Synthesis,Structure and Photoluminescence Properties of a two Dimensional CadmiumCoordination Polymer
2015-12-12WANGShujunLIUDongMAWei
WANG Shujun,LIU Dong,MA Wei
(College of Chemistry and Material Science,Huaibei Normal University,235000,Huaibei,Anhui,China)
Synthesis,Structure and Photoluminescence Properties of a two Dimensional Cadmium
Coordination Polymer
WANG Shujun,LIU Dong,MA Wei
(College of Chemistry and Material Science,Huaibei Normal University,235000,Huaibei,Anhui,China)
A novel two dimensional cadmium coordination polymer,formulated as{[Cd(1,3-PDA)(ppene)]·H2O}n(1)has been synthesized through hydrothermal reaction between Cd(NO3)2·4H2O and 1,3-phenylendiacetic acid(1,3-H2PDA)in the presence of 4-pyr-poly-2-ene(ppene).The complex was structurally characterized by infra⁃red spectroscopy,elemental analysis and single crystal X-ray diffraction.Single-crystal X-ray diffraction analysis shows that the compound belongs to the triclinic system with space group Pī and a=9.524 8(19),b=10.430(2),c= 1.138 9(2)nm,α=88.65(3)°,β=86.79(3)°,γ=69.70(3)°,V=1.059 5(3)nm3,Z=2,R1=0.030 4,wR2=0.074 8. CCDC number 1038885.Compound 1 exhibits a two dimensional(4,4)network.Photoluminescence properties of 1 was also investigated.
coordination polymer;structure;characterization;photoluminescence properties
1 Introduction
The crystal engineering of coordination polymers(CPs)has drawn great interest within chemistry and materials science in recent years mainly due to their intriguing structures and widespread applications in the fields of catalysis,opticals,magnetism,photoluminescence,ion exchange,gas storage,intercalation chemistry,photochemistry and so on[1-6].It is well known that multidentate organic ligands acting as linkers are con⁃firmed to play an important role in the construction of MOFs[3].Although some prospective structures with specific properties can be constructed based on a careful selection of the properties of the ligands,such as flexibility,shape,symmetry,length and substituent groups,it still remains a great challenge to rationally de⁃sign and construct the desired compounds by controlling the factors that affect their structures[4].Among the properties of a ligand,flexibility is very critical for the construction of a coordination polymer because the shape,symmetry and length of a ligand can be affected by its flexibility.Actually,the rigid ligands are prone to form coordination polymers with expected structures.However,the structures of coordination polymers con⁃structed from flexible ligands are not easy to predict since the flexible ligands can freely rotate and adopt a variety of conformations according to the restrictions imposed by the coordination geometry of the metal ions.
In view of the various ligands,dicarboxylic acids are found to be good candidates to build various MOFs with novel topologies and properties.Dicarboxylates are often employed as bridging ligands to afford coordina⁃tion polymers due to their diverse coordinative fashions,high stability and the ability to balance the positive charges of metal ions[5].On the other hand,dipyridyl ligands can be used as ancillary ligands,together with
the dicarboxylate ligands,to meet and satisfy the requirements for coordination geometries of metal centers in the assembly processes[6].By combining these O-donor and N-donor ligands with transition metal ions,for example,CdII,which possess d10configurations,the resulted coordination polymers can exhibit appealing struc⁃tures as well as photoluminescence properties.In this work,a flexible dicarboxylic acid 1,3-phenylendiacetic acid(1,3-H2PDA)was selected as a O-donor ligand to react with Cd(NO3)2and a conjugated dipyridyl li⁃gand 4-pyr-poly-2-ene(ppene).Finally,a novel two dimensional coordination polymer{[Cd(1,3-PDA)(ppene)]·H2O}n(1)was obtained.In this paper,we report the preparation,crystal structure and photolumi⁃nescence properties of the title complex.
2 Experiment
2.1 Materials and physical measurements
The dipyridyl ligand 4-pyr-poly-2-ene(ppene)was synthesized as reported previously[7].Other chemi⁃cals and solvents were purchased from Sigma-Aldrich Co.Infrared(IR)sample was prepared as a KBr pellet. The spectrum was measured by a Nicolet Avatar 360 FT-IR spectrophotometer in the range of 4 000-400 cm-1.The elemental analysis was characterized by an EA1110 elemental analyzer.The photoluminescence spectrum of compound 1 was measured on a JASCO FP-8600 spectrofluorometer.
2.2 X-Ray Data collection and structure determination
Single-crystal X-ray diffraction(SCXRD)data for compound 1 was collected on a Bruker SMART APEX II CCD diffractometer by using the Φ/ω scan technique.Absorption correction was handled with the SADABS program[8].The structure of compound 1 was refined with the SHELXL-97 program,based on full-matrix least-squares methods[9].The H atoms of the solvent water molecule in 1 was located from the Fourier map and included in the final refinement.All other H atoms were introduced at the calculated positions and includ⁃ed in the structure-factor calculations.
2.3 Preparation of{[Cd(1,3-PDA)(ppene)]·H2O}n(1)
To a 15 mL Pyrex glass tube was loaded Cd(NO3)2·4H2O(31 mg,0.1 mmol),ppene(21 mg,0.1 mmol),1,3-H2PDA(21 mg,0.1 mmol),and 4 mL of H2O.The glass tube was sealed and heated in an oven to 150℃for 3 d,and then cooled to ambient temperature at the rate of 5℃·h-1.Yellow block crystals were col⁃lected.Yield:40 mg(76%yield based on Cd).Anal.calcd for C24H22CdN2O5:C 54.30:H 4.18:N 5.28.Found:C 53.98 H 4.26:N 5.15.IR(KBr,cm-1):3 474 m,2 928 w,1 611 s,1 569 s,1 497 m,1 411 s,1 387 s,1 281 m,1 202 w,1 130 m,1 095 m,1 045 m,996 m,879 m,835 s,797 m,772 m,737 s,698 m,652 m,574 m,525 s,441 m.
3 Results and discussion
3.1 Crystal structure of{[Cd(1,3-PDA)(ppene)]·H2O}n(1)
Compound 1 belongs to the triclinic space group Pī.The asymmetric unit of this structure consists of one[Cd(1,3-PDA)(ppene)]unit and one solvent H2O molecule.Each Cd1 atom in 1 is seven-coordinated with a distorted pentagonal bipyramid environment.Five sites are occupied by the O atoms from three differ⁃ent 1,3-PDA ligands,while the remaining two sites are filled with the N atom from the ppene molecule(Fig.1a).Cd1 and its symmetry-related Cd1A atoms are bridged by four 1,3-PDA ligands to generate a di⁃nuclear[Cd2(1,3-PDA)4]unit.Each[Cd2(1,3-PDA)4]unit is interlinked via the 1,3-PDA bridges to its equivalent ones to form a[Cd(1,3-PDA)]nchain extending along the a axis(Fig.1b).Such a chain con⁃nects its adjacent ones through ppene bridges to form a 2D(4,4)layer of[Cd(1,3-PDA)(ppene)]nwith rectangular grids(0.952 5×1.633 5 nm2)extending in the ac plane(Fig.1c).The solvent H2O molecules were firmly anchored by the main framework of 1 via O5-H1W…O3 hydrogen bonding interactions(O5…O3,0.280 3(7)nm:O5-H1W…O3,166.9°).
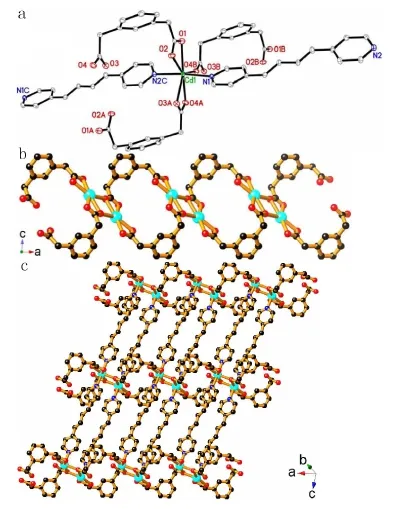
Fig.1 a The coordination mode of Cd1 in compound 1;b View of the 1D[Cd(1,3-PDA)]nchain in 1;c View of the 2D[Cd(1,3-PDA)(ppene)]nnetwork of 1.The nitrogen,oxygen and zinc atoms are represented by blue,red and cyan balls,respectively.Symmetry codes:(A)-x,-y,-z+1:(B)x+1,y,z:(C)x-1,y+1,z-1
3.2 Photoluminescence Properties
In the solid state,the photoluminescence spectrum of compound 1 was examined at room temperature(Fig.2).The ppene ligand displays photoluminescence with emission maxima at 549 nm(λex=430 nm),as⁃signed to π*→π transition of ppene[10].As reported previously,the 1,3-H2PDA ligand did not show any emission in the free state[11],so the 1,3-H2PDA ligand almost have no contribution to the fluorescent emis⁃sion of as-synthesized compound 1.The emission spectrum exhibit maximum emission peaks at 421 nm,un⁃der the exciation at 537 nm.According to the literature,the CdIIion is difficult to oxidize or reduce because of the d10configuration[10].Consequently,the emission of 1 may be assigned to a mixture characteristics of π*→π intraligand fluorescence since similar emission was also observed for the ppene ligand[10].
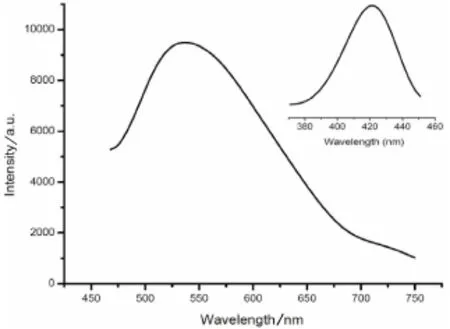
Fig2 Solid state emission and excitation spectrum of compound 1
[1] CARLUCCI L,CIANI G,PROSERPIO D M.Polycatenation,polythreading and polyknotting in coordination network chem⁃istry[J].Coord Chem Rev,2003,246:247-289.
[2] DU M,LI C P,LIU C S,et al.Design and construction of coordination polymers with mixed-ligand synthetic strategy[J]. Coord Chem Rev,2013,257:1282-1305.
[3]LU W,WEI Z,GU Z Y,et al.Tuning the structure and function of metal-organic frameworks via linker design[J].Chem Soc Rev,2014,43:5561-5593.
[4] YANG G P,HOU L,MA L F,et al.Investigation on the prime factors influencing the formation of entangled metal-organ⁃ic frameworks[J].Cryst Eng Comm,2013,15:2561-2578.
[5] LI H,ZHOU J,SHI W,et al.Structural diversity of four new metal-organic frameworks with a curved tetracarboxdiimide dicarboxylic acid[J].Cryst Eng Comm,2014,16:834-841.
[6] ZHANG X,NING Y,MENG L,et al.Constructing various metal-organic frameworks by mixed pyridine-acylamide and carboxylate ligands:ring-like or helical building blocks[J].Cryst Eng Comm,2014,16:7440-7451.
[7] LIU D,WANG H F,ABRAHAMS B F,et al.Single-crystal-to-single-crystal transformation of a two-dimensional coordi⁃nation polymer through highly selective[2+2]photodimerization of a conjugated dialkene[J].Chem Commun,2014,50:3173-3175.
[8] SHELDRICK G M.A program for the siemens area detector absorption correction[M].Göttingen:University of Göttingen,1997.
[9] SHELDRICK G M.Program for refinement of crystal structures[M].Göttingen:University of Göttingen,1997.
[10]MA W,WANG S J,GE Y,et al.Syntheses,structures,thermal and luminescent properties of two interpenetrating coordina⁃tion polymers constructed by 2,5-thiophenedicarboxylate and different dipyridyl ligands[J].Z Anorg Allg Chem,2014,640:2525-2531.
[11]WANG L,YANG M,LI G,et al.Highly stable chiral cadmium 1,2,4-benzenetricarboxylate:synthesis,structure,and NLO and fluorescence properties[J].Inorg Chem,2006,45:2474-2478.
一个二维镉配位聚合物的合成、结构及荧光性能研究
王淑军,刘 东,马 伟
(淮北师范大学 化学与材料科学学院,安徽 淮北 235000)
在水热条件下,一个新的二维镉配位聚合物{[Cd(1,3-PDA)(ppene)]·H2O}n(1)通过Cd(NO3)2·4H2O,1,3-phenylendiacetic acid以及4-pyr-poly-2-ene的反应而被合成.该化合物通过红外光谱,元素分析以及X-射线单晶衍射进行了表征.X-射线单晶结构分析表明,该化合物属于三斜晶系,Pī空间群,晶胞参数:a= 9.524 8(19),b=10.430(2),c=1.138 9(2)nm,α=88.65(3)°,β=86.79(3)°,γ=69.70(3)°,V=1.059 5(3)nm3,Z=2,R1=0.030 4,wR2=0.074 8.CCDC号为1038885.化合物1为一个二维(4,4)网格结构的网络.我们对该化合物的荧光性质也作了研究.
配位聚合物;结构;表征;荧光性质
O 614
A
2095-0691(2015)04-0025-04
2015-05-06
国家自然科学基金项目(21201068);安徽省自然科学基金项目(1208085QB26)
王淑军(1987- ),男,安徽濉溪人,硕士生,主要从事功能配合物的研究;通讯作者:马 伟(1977- ),男,安徽颍上人,副教授,主要从事分析化学和配位化学的研究.
O 614 Document Code:A Article ID:2095-0691(2015)04-0025-04
Received date:2015-05-06
Funded by:National natural science foundation project(21201068);Natural science foundation of Anhui province(1208085QB26)
Author:Wang Shujun(1987-),male,Anhui Suixi,postgraduate student,mainly engaged in the research of functional coordination compounds;Ma Wei(1977-),male,Anhui Yingshang,associate professor,mainly engaged in the research of analytical chemistry and coordination chemistry.
