich equation(R >0.95) is highest, followed by that between the isothermal adsorption of NH4+by purple soils and the Temkin equation, and the correlation between the isothermal adsorption of NH4+by purple soils and the Langmuir equation was lowest.
[1]ZHANG Y(张炎), SHI JH(史军辉), LI P(李磐), et al. Nitrogen loss accesses in the soil and its effects on environment pollution (农田土壤氮素损失与环境污染)[J].Xinjiang Agricultural Sciences(新疆农业科学),2004,41(1):57-60.
[2]MA XH (马兴华), YU ZW (于振文),LIANG XF (梁晓芳),et al.Effects of nitrogen application rate and its basal-/topdressing ratio on spatial-temporal variations of soil NO3--N and NH4+-N contents (施氮量和底施追施比例对土壤硝态氮和铵态氮含量时空变化的影响)[J].Chinese Journal of Applied Ecology (应用生态学报),2006,17(4):630-634.
[3]JIANG GH (姜桂华). Discussion about NH4+-N adsorptive ability in soils(铵态氮在土壤中吸附性能探讨)[J]. Journal of Chang’an University (Arch. & Envir.Science Edition)(长安大学学报(建筑与环境科学版)),2004,21(2):32-34,38.
[4]NIJBOER RC, VERDONSCHOT PF.Variable selection for modelling effects of eutrophication on stream and river ecosystems[J]. Ecological Modelling,2004,177(1/2):17-39.
[5]MEYER-REIL LA,KÖSTER M.Eutrophication of marine waters: effects on benthic microbial communities [J]. Marine Pollution Bulletin, 2000 (41): 255-263.
[6]MILADINOVIC N, WEATHERLEY LR.Intensification of ammonia removal in a combined ion-exchange and nitrification column[J]. Chemical Engineering Journal,2008,135(1/2):15-24.
[7]ZHENG S,CHEN C,LI Y,et al.Characterizing the release of cadmium from 13 purple soils by batch leaching tests[J].Chemosphere, 2013, 91 (11): 1502-1507.
[8]WANG R (王茹), CHEN XY (陈晓燕),ZHOU J(周继).Study on variation characteristics of eroded sediment in purple soil steep slope land based on simulated rainfall (人工模拟降雨紫色土坡耕地土壤颗粒空间分布特征研究)[J].Journal of Southwest University (Natural Sciences)(西南大学学报 (自然科学版)),2012,34(8):59-65.
[9]XIE HM(谢红梅),ZHU B(朱波),ZHU ZL(朱钟麟). Research on the adsorption and desorption characteristics of ammonia and nitrate in purple soil (紫色土NH4+、NO3-的吸附-解吸特性研究)[J].Soils and Fertilizers(土壤肥料),2006,2:19-22.
[10]LU RK (鲁如坤).Agricultural Chemical Analysis Methods of the Soil(土壤农业化学分析方法)[M].Beijing:China Agricultural Science and Technology Press (北京: 中国农业科技出版社),2000:150-152.
[11]LIU H, DONG Y, WANG H, et al. Adsorption behavior of ammonium by a bioadsorbent-Boston ivy leaf powder[J]. Journal of Environmental Sciences-China, 2010, 22 (10): 1513-1518.
[12]MA Z, LI Q, YUE Q, et al. Adsorption removal of ammonium and phosphate from water by fertilizer controlled release agent prepared from wheat straw [J].Chemical Engineering Journal,2011,171(3):1209-1217.
[13]WANG FL, ALVA AK. Ammonium adsorption and desorption in sandy soils[J]. Soil Science Society of America Journal,2000,64(5):1669-1674.
[14]KARADAG D, KOC Y, TURAN M, et al. A comparative study of linear and non-linear regression analysis for ammonium exchange by clinoptilolite zeolite[J].Journal of Hazardous Materials,2007,144(1/2):432-437.
[15]MARA??N E, ULMANU M, FERN?NDEZ Y,et al.Removal of ammonium from aqueous solutions with volcanic tuff[J].Journal of Hazardous Materials,2006,137(3):1402-1409.
[16]SALTALI K, SARI A, AYDIN M. Removal of ammonium ion from aqueous solution by natural Turkish (Yildizeli)zeolite for environmental quality [J].Journal of Hazardous Materials, 2007,141(1):258-263.
[17]BARROW NJ. Testing a mechanistic model. IV. Describing the effect of pH on Zn retention by soils [J]. Soil Science,1986,37:295-302.
[18]WU H (伍华). Adsorption/desorption and transportation characteristics of main nutrient ions in different-texture soils(主要养分离子在不同质地土壤中的吸附-解吸及运移特性)[D]. Beijing:China Agricultural University (北京: 中国农业大学),2006.
[19]FU HM (付海曼), JIA LM (贾黎明).Study progress of nitrogen and phosphate adsorption & desorption in soils(土壤对氮、 磷吸附/解吸附特性研究进展)[J]. Chinese Agricultural Science Bulletin (中国农学通报),2009,25(21):198-203.
[20]XIA Y(夏瑶),LOU YS(娄运生),YANG CG (杨超光), et al. Characteristics of phosphate adsorption and desorption in paddy soils (几种水稻土对磷的吸附与解吸特性研究)[J].Scientia Agricultura Sinica (中国农业科学), 2002, 35(11):1369-1374.
[21]LIANG CS (梁重山),DANG Z (党志),LIU CQ (刘丛强), et al. Studies on sorption-desorption equilibria and hysteresis of phenanthrene by soil and sediment (菲在土壤/沉积物上的吸附-解吸过程及滞后现象的研究)[J]. Acta Pedologica Sinica(土壤学报),2004,41(3):329-335.
[22]ZHOU HB (周洪波), CHEN J (陈坚),QIU GZ ( 邱冠周), et al. Study on biosorption of pentachlorophenol on anaerobic granular sludge(五氯苯酚在厌氧颗粒污泥中的吸附研究)[J].Environmental Pollution&Control (环境污染与防治),2006,28(4):248-251.
[23]ZHANG LF(张丽芳),SUN YF(孙玉凤).Study on removal of alizarine red from water by freshly formed hydrous manganese dioxide (新生MnO2对茜素红吸附脱色的研究)[J]. Liaoning Chemical Industry (辽宁化工), 2006, 35(5):260-263.
[24]JIN XD(金晓丹), WANG DQ(王敦球),ZHANG H(张华),et al.Study on phosphate adsorption characteristics of eggshell(鸡蛋壳对磷的吸附特性研究)[J].Water Treatment Technology(水处理技术),2010,36(4):56-59.
猜你喜欢
杂志排行
Agricultural Science & Technology的其它文章
- Variation in Enzymes Activities of Rhizospheric Substrate and Influencing Factors during Nursing of Watermelon Seedlings
- Determination of Iprobenfos Residue in Rice by GC-FTD using Two-dim Ensional Purification
- In vitro Rapid Propagation of Ficus carica L.‘Masui Dauphine’
- Antioxidant Activity of Polysaccharides in Yam Bulbils and Their Hypoglycemic Effect in Diabetic Mice
- Study on Multiplication,Rooting and Transplanting of Tissue Culture Plantlets of Rhododendron chrysanthum Pall
- Inhibition of Chlamydospore Germination and Mycelial Growth of Trichoderma spp.by Chemical Fungicides
