Intervention effect of traditional Chinese medicine Yi Tang Kang on metabolic syndrome of spleen deficiency
2015-12-08XiaoXiLiuYanShiDepartmentofendocrinologyAffiliatedHospitalofLiaoningUniversityofTMCShenyang110032China
Xiao-Xi Liu,Yan ShiDepartment of endocrinology, Affiliated Hospital of Liaoning University of TMC, Shenyang 110032, China
Intervention effect of traditional Chinese medicine Yi Tang Kang on metabolic syndrome of spleen deficiency
Xiao-Xi Liu,Yan Shi*
Department of endocrinology, Affiliated Hospital of Liaoning University of TMC, Shenyang 110032, China
ARTICLE INFO
Article history:
Received 15 November 2014
Received in revised form 20 December 2014
Accepted 15 January 2015
Available online 20 February 2015
Spleen deficiency syndrome
Objective: To investigate effects of herbal compound Yi Tang Kang on the spleen deficiency metabolic syndrome. Methods: Forty male Wistar rats were randomly divided into two groups: the normal control group and the MS spleen deficiency syndrome group. The control group rats were fed with standard diet and water, while MS spleen deficiency syndrome group with high fat diet and low dose intraperitoneal injection of streptozocin, which swam to the endurance limit. After 12 weeks, the MS spleen deficiency syndrome group was randomly divided into two groups, with 13 rats in each group. Rats in model group were fed with high fat diet and continuouly administered with daily saline, and rats in intervention group with high fat diet were trated with traditional Chinese medicines Yi Tang Kang by gavage, 2 mL/200 g at the same time every day. 10 weeks later, the expression of serum proteomics was investigated through abdominal aortic puncture and separation of serum, using isotope labeling technique, high performance liquid chromatography and four bar-Orbitrap mass spectrometer. Results: After treatment with traditional Chinese medicine yitangkang, in the model group, important carboxylesterase and retinal guanylate cyclase 2 precursor were upregulated. As for intervention group, these indesxes were raised, but immunoglobulin IgG, carnitine acetyltransferase, tubulin beta -5, and Gan Lu sugar binding protein C were down-regulated. At the same time, some new biological active substances, such as protein tyrosine kinase, beta glucosidase were also found. Conclusions: Traditional Chinese medicines Yi Tang Kang could regulate glucose and lipid metabolism in rats with spleen deficiency syndrome.
1. Introduction
Metabolic syndrome (MS) , due to obesity (especially abdominal type), damaged glucose regulation or type 2 diabetes, hypertension and dyslipidemia, insulin resistance, microalbuminuria, and high uric acid hematic disease, causes a variety of pathological and physiological change in material, sugar, fat and protein metabolism, promotes atherosclerosis, eventually leads to a variety of disease of heart head blood-vessel and development of clinical syndrome, also known as X syndrome or the insulin resistance syndrome. Due to the high incidence rate of MS and its close correlation with cardiac-cerebral vascular disease, MS becomes the hot spot in the medical research[1]. Herbal compound Yi Tang Kang could be effective for the treatment of spleen deficiency metabolic syndrome, but its mechanism still needs to be discussed.
This study investigated effect of Yi Tang Kang on the serum proteomics in rats with the metabolic syndrome of spleen deficiency.
2. Materials and methods
2.1. Experimental animals
Forty male wistar rats with weight (220±20) g, at the clean level, were provided by Liaoning Immortality Biological Technology Co., LTD. Feed were purchased from the Qianmin
Animal Feed Factory (China). Feed nutrition guarantee value (%) was shown in Table 1. High-fat feed ingredients included corn oil 8%, sugar 6%, egg yolk powder 5%, cholesterol 1.5%, pig bile salt 0.2%, sulfur methyl oxygen pyrimidine 20 g, sodium glutamate (principle) 1%, and 78.3% of basic feed.

Table 1 Basic feed ingredients.
2.2. Experimental drug, reagents, instruments and equipment
The herbal compound Yi Tang Kang[2] included sugar, poria cocos, atractylodes, radix astragali, red ginseng and other drugs, purchased from Liaoning Chinese Traditional Medicine University Affiliated Hospital. After decoction, it was concentrated and kept at 4 ℃.
Cholesterol, and pig bile salts were purchased from National Medicine Group Chemical Reagent Co., LTD. Citric acid and sodium citrate were purchased from the National Medicine Group Chemical Reagent Co., LTD.; Chain urea with cephalosporins (STZ) was purchased from Sigma.
0.1 mol/L sodium citrate buffer (pH 4.4) included citric acid 2.1 g and 2.94 g sodium citrate, respectively, with sodium chloride injection solution to 100 mL. Citric acid solution 28 mL, 22 mL sodium citrate solution were mixed thoroughly and set aside at 4 ℃. Streptozocin (STZ solution) included 0.1 mol/L sodium citrate buffer solution.
ITRAQ kit (ABI); The pancreatic enzyme (Trypsin) was purchased from Promega Company; Protein concentration detection kit (Bradford); Tris (BBI); NaF (Fluka); Organization cracking liquid used benjia amidine (Benzamidine) and 4-(2 aminoethyl) benzene sulfonyl fluoride hydrochloride (AEBSF) were purchased from Sigma; Bright enzyme inhibitory peptide (leupeptin) and aprotinin were purchased from Shanghai Biological Engineering Co., LTD. Strong cation exchange column for Polysulfoethylcolumn (size 5 μm, aperture 20 nm, 10 cm in length, diameter 2.1 mm, The Nest Group Inc. products); C18 (30 nm size 5 μm, aperture, length of 15 mm, inner diameter of 0.1 mm, Agilent products was used for reversed phase column of the ZORBAX sb-300. Electronic balance (Dalian Xinghai Electronic Weighing Apparatus Co., LTD MODELDS-671); Automatic biochemical analyzer (Japan's Hitachi 7600); Centrifuge table high speed (Xiang Instrument Centrifuge Instrument Co., LTD TG16-WS); Automatic glucose meter (J&J Steady Hao times optimal type); Digital thermometer (omron MC-612); Speed centrifuge at low temperature: Beckman Company; High performance liquid chromatograph: RIGOL model RIGOL 3220 Beijing Science And Technology Co., LTD; Level 4-electrostatic field orbit trap mass spectrometer: model Q-Exactive's Fly The World's Science And Technology Co., LTD; Mass spectrometry data analysis using ProteinPilot software 3.0: ABI.
2.3. Establishment ofMS rats model with spleen deficiency
All the rats were caged, 3-4 rats in a cage, and were randomly divided into the normal group of 10 rats and the MS spleen deficiency group of 30 rats. Normal control group was fed with standard rat feed and water, while rats in MS spleen deficiency group swam (swim every day 5 minutes) to the endurance limit and were given with high-fat diet. Four weeks after fasting for 12 hours, intraperitoneal injection of STZ was applied. Lower dose injectionwas performed for many times, with the first dose as 30 mg/kg, and reduction of 10 mg/kg each time (injection once a week for 6 weeks). They were fed every two weeks during a blood glucose measurement, and blood lipid [triglyceride (TG), high density lipoprotein (HDL-C)] were measured once every four weeks,for 12 weeks. At the same time independent t test was used in statistical analysis, and when blood sugar, blood lipid, and body weight showed statistically significant difference, serum insulin, insulin sensitive index (ISI), insulin resistance (IR), and Homa ss-cell function index (HBCI) were measured.
Metabolic comprehensive evaluation criteria of syndrome included weight gain; increased fasting plasma glucose (8-16.7 tendency/L); dyslipidemia: higher level of TG and (or) HDL-C in fasting blood; insulin resistance [decreased insulin sensitivity index (ISI), reduced homa beta cell function index (HBCI), insulin resistance (IS)]. Occurrence of three or all of the above symptoms could be diagnosed with MS.
2.4. Drug intervention
MS model had been established successfully, and the blood glucose and blood lipid of all rats were measured before treatmen.Then diabetic rats were randomly divided into control group and intervention group. According to the weight, rats in model group and intervention group had
gavage administration at 2 mL/200 g at a time; while rats in control group were administrated with the same amount of 0.9% sodium chloride injection 2 mL/200 g a day for 10 weeks.
2.5. Determination of serum proteomics
2.5.1. iTRAQ labeling
High abundance protein of serum samples were removed, and were freeze-dryied. Those protein samples were added with 40 mu L cracking fluid and 2 mu L denaturant, the oscillation suspension was dissolved and sedimentation was centriguged. It was quantitatively analyzed by the Bradford method, according to the solution of the protein concentration.
It was reduced and closed according to kit. 1 μg pancreatic enzyme was added in each group at 37 ℃ for 16 h for enzymatic hydrolysis. Two marked reagents were diluted by 50 μL propofol, and mixed respectively with corresponding sample, and then marked, model group samples with 114 mark, and intervention group with 113 mark. They were placed at room temperature for 1 h, added with 100 μL deionized water for inactivation. Samples were mixed and freeze-dried.
2.5.2. Peptides separation
The chromatographic column: C18 reverse phase column (Agela, C18 chromatographic column, 250 mm×4.6 mm I.D., filler particle diameter, including 5 m).
Mobile phase: A: 2% ACN-98% H2O (ammonia adjusted pH 10.0); ACN mobile phase B: 98%-2% H2O (ammonia adjusted pH 10.0);
Solvent gradient: 5%-8% B, 1 min; 8%-32% B, 24 min; B, 32% 2 min; 95%, 4 min. 95%-5% B, 1 min; Column temperature, 45 ℃; Flow rate: 0.7 mL/min; detection wavelength 214 nm.
With a tube per minute, from 8% to 32% effective gradient, a total of 24 components were obtained by high performance liquid chromatograph RPRP separation, fractions were collected, divided into 24 distillates, then were vacuum dried.
2.5.3. Mass spectrometry analysis
Twenty-four fractions were combined into eight samples, using A fluid on l c/mass spectrometer (Q-Exactive). Dry samples were dissolved in A liquid (1.9% ACN/1.9% H2O/1.9% FA), centrifugated at 12 000 rpm for 3 min on the EASY-nLC-1000 liquid with ThermoFisher Q Exactive mass spectrometer.
The chromatographic conditions were as follows:
Liquid phase: EASY-nLC-1000; Enrichment column: homemade C18, 5 μm, ID100 μm, 20 mm Length; Separation column: homemade C18, 3 μm, ID75 μm, 120 mm in length; Mobile phase: A, H2O+98% FA+98%-1.9% ACN; B, 98%-1.9% ACN + H2O + 98% FA; Flow rate: 450 nL/ min. Elution conditions were time 0, 3% B; time 24, 16% B; time 30, 30% B; time 31, 90% B; time 38, 90% B.
Mass spectrometry conditions were as follows: data collection time was 38 min, spray voltage was 2.0 KV; Capillary temperature was 320 ℃. Collision energy was 30; Acquisition quality range was 300-1 400 da.
2.5.4. Library search and analysis
Proteome Discoverer (version: 1.2) was used to search library, search engine for the built-in mascot, database for Rattus library. Error level was 15 ppm, secondary error wasr 20 mmu. All components were merged to search library. Grade appraisalof protein was performed according to the software.Reporting threshold was 1.5, the corresponding protein of false positive rate was 5%. Software on the basis of isotopic reports quantified the relative content of protein, m/z 117 for reference, the result of the significant difference was chosen (P<0.05). Cluster 3.0 software was used for hierarchical clustering analysis of protein expression patterns. Protein annotation and classification were performed by using DAVID functional annotation. Cell components and functional annotation of protein molecules was classified, KEGG pathways database was used to classify protein pathways involved.
2.6. Statistics analysis
IBM SPSS Statistics18.0 package was used for statistical analysis. Groups of experimental data were applied with mean±standard deviation. The differences between each group were analyzed by using single factor analysis of variance. P<0.05 was considered as significant difference.
3. Results
3.1. Basic situation of experimental animals
No rat died in the process of the whole experiment. During feeding process, rats from MS spleen deficiency group drank significantly more water than that of rats from the normal group, and also had more urine. Two weeks after feeding, glucose and lipid level of rats of model group and
intervention group were higher than those of control group, and the increase was more significant with increasing feeding tim. After treatment with Yi Tang Kang, no obvious abnormality was found. According to the model assessment standard, a total of 26 models of rats had been established successfully, and the success rate was 86.7%.

Table 2 Changes of fasting plasma glucose (mmol/L).
3.2. Blood glucose, lipid and weight change before treatment
There was no significant differences in blood glucose, blood lipid, and weight between control group (n=10) and model group (n=30) before treatment (P>0.05).
As shown in Table 2, compared with control group, weight and fasting glucose of model group was increased more significantly, and from the beginning of the 4th week, the difference was significant between the two groups (P<0.05). As shown in Table 3, compared with control group, TG and HDL-C level of model group was increased more significantly, and from the beginning of the 8th week, difference was significant between the two groups (P<0.05).

Table 3 Changes of TG (mmol/L).
3.3. Insulin level, IR, ISI, and HBCI changes before treatment
As shown in Table 4, serum insulin level and IR of model group were significantly higher compared with control group (P<0.05). ISI and HBCI were significantly lower in model group (P<0.05).
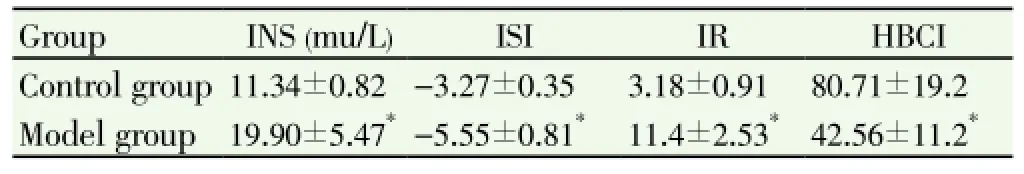
Table 4 Changes of Serum INS and ISI, IR, HBCI rats.
3.4. Changes in glucose and lipid
Compared with that of model group, fasting glucose and TG were significantly lower in intervention group from the beginning of 6th week,while HDL-C level was significantly higher (P<0.05) (Table 5).

Table 5 Changes of fasting plasma glucose, TG and HDL-C (mmol/L).
3.5. Insulin and ISI, and HBCI changes
As shown in Table 6, serum insulin in rats of intervention group was decreased significantly, compared with model group. ISI and HBCI were increased significantly, while IRS was decreased significantly (P<0.05).

Table 6 Changes of serum insulin , ISI, IR and HBCI.
3.6. Serum proteomics changes after the intervention
A total of 44 different proteins were selected, including 21 proteins, which were up-regulated more than 1.5 times, and 23 proteins, which decreased more than 0.75 times. Carboxylesterase, tyrosine protein kinase, retina guanylate cyclase acid precursors 2, immunoglobulin IgG, carnitine acetyltransferase and tubulin beta 5, mannose binding protein C, beta-glycosidase enzymes. carboxylesterase, tyrosine protein kinase, retinal guanylate cyclase acid 2 precursor were up-regulated, and immunoglobulin IgG, carnitine acetyltransferase
and tubulin beta 5, mannose binding protein C, betaglycosidase enzymes were down-regulated. (Table 7&8).

Table 7 Up-regulated proteins at more than 1.5 times.
4. Discussion
MS is a kind of syndrome represented with high blood pressure, glucose and lipid metabolism abnormality, higher low density lipoprotein and lower HDL cholesterol. It is a complex metabolic disorder syndrome. Tt is a risk factor for diabetes mellitus and cardiovascular disease, and its cluster occurrence may be associated with IR[3]. Its pathogenesis is very complex, and may be associated with interaction between genetic factors and environmental factors. It has been confirmed that its core base for the disease was insulin resistance[4-6]. According to traditional Chinese medicine theory, the spleen deficient is the key pathogenesis of MS, therefore, in the treatment of MS, sufficient attention must be paid to the application of the spleen and replenishing method. It has been demonstrated that traditional Chinese medicine compound Yi Tang Kang, could improve the high insulin hematic disease, and play the roles in lowering blood sugar and adjusting blood fat.
In this study, by using the proteomics method, it shows that after treatment with Yi Tang Kang in rats of MS with spleen deficient, a total of 44 differently expressed proteins were found, including 21 proteins upregulated more than 1.5 times, and 23 proteins downregulated more than 0.75 times. More importantly, the carboxylesterase and retina guanylate cyclase acid 2 precursor were up-regulated, and immunoglobulin IgG, carnitine acetyltransferase, tubulin beta 5, and mannose binding protein C levelswere downregulated, and there were new bioactive substances, such as protein tyrosine kinase, and beta glycosidase enzymes.
Tyrosine protein kinases are a set of catalytic tyrosine residues phosphorylation enzymes, which can launch multiple downstream signaling pathways, such as the Ras/ Raf/MAPK pathway and JAK-STAT[7,8]. Src tyrosine protein kinase is a proto-oncogene tyrosine kinase, and is a member of the family of Src kinase[9], which is involved in antigen antibody, cytokine and integrin receptor mediated transmembrane signal transduction, and plays an important role in cell differentiation, proliferation and transformation of regulation[10-12]. Recent studies suggest that the Src tyrosine protein kinase plays a significant role in the process of the occurrence of hypertension, and its mechanism includes REDOX, coupling factor 6, slow excitation signal peptide, vascular endothelial growth factor and transforming growth factor beta 1, etc., which make the structure and function of the angiogenesis changes, and play an important
role in cardiovascular diseases, especially hypertension and stroke[13,14]. Vascular endothelial growth factor (VEGF) is a kind of endothelial cell specific mitogen[15]. VEGF can promote angiogenesis and increase vascular permeability and diastolic blood vessels, which suggests that VEGF can reduce vascular tension and blood pressure in the process of high blood pressure[16]. Clinical trials have proved that VEGF signaling pathway inhibitors can lead to high blood pressure[17,18]. The present study suggests VEGF induced NO and PGI-2, VEGF can increase the synthesis of NO more than 50 times, improve PGI-2 the synthesis by 3- 4 times, the synergy of NO and PGI-2 mediated the VEGF on vascular endothelial function. And in this process, the activation of Src tyrosine protein kinase is required. These studies suggest that the Src tyrosine protein kinase is involved in the NO and PGI-2 generation induced by VEGF, and also suggest that the Src tyrosine protein kinase is involved in vascular endothelial vascular tension, the process of diastolic blood vessels and blood pressure[19,20].
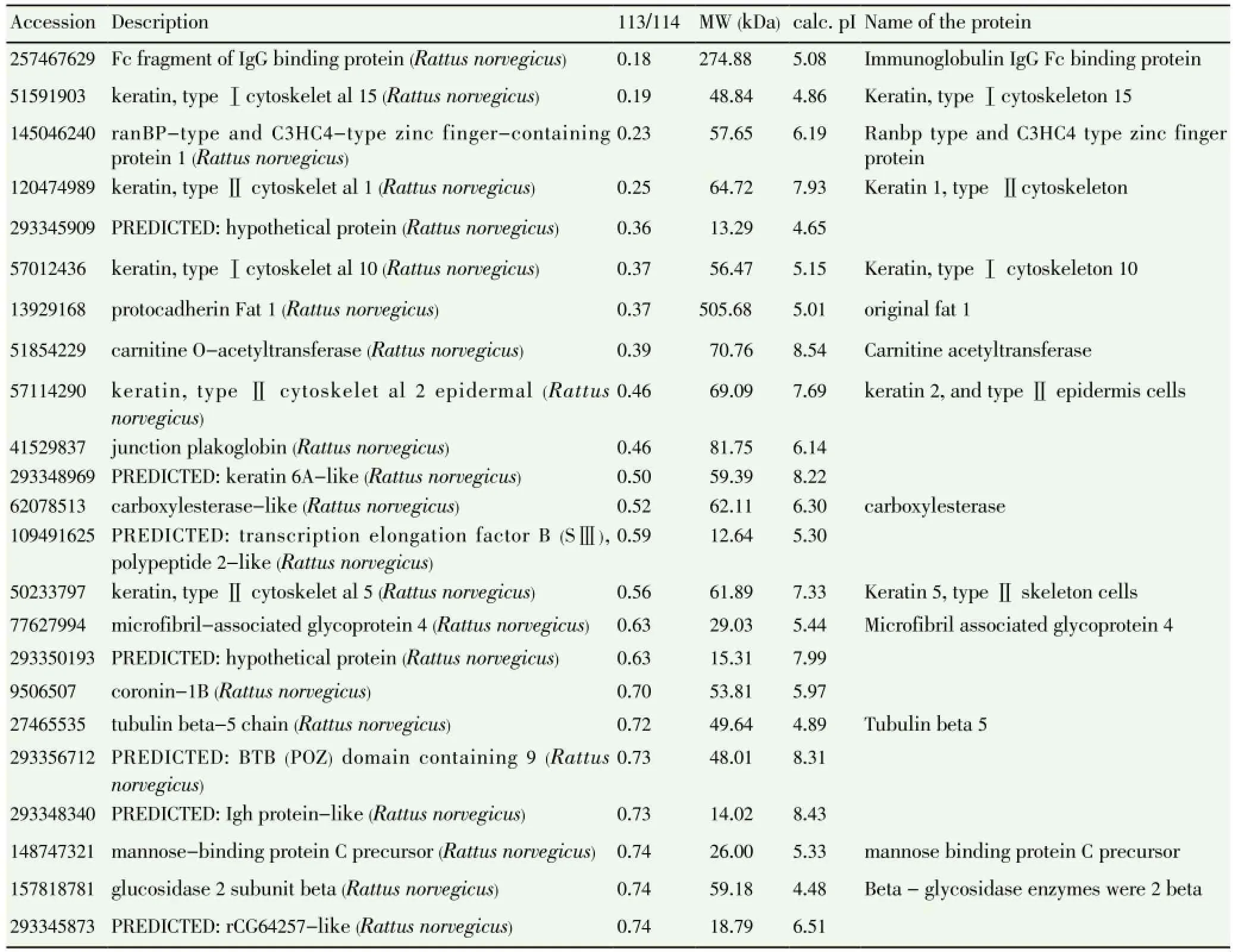
Table 8 Down-regulated proteins at more than 0.75 times.
Beta-glycosidase enzymes (beta glucosidase, EC3.2.1.21), also known as beta- D-glycosidase glucose hydrolytic enzymes, can hydrolysis beta-D-glucoside keys, and can release beta-D-glucose and corresponding ligands at the same time. In 1837, Liebig and Wohler found beta glycosidase enzymes in bitter almond for the first time[21,22]. It is involved in the glucose metabolism of the organism, and plays an important role for organisms in maintaining normal physiological function. It was found that the beta glycosidase enzymes were involved in EMP glycolytic pathway, and it is one of enzymes related to the sugar metabolism in bifidobacterium[23]. In mammals and human body, lactase/root skin glycosides (LPH) hydrolytic enzymes contain aryl beta glycosidase enzymes, and LPH involved in the lactase deficiency disease-a common human genetic disorder, which has been widely studied. It is closely related with many diseases caused by metabolic disorders, such as diabetes, cancer and virus infection.
In conclusion, this studyproved thatYi Tang Kang can improve IR, lopid and glucose metabolism. By methods of proteomics protein changes are found after drug
intervention, which suggested that tyrosine protein kinase, beta-glycosidase enzyme proteins could be therapeutic targets, and it provided new treatment of MS with spleen deficiency.
Conflict of interests
We declare that we have no conflict of interest.
[1] Stroke, coronary heart disease (CHD) risk factors further research collaboration. China's 11 provinces of MS epidemiological investigation. Chin J Prev Med 2002; 4(50): 298.
[2] Pharmacopoeia Commission of the People's Republic of China. The Chinese pharmacopoeia. Chemical Industry Press; 2000, p. 257-259.
[3] Momma H, Niu K, Kobayashi Y, Huang C, Chujo M, Otomo A, et al. Higher serum soluble receptor for advanced glycation end product levels and lower prevalence of metabolic syndrome among Japanese adult men: a cross-sectional study. Diabetol Metab Syndr 2014; 6(1): 33.
[4] Huang WH, Heng XP. The theory and practice research of diabetes inflammatory state. J Liaoning Univ Trad Chin Med 2008; 10(2): 38-39.
[5] Liu L, Ping Z, Li L. Power and the cutoff value of waist-to-height ratio predicting metabolism syndrome. Wei Sheng Yan Jiu 2012; 41(6): 992-996.
[6] Wöhler P, Hirl B, Kellermann W. Case report-cerebral fat metabolism syndrome after bilateral femoral fracture. Anasthesiol Intensivmed Notfallmed Schmerzther 2013; 48(5): 300-302.
[7] Liu Q, Wong-Riley MT. Postnatal development of brain-derived neurotrophic factor (BDNF) and tyrosine protein kinase B (TrkB) receptor immunoreactivity in multiple brain stem respiratoryrelated nuclei of the rat. J Comp Neurol 2013; 521(1): 109-129.
[8] Parthibane V, Iyappan R, Vijayakumar A, Venkateshwari V, Rajasekharan R. Serine/threoninerosine protein kinase phosphorylates oleosin, a regulator of lipid metabolic functions. Plant Physiol 2012; 159(1): 95-104.
[9] Luo W, Slebos RJ, Hill S, Li M, Brábek J, Amanchy R, et al. Global impact of oncogenic Src on a phosphotyrosine proteome. J Prot Res 2008; 7(8): 3447-3460.
[10] Brown MT, Cooper JA. Regulation, substrates and functions of Src, A thorough review of the biochemical properties of Src. Biochim Biophys Acta 1996; 1287: 121-149.
[11] Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases, A comprehensive review of the physiology and substrates of Src and Src-family kinases. Annu Rev Cell Dev Biol 1997; 13: 513-609.
[12] Frame MC. Src in cancer: deregulation and consequences for cell behaviour. Biochim Biophys Acta 2002; 1602: 114-130.
[13] Huang R, Fang P, Kay BK. Isolation of monobodies that bind specifically to the SH3 domain of the Fyn tyrosine protein kinase. N Biotechnol 2012; 29(5): 526-533.
[14] Adame J, Adame H. Plantas curativas del Noreste Mexicano. Ediciones Castillo, México; 2000. Chun-Jen Lin C, Summerville JB, Howlett E, Stern M. The metabotropic glutamate receptor activates the lipid kinase PI3K in Drosophila motor neurons through the calcium/calmodulin-dependent protein kinase Ⅱand the nonreceptor tyrosine protein kinase DFak. Genetics 2011; 188(3): 601-613.
[15] Wang XZ, Jiang JY, Lu JF. Study in the damage of endothelial function and administration recovery among different arteries during the developing progress of SHR. Chin Pharmacol Bull 2006; 26(2): 163-168.
[16] Morbidelli L, Chang CH, Douglas JG, Granger HJ, Ledda F, Ziche M. Nitric oxide mediates mitogenic effct of VEGF on coronary venular endothelium. Am J Physiol 1996; 270: H411-415.
[17] He H, Venema VJ, Gu X, Venema RC, Marrero MB, Caldwell RB. Vascular endothelial growth factor signals endothelial cell production of nitric oxide and prostacyclin through flk-1/KDR activation of c-Src. J Biol Chem 1999; 274(35): 25130-25135.
[18] Janku F, Garrido-Laguna I, Petruzelka LB, Stewart DJ, Kurzrock R. Novel therapeutic targets in non-small cell lung cancer. J Thorac Oncol 2011; 6(9): 1601-1612.
[19] Danza K, Pilato B, Lacalamita R, Addati T, Giotta F, Bruno A, et al. Angiogenetic axis angiopoietins/Tie2 and VEGF in familial breast cancer. Eur J Hum Genet 2013; 21(8): 824-830.
[20] Metsuyanim S, Levy R, Davidovits M. Molecular evaluation of circulating endothelial progenitor cells in children undergoing hemodialysis and after kidney transplantation. Pediatr Res 2009; 65(2): 221-225.
[21] Nakabayashi M, Kataoka M, Mishima Y, Maeno Y, Ishikawa K. Structural analysis of β-glucosidase mutants derived from a hyperthermophilic tetrameric structure. Acta Crystallogr D Biol Crystallogr 2014; 70(Pt 3): 877-888.
[22] Kurniasih SD, Alfi A, Natalia D, Radjasa OK, Nurachman Z. Construction of individual, fused, and co-expressed proteins of endoglucanase and β-glucosidase for hydrolyzing sugarcane bagasse. Microbiol Res 2014; S0944-5013(14): 17-22.
[23] Peng ZY. Food biotechnology. Beijing: China Light Industry Press; 1999.
ment heading
10.1016/S1995-7645(14)60309-6
*Corresponding author: Yan Shi, M.D., Supervisor of Doctoral Students, Department of endocrinology, Affiliated Hospital of Liaoning University of TMC, Shenyang 110032, China.
E-mail: shiyan@lnutcm.edu.cn
Foundation project: This work was supported by grants from National Nature Science Foundation of China (No. 30672767).
Yi Tang Kang
Proteomics
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Effect of interferon plus ribavirin therapy on hepatitis C virus genotype 3 patients from Pakistan: Treatment response, side effects and future prospective
- Imported cases of dengue fever in Russia during 2010-2013
- Detection and characterization of Chlamydophila psittaci in asymptomatic feral pigeons (Columba livia domestica) in central Thailand
- Chemical composition of Rosmarinus and Lavandula essential oils and their insecticidal effects on Orgyia trigotephras (Lepidoptera, Lymantriidae)
- Total phenolic content, in vitro antioxidant activity and chemical composition of plant extracts from semiarid Mexican region
- Cytoprotective and anti-inflammatory effects of kernel extract from Adenanthera pavonina on lipopolysaccharide-stimulated rat peritoneal macrophages
