Total phenolic content, in vitro antioxidant activity and chemical composition of plant extracts from semiarid Mexican region
2015-12-08JorgeWongPazJuanContrerasEsquivelRaRodrguezHerreraMarCarrilloInungarayLluviapezGuadalupeNevrezMoorillCristbalAguilar
Jorge E. Wong-Paz, Juan C. Contreras-Esquivel, Raúl Rodríguez-Herrera, María L. Carrillo-Inungaray, Lluvia I. López, Guadalupe V. Nevárez-Moorillón, Cristóbal N. Aguilar*
1Laboratory of Bioprocesses and Natural Products, Food Research Department, School of Chemistry, Universidad Autónoma de Coahuila, Saltillo, 25280, México
2Laboratory of Biochemistry, Multidisciplinary Academic Unit-Huasteca, Universidad Autónoma de San Luis Potosí, 79060, Cd. Valles, S.L.P., México
3Department of Microbiology, School of Chemistry, Universidad Autónoma de Chihuahua, 31125 Chihuahua, Mexico
Total phenolic content, in vitro antioxidant activity and chemical composition of plant extracts from semiarid Mexican region
Jorge E. Wong-Paz1, Juan C. Contreras-Esquivel1, Raúl Rodríguez-Herrera1, María L. Carrillo-Inungaray2, Lluvia I. López1, Guadalupe V. Nevárez-Moorillón3, Cristóbal N. Aguilar1*
1Laboratory of Bioprocesses and Natural Products, Food Research Department, School of Chemistry, Universidad Autónoma de Coahuila, Saltillo, 25280, México
2Laboratory of Biochemistry, Multidisciplinary Academic Unit-Huasteca, Universidad Autónoma de San Luis Potosí, 79060, Cd. Valles, S.L.P., México
3Department of Microbiology, School of Chemistry, Universidad Autónoma de Chihuahua, 31125 Chihuahua, Mexico
ARTICLE INFO
Article history:
Received 15 November 2014
Received in revised form 20 December 2014
Accepted 15 January 2015
Available online 20 February 2015
Plant-extracts
Polyphenols
Natural antioxidants
Mexican plants
Heat-reflux system
Objective: To determine the extraction suitable conditions of total phenolic content (TPC) by heat-reflux system, antioxidant activities and HPLC characterization of the aqueous-ethanolic extracts of Jatropha dioica (J. dioica) (Dragon's blood), Flourensia cernua (F. cernua) (Tar bush), Eucalyptus camaldulensis (E. camaldulensis) (Eucalyptus) and Turnera diffusa (T. diffusa) (Damiana). Methods: TPC was evaluated by the well-known colorimetric assay using Folin-Ciocalteu reagent. The antioxidant activities were assayed by three methods based on scavenging of DPPH, ABTS and by lipid oxidation inhibition. The chemical composition of the extracts obtained was subject to HPLC analysis. Results: TPC in the plant extracts ranged from 2.3 to 14.12 mg gallic acid equivalents/g for J. dioica and E. camaldulensis, respectively. The plant extracts of F. cernua, E. camaldulensis and T. diffusa showed similar strong antioxidant activities on scavenging of DPPH and lipid oxidation inhibition. In contrast, J. dioica extracts had lowest potential antioxidant in three assays used. HPLC assay showed the presence of several phenolic compounds in the extracts used. Conclusions: The results obtained suggest that F. cernua, E. camaldulensis and T. diffusa are potential sources to obtain bioactive phenolic compounds with high antioxidant properties which can be used in the factories as antioxidant agents or for treatments in diseases.
1. Introduction
Recently synthetic products are being restricted in the industries, because of harmful effects observed such as human toxicity and environmental pollution[1] and besides have been reported to be carcinogenic[2,3]. Additionally, the new demands by the consumers which pressure on the industries particularly for safer products are presented[1,4].
From this point of view, an increasing tendency towards the use of natural products instead of synthetic products has been observed in a high demand for food, cosmetics and pharmaceutical manufacturers. For thousand years the plant natural products have been used in the medicine, cosmetics, nutrition and flavoring without or less harmful effects. Thus, plant extracts appear to be a feasible alternative for this problem and the industries have put the attention in the bioactive phytochemicals present in the plants.
Generally, phytochemicals present in the plant extracts are nontoxic, effective at low concentrations, low cost and friendly with the environment. Besides, recent studies have shown that the ingestion of vegetables, fruit and herbs is associated with prevention of some bactericidal, antiviral, analgesic, anti-inflammatory and anti-carcinogenic disorders, due to their antioxidant activities[5-7]. In the food industry, phytochemicals are interesting due to these compounds retard oxidative degradations of lipids, improve
the food quality and nutritional value[5] and contributing to prevent microbial deterioration[4,8-10].
Plant extracts consist in a complex mixture of several compounds as alcohols, esters, aldehydes, ketones, carbohydrates, terpenes, polyphenols, etc[11]. In addition, crude extracts, purified fractions and pure compounds have already been used in an antioxidant approaches[12] and several responses have been obtained. Generally, there is not compound or extract that can be used as universal antioxidant. However, nowadays is necessary search new sources and compound of specific antioxidants for determined objectives. Phenolic compounds are commonly reported to have the most antioxidant activity[13].
In this context, Mexico is an attractive country for its large endemic plant variety. In the semiarid regions of Mexico several plants take part of a great source of antioxidative compounds, mainly because their ability to grown under extreme climatic conditions[13,14]. This kind of plants have developed in most cases a pool of chemical compounds produced as secondary metabolites as environmental defense mechanism. Chemical compounds of semiarid region plants has been well studied to develop potential agents against certain pathogenic and phytopatogenic microorganisms present in the food and agricultural industries[4,10,13-16]. In addition, semiarid region endemic plants are commonly used in the Mexican traditional medicine. Among the plants most used are: Jatropha dioica (J. dioica) (Dragon's blood), Flourensia cernua (F. cernua) (Tar bush), Eucalyptus camaldulensis (E. camaldulensis) (Eucalyptus) and Turnera diffusa (T. diffusa) (Damiana).
J. dioica has been used as analgesic in toothache and T. diffusa as aphrodisiac[17]. On the other hand F. cernua is widely used to treat diarrhea, rheumatism, venereal disease, sores, bronchitis, chicken pox and common cold[15,18]. Nevertheless, actually there is a lack of knowledge about some plants that have not been extensively studied and neither theirs phytochemical compounds which could be used as antioxidants on control of certain diseases or in the food industry. Therefore, the purpose of this work was to evaluate the extraction conditions, antioxidant potential and chromatographic profiles (HPLC) of extracts from J. dioica, F. cernua , T. diffusa and E. camaldulensis.
2. Materials and methods
2.1. Plant collection
Plant materials were proportioned by the company Fitokimica Industrial de Mexico SA de CV (Table 1). The plants were transported to the Department of Food Research at Universidad Autonoma de Coahuila, in black plastic bags. Immediately the samples were dried for 24-48 h at 60 ℃ in an oven (LABNET International, Inc.). The dry samples were ground in a mechanical mill and screened at 0.6-0.8 mm size particle. The fine and dried powder was stored in black plastic bags with sealed hermetically and at room temperature under darkness.

Table 1 Source and tissue of the plants used.
2.2. Preparation of the plant extracts
Phenolic extracts were obtained by heat-reflux extraction system evaluating two independent factors. It was necessary to select the best extraction time to assure the complete TPC extraction. Then, the first factor evaluated was the extraction time of heat-reflux. Mixtures of water and ethanol were used because it is well known the phenolic compounds are soluble in polar solvents as water and hydroalcoholic solutions, in addition, ethanol is a safe solvent (FDA 2012). However pure water is not the best solvent for phenols extraction[19,20]. Each dried powder sample (5 mg) (tissues ratio 1:1, w/w, where was necessary) were placed in an Erlenmeyer flask covered with aluminum foil to avoid light exposure and mixed with aqueous-ethanol (70%, v/v) (solidliquid ratio 1:4, w/v, except in J. dioica, solid-liquid ratio 1:15, w/v was used). The mixture of the material vegetal and solvent was heat-refluxed in a water bath at 60 ℃. A kinetic study on the total phenolic content extracted was carried out at different times: 0, 2, 4, 6, and 8 h of extraction.
Once selected the best extraction time the second factor evaluated was the aqueous-ethanol concentration. Three aqueous-ethanol concentrations were tested: 0%, 35% and 70% (v/v), where 0% is water without ethanol. Then, the heatreflux extraction was performed under the same conditions as in the first experiment.
After extraction, each extract was filtered using a gauze for eliminate the big particles. Briefly, extracts were centrifuged at 3 500 rpm during 10 min and subsequent were filtered through a filter paper (fine pore, 0.45 μm) under vacuum. The filtered extracts were dehydrated in an oven at 60 ℃for 24 h and the extraction yields were obtained in dry base (%, w/w). The dried extracts were stored at 4 ℃ in a dark place before their use in the quantification of total phenolic
content. The experiments were performed in triplicates. In all the next determinations the extracts were re-suspended in water (1 mg/mL).
2.3. Determination of total phenols content (TPC)
TPC was quantified according the methodology reported by Makkar[21] with some modifications[22]. First, 800 μL of the sample were mixed with 800 μL of Folin-Ciocalteu reagent (Sigma-Aldrich), shaken and left for 5 min. Then 800 μL of Na2CO3(0.01M) were added and shaken and left for 5 min again. Finally, the solution was diluted with 5 mL of distilled water and the absorbance was read at 790 nm. TPC was expressed as gallic acid equivalents per gram of vegetal material (mg GAE/g).
2.4. Antioxidant activity
Antioxidant activity was measured in the extracts that presented the higher amount of total phenolic content in each vegetal material for three methodologies (Table 3).
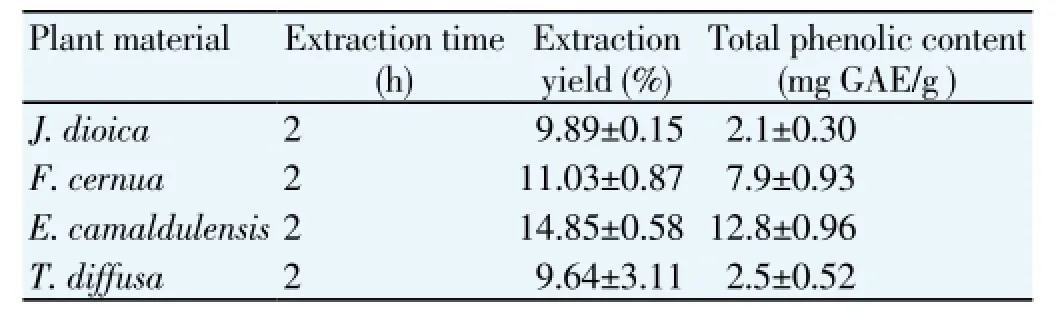
Table 2 Extraction yield and total phenolic content of the extracts obtained in the kinetic of extraction.
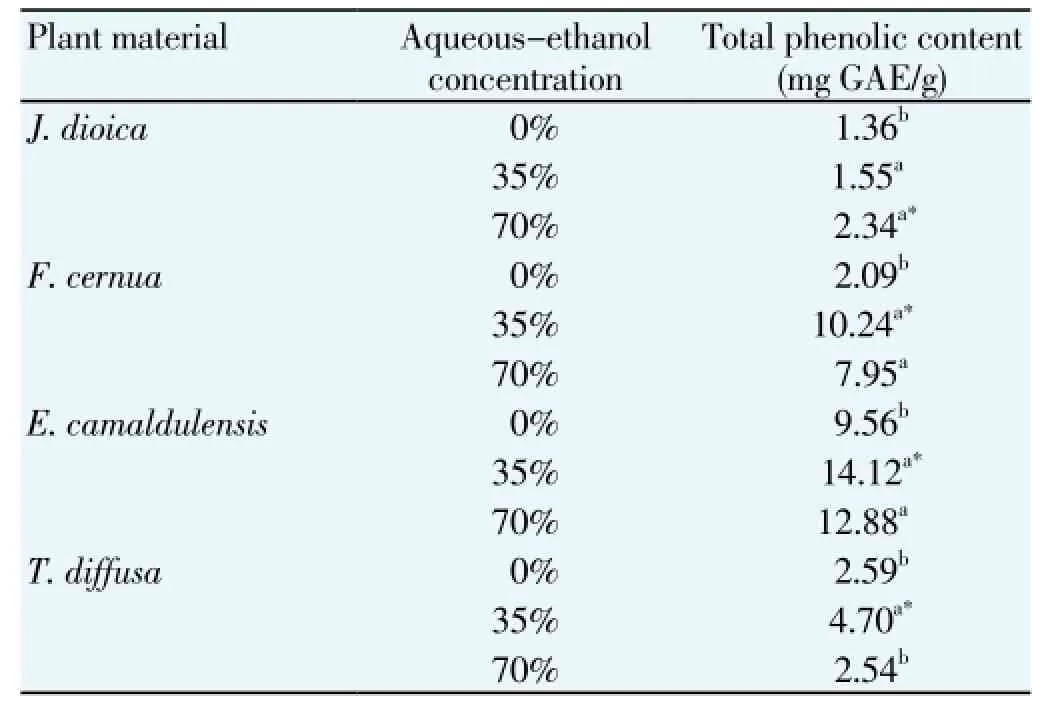
Table 3 Effect solvent concentration on the extraction yield and total polyphenolic content.
2.4.1. 1,1-diphenyl-2-picryl-hydrazyl (DPPH) radical scavenging activity
First, the antioxidant activity in the extracts was evaluated as the DPPH free radical-scavenging activity. This assay was following the method reported by Molyneux[23]: briefly, the solution of free radical DPPH (2.9 mL, 60 μM, in methanol) was added to the sample (0.1 mL) and the controls (0.1 mL of water) respectively. The reaction was 30 min standing in a dark room at room temperature and after the absorbance was measured at 517 nm on a Cary-50-Bio Varian spectrophotometer. The free radical-scavenging activity of the extracts was expressed as percentage inhibition of DPPH and was calculated according to the formula:
where Cabs is the control absorbance and Sabs is the sample absorbance.
2.4.2. ABTS (2,2-azino-bis 3 ethyl benzothiazoline-6-sufonic acid) radical cation scavenging activity
ABTS radical cation was prepared adding potassium persulphate solution (2.45 mM) and an ABTS aqueous solution (7 mM) and stand in the dark at room temperature for 12 h before use. After 12 h, the final solution was diluted with ethanol to an absorbance of 0.70±0.02 at 734 nm. Briefly, the extract (50 μL) or the control (50 μL of water) was mixed with ABTS solution (1 mL) and immediately the time was taken and the absorbance was read after 1 min using a Cary-50-Bio Varian spectrophotometer. The sample absorbance was compared with the control absorbance. The total antioxidant capacity was calculated as percent inhibition of ABTS radical using the following equation:
where Cabs is the control absorbance and Sabs is the sample absorbance[24].
2.4.3. Lipid oxidation inhibition assay
The potential antioxidant of the extracts was obtained by lipid oxidation inhibition assay with the finality of measure the antioxidant activity in a medium close to lipids in a biological system[6]. First, the linoleic acid solution was prepared by placing 0.56 g of linoleic acid and 1.5 g of Tween 20 in 8 mL of ethanol (96%, v/v). Then, the plant extract (50 L) was mixed with linoleic acid solution (100 μL) and acetate
buffer (1.5 mL, 0.02 M, pH 4.0). Controls contained 50 μL of distilled water. The samples were homogenized in vortex and incubated at 37 ℃ for 1 min. Once achieved 1 min, 750 μL of 50 M FeCl2solution (0.099 4 g FeCl2and 0.168 g EDTA diluted to 1 L with distilled water) were added to induce the lipid oxidation and incubated for 24 h at 37 ℃. Two aliquots (250 μL) were withdrawn during this period, at 1 and 24 h. Each aliquot was processing in the moment as following: the aliquot were added to NaOH solution (1 mL, 0.1 M, in ethanol at 10%, v/v) to stop the oxidation process; after ethanol (2.5 mL, 10%, v/v) was placed to dilute the sample. Then, the absorbance of the samples was measured at 232 nm. Ethanol (10%, v/v) was used as blank. Percent inhibition of linoleic acid oxidation was calculated with the following equation:
Lipid oxidation inhibiton(%)=
where A is the difference between the absorbance of the control sample (distilled water) after 24 h and 1 h of incubation, and B is the difference between the absorbance of each extract sample after 24 h and 1 h of incubation[25].
2.5. High performance liquid chromatography analysis
The characterization of the phenols compounds present in the selected extracts (Table 3) was carried out using high performance liquid chromatography (HPLC) analysis following the method reported by Ruiz et al[18]. A Varian Pro-Star 330 with DAD detector and Photodiode detection at 280 nm was used. Fractionation of the samples was performed on an Optisil ODS column (5 μm, 4.6 mm × 250 mm) under following analytical conditions: using a mobile phase consisting of 3% acetic acid and acetonitrile, during 25 min with flow rate of 1 mL/min (sample previously filtered through a 0.22 μ m nylon membrane; with injection volume of 10 μL). All the standard solutions of the different phenolic compounds used were injected under the same conditions.
2.6. Statistics analysis
The experiments were established under a blocks completely randomized design for which material vegetal independently. The dates were transformed by log X and analyzed using the software SAS V 9.0. where the comparison test of means of multiple range Tukey (α = 0.5) was used. In the graphic the dates observed are the true values.
3. Results
3.1. Extraction of total phenols content
The kinetic extraction of polyphenols allowed to establish the time required for recovery the TPC maximum (Table 2). In the four plants was noted that exist a significant difference in the TPC in the control time (0 h) compared to other extraction times (2, 4, 6 and 8 h). However, no significant difference was found in the times 2, 4, 6 and 8 h in four plants evaluated. Thus, with the purpose of select the suitable time for extraction of phenols, the productivity in each vegetal material was determined (Figure 1). It was observed in all cases that the productivity was higher at 2 h of extraction and decreased at rest of the times evaluated. Using this analysis was possible select 2 h of extraction time as the suitable time for the TPC extraction in the four plants and it was used in the evaluation of the second factor, aqueous-ethanol concentration.
The second factor evaluated was the aqueous-ethanol concentration on the extraction of polyphenols from the four plant used. In this experiment, the extraction of TPC from E. camaldulensis, F. cernua and T. diffusa showed a quadratic effect (Table 3). When aqueous-ethanol concentration was increased from 0% to 35% (v/v) more phenolic compounds were extracted. But lower TPC yield was observed when aqueous-ethanol concentration was increased until 70% (v/ v). On the other hand, in J. dioica extracts was observed an increase in the TPC extracted which was proportional at the increase of aqueous-ethanol concentration (Table 3). It is possible to mention that under the suitable conditions of extraction time and aqueous-ethanol concentration, E. camaldulensis and F. cernua were the species with higher amount of TPC while T. diffusa and J. dioica were the
species with lower TPC yield content (Table 3).
3.2. Antioxidant capacity of the extracts
Four plants of the semiarid Mexican regions were used to assess the antioxidant potential by three methods.
3.2.1. Free radical scavenging activity on DPPH
Figure 2a shows the DPPH scavenging activity of the extracts obtained under the TPC suitable extraction conditions for the four plants. F. cernua, E. camaldulensis and T. diffusa extracts had similar capacity antioxidant ranging between 76.03% to 91.96%. These results were higher (70%) that obtained for J. dioica [(15.40±1.88)%]. To our knowledge, there are not concise studies to compare the J. dioica potential antioxidant. On the other hand, Salazar et al[13] evaluated the antioxidant activity using the DPPH assay in F. cernua and T. diffusa reporting values ranged from 75.3% to 86.8% and from 27.9% to 88.1%, respectively for individual tissues. It is according with our findings since we used a mix of the tissues where antioxidant capacity of each tissue was slight modified.
3.2.2. Free radical scavenging activity on ABTS
Antioxidant activity in the extracts was also investigated using the well-known ABTS method. The results in ABTS assays show that only E. camaldulensis extract maintained a high antioxidant activity very similar in both DPPH and ABTS assays with radical inhibition of 88% approximately under the condition used. While, F. cernua and T. diffusa extracts were more effective in DPPH radical scavenging than ABTS radical scavenging with a loss of 50% in the antioxidant activity decreasing from 80% in DPPH assay to 30% in ABTS assay in both cases (Figure 2). In contrast, J. dioica extract had a negligible increasing of 9% inhibition approximately when the ABTS assay was evaluated. In general terms, E. camaldulensis extract showed more free radicals scavenging capacity as antioxidant activity.
3.2.3. Lipid oxidation inhibition assay
In the light of the differences among the wide number of test systems available for the antioxidant activity, the results of a single-assay can give only a limited suggestion of the antioxidant properties of plant extracts[25,26]. In this study, three assays were carried out for have a better interpretation of the antioxidant activities of the obtained extracts. Figure 2c shows the inhibition activity of the extracts against linoleic acid peroxidation caused by FeCl2as an oxidant initiator. In this assay the values obtained without antioxidants were taken for 100% lipid peroxidation. As do DPPH and ABTS assays, lower values (13.95%) were obtained with J. dioica extract at the concentration used. The rest of the extracts (F. cernua, E. camaldulensis and T. diffusa) presented an average of 65% in the lipid oxidation inhibition. Anew E. camaldulensis extract maintained good antioxidant activity compared with the other extracts. These last findings for E. camaldulensis extract in the lipid oxidation inhibition are close to results in the DPPH assay.

Table 4 Phenolic compounds detected by HPLC assay in the different extracts selected.
3.3. HPLC assay
Using the HPLC analytical assay was possible detect and confirm the presence of the some phenolic compounds in J. dioica, F. cernua, T. diffusa and E. camaldulensis extracts. Table 4 shows a summary of the analyzed samples. It was noted that only J. dioica extract presented none phenolic compound. Possibly because of this is a crude extract and no purified then the phenolic compounds are present in low concentration. Nevertheless, recently Aguilera et al[27] confirmed the presence of ellagic acid by HPLC in a methanolic hydrolyzed extract in some arid Mexican plants including J. dioica, F. cernua and T. diffusa. However, these authors reported that the concentration was low (1.8-2.5 mg/ g). On the other side, the HPLC assay in the others three extracts, in special F. cernua, permitted to detect almost one compound (Table 4). Ruiz et al[18] studied the phytochemical profile of the F. cernua extracts and fractions, reporting two important phenolic compounds namely 2-hidroxicinnamic and ellagic acids. In our screening these compound was not detected. However, three different polyphenolic compounds were clearly detected as chlorogenic and coumaric acids and quercetin. Quercetin and catechin were identified in T. diffusa and E. camaldulensis extracts respectively.
4. Discussion
The TPC extracted from each plant was very different among plants despite of the extraction yields were similar in all the samples. Similar reports have been published by other authors[28]. These authors observed that no existed significant differences in the extraction yield evaluating times from 3 to 8 h with heat-reflux system using other plants. Hence, they concluded that highest quantity of compounds extracted is achieved in a lesser time than the times evaluated. Few studies had been developed concerning the polyphenolic content from F. cernua[15,29,30]. Interestingly, in the present study was possible to obtain higher TPC. These differences in the results can be attributed at solvent used in their studies as acetone and hexane which extracted certain compounds of low or no polarity. In contrast, in our study, the solvent employed was aqueous-ethanol which is a solvent with higher polarity and it is known that the most phenolic compounds are polar permitting easier the diffusivity in the ethanol and water mixture. Other factor could affect positively the yield extraction was the particle size. In our study was used a small particle size between 0.6-0.8 mm. It is known that a small size particle increase the contact surface in the vegetal material and therefore, increases the extraction yields of the target compounds[31]. Our findings are also according with previously reports by Castillo et al[32], where these authors reported J. dioica had lower values of TPC than T. diffusa and F. cernua before and after fermentation. It is well documented that phenol content in the plants varies depending the specie, the plant tissue and environmental factors as temperatures, water stress, light conditions as well as phenological development[20]. It explains the large differences obtained among the plants used.
It is interesting to note that despite of that T. diffusa extract had lower TPC levels respect to F. cernua and E. camaldulensis extracts, it showed a good potential antioxidant. Several reports have showed that the phenolic compounds are not the unique phytochemicals to possess antioxidant properties[33].
On the other hand Amakura et al[34] evaluated the antioxidant activity by DPPH assay of eucalyptus extract and, gallic and ellagic acids isolated from the eucalyptus extract. They observed that the isolated compounds had higher antioxidant activity than BHA and BHT compounds (synthetic antioxidants), isolated terpenes and phloroglucinol. Finally, the authors concluded that the main antioxidant activity in eucalyptus extract can be attributing to gallic and ellagic acids present in the extracts. As expose above, E. camaldulensis extract maintained a high antioxidant activity very similar in both DPPH and ABTS assays. These findings shows that one or several antioxidant compounds present in the E. camaldulensis extract have the capacity to act in two different mechanisms for the free radical scavenging activity, through a single electron transfer reaction (ABTS assay)[6,35] and a hydrogen transfer reaction (DPPH assay)[23,36]. In this sense, F. cernua and T. diffusa extracts had more capacity for donate a hydrogen atom instead of transfer an electron. However, it is important to note that antioxidant compounds can respond in a different manner to different radical or oxidant compounds[37]. It could be explained according the mechanism of oxidation proposed by Huang et al[6] and Mishra et al[36] for DPPH assay and Choe and Ming[33] for lipid oxidation inhibition, both methods are based on the oxidation process could be stopped when another molecule have the capacity of transferring a hydrogen atom to the radical. However, the assay lipid oxidation inhibition is an antioxidant test stricter than DPPH method due to chain reactions are involved[38]. Hence, the antioxidant compounds should be more specific, resistant and higher stoichiometrically for stabilized the several kinds free radical formed; besides exist the possibility that extracts
had a low quantity of fat oxidant agents[39]. It explains the slight decrease in the lipid oxidation inhibition percentage for F. cernua, E. camaldulensis and T. diffusa extracts.
Antioxidant activity and the phenol content of several extracts had been correlated positively in several studies but, it still is not constant and dependent of the material analyzed[19,34]. In this context, we can observe that E. camaldulensis extract was the best antioxidant extract and it is correlated with the bigger TPC observed during the quantification in the extraction process. In the same understanding J. dioica extract was the sample with lower antioxidant capacity and TPC. Finally the antioxidant activity and TPC in the four samples tested were in the order of E. camaldulensis > F. cernua> T. diffusa> J. dioica.
The phenolic compounds found in the extracts had been reported to be powerful antioxidants present in vegetables and fruit[40,41] and known as take part of the mechanism of defense environmental in vegetables, fruit and herbs[38]. Then, it was presumed that the antioxidant activity in E. camaldulensis, F. cernua and T. diffusa extracts could be due to those typical phenolics.
The better extraction conditions of total polyphenols were clearly associated to the antioxidant capacity in semiarid region plants. The plant E. camaldulensis was the better antioxidant source in our study because it maintained the great antioxidant potential in the three antioxidant activity assays with electron transfer and hydrogen atom capacity. However to confirm the close mechanism is necessary subsequent studies as isolation compounds or fractionation of the extract and characterization of the structures. To our knowledge this is the first approach where the phenolic antioxidant activity in these arid Mexican plants is explored. However, it is important to note that is necessary more studies to determine the better conditions of use. Generally, we can suggest that the plants used are a natural source of bioactive compounds of plant widely distributed in Mexico of great value that could be used properly in the food, pharmaceutical and cosmetic industries.
Conflict of interests
The authors disclose no conflicts.
Acknowledgements
Author J. E. Wong thanks to CONACYT for the financial support of program Master in Foods Science and Technology in UAdeC.
[1] Oliveira I, Sousa A, Ferreira I, Bento A, Estevinho L, Pereira JA. Total phenols, antioxidant potential and antimicrobial activity of walnut (Juglans regia L.) green husks. Food Chem Toxicol 2008; 46: 2326-2331.
[2] Oh S, Kim J, Jeon H, Park J, Koh Y, Hur H, et al. Antifungal activity of eucalyptus-derived phenolics against postharvest pathogens of kiwifruits. Plant Pathol J 2008; 24: 322-327.
[3] Angulo MA, Armenta E, García RS, Carrillo JA, Salazar E, Benigno J. Extracts of Swietenia humilis Zucc. seed with antifungal activity in Rhizopus stolonifer (Ehrenb.:Fr.) Vuill. Rev Mex Fitopatolog 2009; 27: 84-92.
[4] Osorio E, Flores M, Hernández D, Ventura J, Rodríguez R, Aguilar CN. Biological efficiency of polyphenolic extracts from pecan nuts shell (Carya illinoensis), pomegranate husk (Punica granatum) and creosote bush leaves (Larrea tridentata Cov.) against plant pathogenic fungi. Ind Crop Prod 2010; 31: 153-157.
[5] Kähkönen MP, Hopia AI, Heino M. Berry phenolics and their antioxidant activity. J Agric Food Chem 2001; 49: 4076-4082.
[6] Huang D, Ou B, Prior RL. The chemistry behind antioxidant capacity assays. J Agric Food Chem 2005; 53:1841-1856.
[7] Bakkali F, Averbeck S, Averbeck D, Idaomar M. Biological effects of essential oils. Food Chem Toxicol 2008; 46: 446-475.
[8] López A, López RS, Vázquez ME, Rodríguez A, Mendoza M, Padrón E. Inhibición del crecimiento micelial de Fusarium oxysporum Schlechtend. f. sp. lycopersici (Sacc.) Snyder y Hansen, Rhizoctonia solani Kuhn y Verticilllium dahliae Kleb. mediante extractos vegetales acuosos. Rev Mex Fitopatolog 2005; 23: 183-190.
[9] Guerrero E, Solís S, Hernández FD, Flores A, Sandoval V, Jasso D. Actividad biológica in vitro de extractos de Flourensia cernua D. C. en patógenos postcosecha: Alternaria alternata (Fr.:Fr.) Keissl., Colletotrichum gloeosporioides (Penz.) Penz. y Sacc. y Penicillium digitatum (Pers.:Fr.) Sacc. Rev Mex Fitopatolog 2007; 25: 48-53.
[10] Jasso de Rodríguez D, Rodríguez R, Hernández FD, Aguilar CN, Saenz A, Villareal JA, et al. In vitro antifungal activity of extracts of Mexican Chihuahuan desert plants against postharvest fruit fungi. Ind Crop Prod 2011; 34: 960-966.
[11] Singh HP, Mittal S, Kaur S, Batish DR, Kohli RK. Characterization and antioxidant activity of essential oils from fresh and decaying leaves of Eucalyptus tereticornis. J Agric Food Chem 2009; 57: 6962-6966.
[12] González J, Cruz JM, Domínguez H, Parajó JC. Production of antioxidants from Eucalyptus globulus wood by solvent extraction of hemicellulose hydrolysates. Food Chem 2004; 84: 243-251.
[13] Salazar R, Pozos ME, Cordero P, Pérez J, Salinas MC, Waksman N. Determination of the antioxidant activity of plants from northeast Mexico. Pharm Biol 2008; 46: 166-170.
[14] Adame J, Adame H. Plantas curativas del Noreste Mexicano.
Ediciones Castillo, México; 2000.
[15] Ventura J, Belmares R, Aguilera A, Gutiérrez G, Rodríguez R, Aguilar CN. Fungal biodegradation of tannins from creosote bush (Larrea tridentata) and tar bush (Flourensia cernua) for gallic and ellagic acid production. Food Technol Biotechnol 2008; 46: 213-217.
[16] Castillo F, Hernández D, Gallegos G, Méndez M, Rodríguez R, Reyes A, et al. In vitro antifungal of plant extracts obtained with alternative organic solvents against Rhizoctonia solani Kühn. Ind Crop Prod 2010; 32: 324-328.
[17] Garza Y, Rodríguez J. Bioprospectiva del desierto y semidesierto mexicanos. In: Aguilar CN, Rodríguez R, Saucedo SY, Jasso D, editors. Fitoquímicos Sobresalientes del Semidesierto Mexicano: de la planta a los químicos naturales y a la biotecnología. México: Path Design S. A.; 2008, p. 41.
[18] Ruiz J, Ascasio JA, Rodríguez R, Morales D, Aguilar CN. Phytochemical screening of extracts from some Mexican plants used in traditional medicine. J Med Plant Res 2011; 5: 2791-2797.
[19] Vázquez G, Fontenla E, Santos J, Freire MS, González-Álvarez J, Antorrena G. Antioxidant activity and phenolic content of chestnut (Castanea sativa) shell and eucalyptus (Eucalyptus globulus) bark extracts. Ind Crop Prod 2008; 28: 279-285.
[20] Upadrasta L, Mukhopadhyay M, Banerjee R. Tannins: Chemistry, biological properties and biodegradation. In: Sabu A, Roussos S, Aguilar CN. Chemistry and biotechnology of polyphenols. India: Cibet Publishing House; 2011, p. 5-32
[21] Makkar H. Quantification of tannins in tree foliage. A laboratory manual for the FAO/IAEA. Vienna; 1999.
[22] Ventura J, Gutiérrez G, Rodríguez R, Aguilar CN. Fungal cultures of tar bush and creosote bush for production of two phenolic antioxidants (Pyrocatechol and gallic acid). Folia Microbiol 2009; 54: 199-203.
[23] Molyneux P. The use of the stable free radical diphenylpicrylhidrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J Sci Technol 2004; 26: 211-219.
[24] Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 1999; 26: 1231-1237.
[25] Martínez CG, Aguilera AF, Rodríguez R, Aguilar CN. Fungal enhancement of the antioxidant properties of grape waste. Annals Microbiol 2011; 62: 923-930.
[26] Kalia K, Sharma K, Singh HP, Singh B. Effects of extraction methods on phenolic contents and antioxidant activity in aerial parts of Potentilla atrosanguinea Lodd. and quantification of its phenolic constituents by RP-HPLC. J Agric Food Chem 2008; 56: 10129-10134.
[27] Aguilera AF, Augur C, Prado LA, Aguilar CN, Favela E. Extraction and analysis of ellagic acid from novel complex sources. Chem Pap 2008; 62: 440-444.
[28] Martins S, Aguilar CN, De la Garza I, Mussato SI, Teixeira JA. Kinetic study of notdihydroguaiaretic acid recovery from Larrea tridentata by microwave-assisted extraction. J Chem Technol Biotechnol 2010; 85: 1142-1147.
[29] Mercado D, Belmares R, Aguilera A, Contreras JC, Rodríguez R, Heredia N, et al. Toxicity and in vitro digestibility of creosote bush and tar bush fermented under fungal solid state culture conditions. Res J Biolog Sci 2007; 2: 571-575.
[30] Belmares R, Garza Y, Rodríguez R, Contreras JC, Aguilar CN. Composition and fungal degradation of tannins present in semiarid plants. Electron J Environ Agric Food Chem 2009; 8: 312-318.
[31] Galvan d'Alessandro L, Kriaa K, Nikov I, Dimitrov K. Ultrasound assisted extraction of polyphenols from black chokeberry. Sep Pur Technol 2012; 93: 42-47.
[32] Castillo R, Rodríguez R, Ventura J, Belmares R, Aguilar CN. Antibacterial and antioxidant activities of polyphenolic extracts from Mexican semiarid plants pre and post-fementation. innovations in food science and food biotechnology in developing countries; 2008.
[33] Choe E, Min DB. Mechanisms of antioxidants in the oxidation of foods. Compr Rev Food Sci Food Safety 2009; 8: 345-358.
[34] Amakura Y, Umino Y, Tsuji S, Ito H, Hatano T, Yoshida T, Tonogai Y. Constituents and their antioxidative effects in eucalyptus leaf extract used as a natural food additive. Food Chem 2002; 77: 47-56.
[35] Osman AM, Wong KKY, Fernyhough A. ABTS radical-driven oxidation of polyphenols: Isolation and structural elucidation of covalent adducts. Biochem Biophys Res Comm 2006; 346: 321-329.
[36] Mishra K, Ojha H, Chaudhury NK. Estimation of antiradical properties of antioxidants using DPPH assay: A critical review and results. Food Chem 2012; 130: 1036-1043.
[37] Prior RL, Wu X, Schaich K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J Agric Food Chem 2005; 53: 4290-4302.
[38] Moure A, Cruz JM, Franco D, Domínguez JM, Sineiro J, Domínguez H, et al. Natural antioxidants from residual sources. Food Chem 2001; 72:145-171.
[39] Starzynska-Janiszewska A, Stodolak B, Jamróz M. Antioxidant properties of extracts from fermented and cooked seeds of Polish cultivars of Lathyrus sativus. Food Chem 2008; 109: 285-292.
[40] Guyot S, Bernillon S, Poupard P, Renard CMGC. Multiplicity of phenolic oxidation products in apple juices and ciders, from synthetic medium to commercial products. In: Daayf F, Lattanzio V, eds. Recent advances in polyphenol research. UK: Wiley-Blackwell; 2009, p. 278-92.
[41] Swapna TS, Sabu A. Polyphenols in plants. In: Sabu A, Roussos S, Aguilar CN. Chemistry and biotechnology of polyphenols. India: Cibet Publishing House; 2011, p. 135-151.
ment heading
10.1016/S1995-7645(14)60299-6
*Corresponding author: Cristóbal N. Aguilar, School of Chemistry. Universidad Autónoma de Coahuila, Saltillo, Coahuila, México.
E-mail: cristobal.aguilar@uadec.edu.mx
Foundation project: It is supported by program Master in Foods Science and Technology in UAdeC.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Effect of interferon plus ribavirin therapy on hepatitis C virus genotype 3 patients from Pakistan: Treatment response, side effects and future prospective
- Imported cases of dengue fever in Russia during 2010-2013
- Detection and characterization of Chlamydophila psittaci in asymptomatic feral pigeons (Columba livia domestica) in central Thailand
- Chemical composition of Rosmarinus and Lavandula essential oils and their insecticidal effects on Orgyia trigotephras (Lepidoptera, Lymantriidae)
- Cytoprotective and anti-inflammatory effects of kernel extract from Adenanthera pavonina on lipopolysaccharide-stimulated rat peritoneal macrophages
- Cattle toxoplasmosis in Iran: a systematic review and meta-analysis
