Effect of interferon plus ribavirin therapy on hepatitis C virus genotype 3 patients from Pakistan: Treatment response, side effects and future prospective
2015-12-08YasirWaheed
Yasir Waheed
1Atta ur Rahman School of Applied Biosciences, National University of Sciences & Technology (NUST), Islamabad 44000, Pakistan
2Foundation University Medical College, Foundation University Islamabad, DHA Phase 1, Islamabad 44000, Pakistan
Effect of interferon plus ribavirin therapy on hepatitis C virus genotype 3 patients from Pakistan: Treatment response, side effects and future prospective
Yasir Waheed1,2*
1Atta ur Rahman School of Applied Biosciences, National University of Sciences & Technology (NUST), Islamabad 44000, Pakistan
2Foundation University Medical College, Foundation University Islamabad, DHA Phase 1, Islamabad 44000, Pakistan
ARTICLE INFO
Article history:
Received 10 September 2014
Received in revised form 15 December 2014 Accepted 15 January 2015
Available online 20 February 2015
Hepatitis C virus
More than 10 million people are suffering from hepatitis C virus (HCV) in Pakistan. The available treatment option is a combination of interferon and ribavirin. Treatment response is linked with several factors and also induces a number of side effects. We searched in Pubmed, Pak Medi Net and Google Scholar for the articles presenting the effect of interferon plus ribavirin therapy on HCV patients from Pakistan, their side effects and future prospects. The major prevalent HCV genotype in Pakistan is 3. Conventional interferon alpha plus ribavirin showed sustained virological response of 54%-64% while pegylated interferon alpha plus ribavirin showed sustained virological response of 58%-75%. IL-28B CC genotype is linked with better sustained virological response. Studies on patients with HCV genotype 3 infections showed no correlation between treatment response and interferon sensitivity determining region mutations. Interferon therapy is linked with a number of side effects like thyroid dysfuncton, haematological disorders, weight loss, gastrointestinal tract side effects and neuropsychiatric side effects. Unusual side effects of clubbing of fingers and seizures were also observed in a couple of patients. Interferon alpha plus ribavirin therapy showed better response rate in HCV genotype 3 patients from Pakistan with number of side effects. A couple of interferon free therapies are light of hope for the patients living with HCV.
1. Introduction
Hepatitis C virus (HCV) is a plus stranded RNA virus belonged to the family Flaviviridae. It has infected more than 200 million people worldwide with high morbidity and mortality[1]. World Health Organization reported in 2012 that the annual deaths caused by HCV related liver diseases are greater than 350 000[2]. Pakistan has more than 10 million people carrying HCV infection[1].
HCV is classified into different genotypes on the basis of nucleotides sequence divergence. A strong link has been reported between viral genotypes and treatment response. HCV is classified into six genotypes[3]. HCV genotypes 1-3 are prevalent throughout the world, and their prevalence varies from area to area. In Middle East and North Africa major prevalent HCV genotype is 4. HCV genotype 5 and 6 are dominant in South Africa and Hong Kong respectively[4]. The major prevalent genotype in Pakistan is 3[3-6].
The major objective of HCV therapy is to attain sustained virological response (SVR), which is defined as the absence of HCV RNA in serum after 6 months of treatment completion, confirmed by polymerase chain reaction (PCR)[7]. Two decades back, HCV treatment was started with recombinant human interferon alpha[8]. The interferon decreased the viral RNA in a considerable number of patients, but it also produces a number of side effects. Ribavirin is a purine analog of HCV. It has ability to increase the viral mutation rate to a level where viable genomes are only able to produce unfit progeny. Ribavirin down regulates the production of cellular IMP dehydrogenase enzyme, leading to decreased cellular GTP levels. It also modulates the host's immune system and increases the polymerase mutation rate, leading to error catastrophe[9-13].
Currently there is no vaccine available for HCV[14]. The only available treatment option for majority of genotypes is the combination of interferon and ribavirin. The therapy showed diverse response in patients living with different HCV genotypes along with a number of side effects.
Recently two protease inhibitors were approved by FDA for the treatment of patients living with HCV genotype 1 infection. The protease inhibitors remained unsuccessful due to rapid emergence of resistant mutants[12]. This article presents the effect of different therapeutic combinations being used for the treatment of HCV patients living in Pakistan, side effects of therapy, factors linked with therapy response and future therapeutic prospects.
2. Literature search
We searched in Pubmed, Google Scholar, Pak Medi Net and Google Web for the articles by using keywords “Effect of interferon treatment on HCV patients from Pakistan, Factors linked with interferon treatment response in Pakistan, side effects of interferon therapy and hepatitis C virus in Pakistan”. Inclusion criteria entailed the manuscripts with HCV treatment response and risk factors from reliable publication source, while the data without any references were excluded from the study.
3. Effect of conventional interferon plus ribavirin therapy
Interferon alpha with ribavirin was administered to 616, HCV genotype 3, treatment naive patients for 24 weeks. SVR was achieved in 63.5% patients. Moreover, 70.7% response rate was achieved in female patients having age less than 40 years compared with 55.4% response rate in female patients having age greater than 40 years. Similarly, male patients having age less than 40 years showed response rate of 67.3% compared with 53.9% in male patients of age greater than 40 years. The patients having HCV RNA titer <8×105IU/mL showed response rate of 68.9% compared with 60.4% response rate in patients with HCV RNA titer >8×105IU/mL. The patient population was divided into two groups, ie., Punjabi and Pushtoon. Response rate was observed 69.9% in Pushtoon patients compared with 62% in Punjabi patients[15].
In another study by Ahmed et al, conventional interferon plus ribavirin was administered to 829 patients for 24 weeks to HCV genotypes 2 & 3 patients and for 48 weeks to HCV genotypes 1 & 4 patients. End-of-treatment response (ETR) was achieved in 74% of patients. Post treatment follow up was available in 492 patients. SVR was achieved in 63% (308 out of 492 cases) of patients. Better response rate was observed in patients with <40 years of age, serum albumin >4 g/dL, normal alanine aminotransferase at the first month of therapy, non-diabetic and with normal platelet count[16].
Conventional interferon plus ribavirin was administered to 86 patients enrolled at Ghurki Trust Hospital Lahore. Totally, 69% of patients showed rapid virologic response (RVR) while SVR was observed in 53.5% patients. Younger age and RVR were important factors to achieve SVR. Male patients showed better SVR than female patients[17].
4. Effect of pegylated interferon plus ribavirin therapy
In a study by Aziz et al, 403 HCV patients were administered pegylated interferon alpha-2b with ribavirin for 24 weeks to HCV genotype 3 patients and for 48 weeks to HCV genotype 1 patients. Overall SVR was achieved in 74.7% patients. Patients having age <40 years showed SVR of 79% as compared with 69% in patients >40 years. Patients having viral load <8×105IU/mL showed SVR of 85% as compared with 70% in patients having viral load >8×105IU/mL. SVR of 76% and 55% was observed in HCV genotype 3 and HCV genotype 1 patients respectively. Ethnic group Pushtoon showed SVR of 85% compared with 73% SVR in Punjabi group. SVR was observed 86% in patients with RVR and 39% in non-RVR patients. The most common side effects observed were fever, headache, fatigue, myalgia, diarrhea and skin rash. Insomnia and depression were most commonly observed psychiatric side effect[18].
In another study, 426 HCV genotype 3 patients were enrolled and pegylated interferon alpha-2a with ribavirin was administered for 24 weeks. Overall SVR was observed in 75.1% of patients. The patients less <40 years showed SVR of 80.8% compared with 69.1% SVR in patients >40 years of age. Male patients showed SVR of 78.5% while female patients showed SVR of 71.9%. Patients having HCV RNA <8×105IU/mL showed SVR of 87.7% while patients with viral titer >8×105IU/mL showed SVR of 63.7%. The SVR was observed in 85.5% of RVR patients and 44.4% of non-RVR patients. Patients with normal alanine aminotransferase showed SVR of 85.5% while the patients with raised alanine aminotransferase showed SVR of 72.9%. The most common side effects observed were fatigue (in 55.2%), headache (in 16.2%), cough (10.3%), fever (in 12.2%) and alopecia (in 2.3%). Insomnia and depression were frequently observed psychiatric adverse effect[19].
Chronic HCV cirrhotic patients are difficult to treat. Butt et al enrolled 66 HCV cirrhotic patients in her study. Out of 66 patients, 61 had child's A cirrhosis and 5 had child's B cirrhosis. All the patients belonged to HCV genotypes 3. About 50% of patients were given peg-interferon alpha-2a with ribavirin and other 50% were given peg-interferon alpha-2b with ribavirin. Early virological response was achieved in 44 (66.7%) patients and ETR was achieved in 46 (69.7%) patients. The overall SVR was observed in 38 (57.6%) patients. Factors affecting SVR were patient's age, treatment naive status and achievement of early virological response. Due to the development of neutropenia and anaemia, 10 patients required erythropoietin or GCSF during treatment[20].
5. IL-28B polymorphism
Genome wide association studies showed that a genetic polymorphism on chromosome 19, at rs12979860, near IL28 gene is linked with response to interferon therapy[21,22]. It has observed that genotype CC of IL-28B is linked with 2-3
folds rise in SVR as compared to CT or TT genotypes[23]. It is also reported that IL-28B patients with CC genotypes responded more quickly to treatment as compared with TC and TT genotypes. CC genotype was observed in 77% of patients with rapid virological response. This genotype also predicts SVR in patients who had not achieved RVR. IL-28B polymorphism is also a factor affecting peg-interferon and ribavirin therapy response[21-24].
6. Interferon sensitivity determining region
HCV variants with mutations in NS5A gene play a role in conferring interferon resistance and the region is known as interferon sensitivity determining region (ISDR). Several studies on patients having HCV genotype 1 showed ISDR mutations perturbing interferon therapy response[25-27]. Studies on HCV genotype 3 patients showed no correlation with ISDR mutations and interferon therapy response[28,29]. Ali et al studied HCV NS5A mutations and response to interferon therapy in genotype 3 patients. In this study he sequenced HCV NS5A gene before and after the therapy in 27 patients. SVR was achieved in 20 patients, 4 patients did not show any response while remaining 3 showed ETR. Three SVR patients and two ETR patients showed mutations (Ⅰ-Ⅴamino acids) within the NS5A ISDR region. While 85% SVR and all NR has no mutations in ISDR region. They concluded that the mutations in NS5A region of HCV 3a genotypes may not influence the response rate of interferon plus ribavirin therapy in Pakistani patients[28].
7. Protein kinase (PKR) eukaryotic initiation factor 2 alpha (eIF2a) phosphorylation homology domain (PePHD)
During HCV infection, interferon is released from the infected cells and in response; the neighboring cells release PKR. During HCV replication, the viral single stranded RNA is converted into double stranded RNA. The double stranded RNA activates PKR, which leads to phosphorylation of eIF2a which halts the HCV RNA transcription. The HCV is a smart virus; it evolved a mechanism to overcome the host antiviral response. The HCV glycoprotein E2 carries a short stretch of 12 amino acids, forming a domain known as PePHD, which binds with protein kinase leading to inhibition of interferon alpha resulting in viral persistence[30-32].
The PePHD domain of six different HCV genotype 3 patients was compared. Three patients showed RVR and rest of three showed breakthrough to the same therapy. No conservation in PePHD domain was observed. Amino acid substitution of glutamine to leucine was observed in a non responder patient which made it more identical to HCV genotype 1a. This substitution also increased the average hydrophilic activity, making binding of PePHD to PKR more easy and inhibition of PKR more favorable[30].
8. Side effects of therapy
It is reported that the interferon plus ribavirin therapy leads to thyroid dysfunction in HCV patients. Nadeem et al gave combined therapy to HCV patients and analyzed thyroid functions at 0, 12 and 24 weeks. In total, 18.69% patients showed thyroid dysfunction with hypothyroidism being observed more common than hyperthyroidism. Thyroid dysfunction was observed higher in female patients. There was no association observed between severity of diseases and response to therapy with interferon induced thyroid dysfunction[33].
Interferon and ribavirin therapy also induces various hematological disorders. In a study conducted at Liaquat University Jamshoro, 100 patients were give interferon and ribavirin therapy and their hematological parameters were analyzed. Significant drop in mean haemoglobin, mean total leucocyte count and mean platelates count was observed in majority of patients. About 38% patients developed anemia, 13% developed leucopenia and 17% developed thrombocytopenia at 24 weeks of therapy[34].
Weight loss is also observed as side effect of interferon plus ribavirin therapy. In a study conducted at Jinnah Postgraduate Medical Center, Karachi, 260 patients were given interferon plus ribavirin therapy and their body weight was measured at 1, 3, 6 months of treatment and 6 months after the cessation of treatment. Weight loss was observed in 67.7% patients on completion of therapy compared with initial visit. Weight loss was observed to be independent of gender, age, treatment response, haemoglobin level, white blood cell counts and platelet counts. In most of the cases the body weight returned to baseline within 6 months after the completion of therapy[35].
Mahmood et al gave interferon plus ribavirin therapy to 400 patients and carefully observed various mild to moderate and severe side effects. The mild to moderate side effects observed were leukopenia (64%), anaemia (70%) and thrombocytopenia (61%). Flu like symptoms, e.g., fever (70%), fatigue (67%), headache (62%), myalgia (54%) and arthralgia (34%), were also observed in large patient population. GIT side effects observed were loss of appetite (35%), dyspepsia (16%) and constipation (5%). Neuropsychiatric side effects observed were irritability (28%), depression (20%), insomnia (35%), anxiety (12%) and emotional instability (10%). Other side effects observed were rhinitis (10%), pharyngitis (8%), thyroid abnormality (4%) and retinopathy (8%). The severe side effects of therapy observed were severe anaemia (8%), severe neutropenia (3%), severe thrombocytopenia (1%), major depression (1%), suicide ideation (0.5%) and severe thyrotoxicosis (0.5%)[36].
9. Unusual effects
Peg-interferon plus ribavirin was administered to 4 913 patients; eight had seizure. Five patients had unknown type of seizures, while three patients had grand mal seizure. It is also observed that six patients were taking anti-depressants
at the time of seizures, one patient had taken enough amount of alcohol and one was hyponatremic. It is concluded that seizures occur occasionally in patients on peg-interferon plus ribavirin therapy and are associated with the use of antidepressants[37].
An unusual effect of clubbing of fingers was observed in two patients on interferon alpha plus ribavirin therapy. One patient developed clubbing of fingers in the second month of therapy and the other patient developed in the fourth month of therapy. Clubbing was bilateral, of grad Ⅱ in one patient and grade Ⅲ in another. Clubbing was not observed before the start of therapy and no other secondary cause was seen. Both patients were not taking any other drug which interferes with therapy or can be associated with clubbing[38].
10. Future prospects
In addition to peg-interferon plus ribavirin therapy, two protease inhibitors are also approved for the treatment of HCV genotype 1 patients. The protease inhibitors along with peg-interferon plus ribavirin showed better SVR than peg-interferon with ribavirin alone. Both inhibitors showed some adverse effects when given to HCV genotype 1 patients. These inhibitors are not approved for the treatment of patients not having HCV genotype 1[12,39].
Abbott is also working to develop interferon free drug combination for the treatment of patients living with HCV. In a mild stage trial, they gave a combination of protease inhibitor (ABT-450), NS5A inhibitor (ABT-267) and protease inhibitor (ABT-33) to HCV patients, and the rate of SVR observed was 87%. Abbott will soon start phase Ⅲ trials for this combination[40].
Different nucleoside and non-nucleoside inhibitors targeting the HCV polymerase are at different phases of development (Table 1)[12]. The major advantage of nucleoside inhibitors is their pan-genotypic effect and high barrier to emergence of resistant mutant[12]. Nucleoside analogue GS-7977 is a big hope for the patients living with HCV. GS-7977 was administered in phase Ⅱ trials in a dose of 400 mg once daily plus ribavirin with and without peg interferon to HCV genotype 1, 2 & 3 patients. Approximately 88% SVR was achieved in treatment naive genotype 1 patients. The rate of SVR after 12 weeks administration of GS-7977 plus ribavirin was higher as compared with 12 week administration of protease inhibitor with peg interferon plus ribavirin on treatment naive HCV genotype 1 patients. GS-7977 plus ribavirin therapy showed a better response with both treatment naive and treatment experience patients of HCV genotypes 1, 2 & 3. To date no emergence of resistant mutant was observed. The phase Ⅲ trials showed that the GS-7977 showed a better response in patients living with different genotypes of HCV. The drug also showed very good response in non-responders of interferon therapy patients and patients with cirrhosis[12,41-43]. It is also expecting that GS-7977 therapy will replace Iiterferon therapy in 2015.
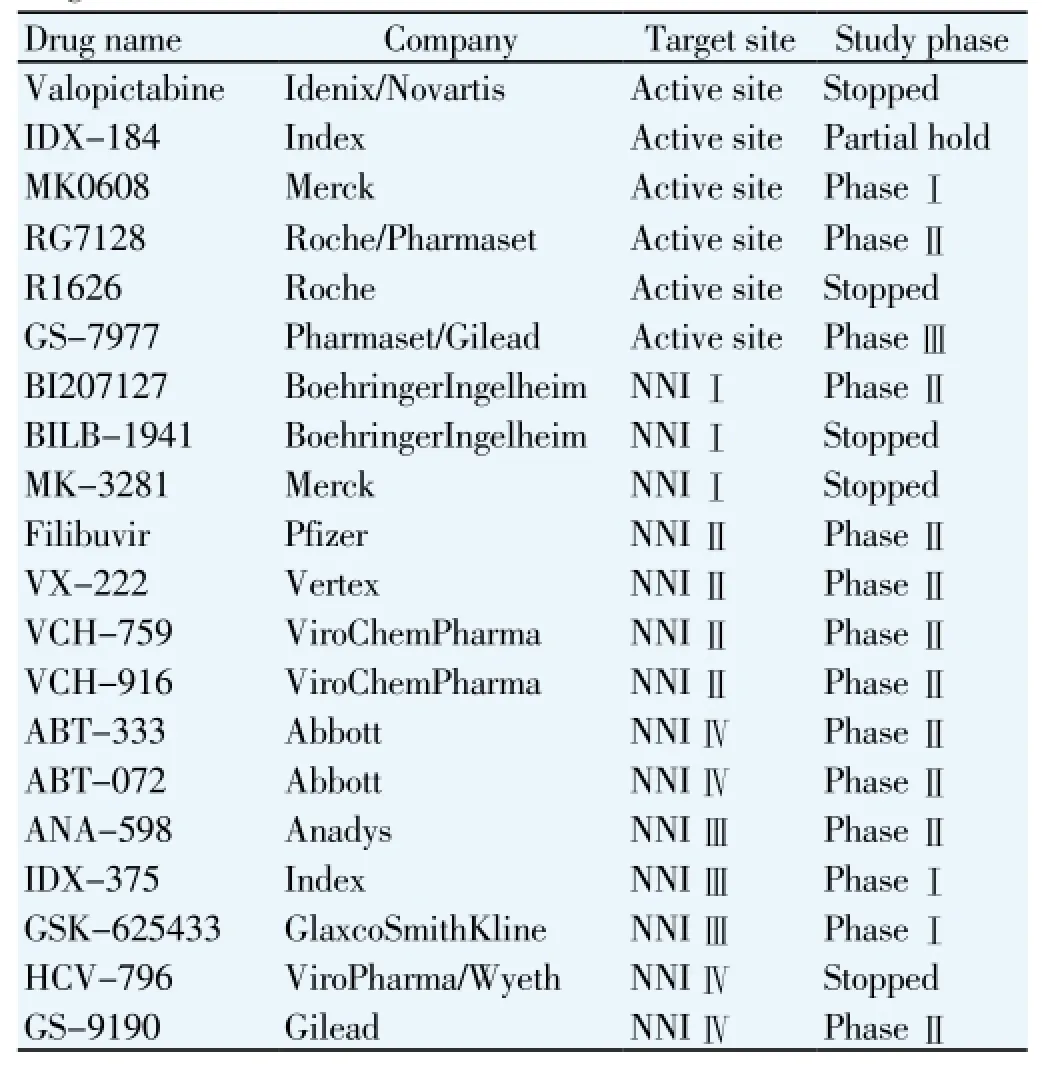
Table 1 Progress on HCV nucleoside and non-nucleoside inhibitors[12].
11. Conclusion
Interferon plus ribavirin therapy produces a better response in patients living with HCV genotype 3. The major drawback of the therapy is a number of side effects. Interferon free Abbott's triple therapy and GS-7977 drug are light of hope for the patients living with HCV.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
We are thankful to NUST. We acknowledge Ms. Amnah for formatting and revising manuscript.
[1] Waheed Y, Shafi T, Safi SZ, Qadri I. Hepatitis C virus in Pakistan: A systematic review of prevalence, genotypes and risk factors. World J Gastroenterol 2009; 45: 5647-5653.
[2] WHO. Prevention and control of viral hepatitis infection. Genewa: WHO Press; 2012 [Access on 2013 Aug 30]. Available from: http://www.who.int/csr/disease/hepatitis/GHP_framework.pdf.
[3] Safi SZ, Badshah Y, Waheed Y, Fatima K, Tahir S, Shinwari A, et al. Distribution of hepatitis C virus genotypes, hepatic steatosis and their correlation with clinical and virological factors in Pakistan. Asian Biomed 2010; 4: 253-262.
[4] Idrees M, Riazuddin S. Frequency distribution of hepatitis C virus genotypes in different geographical regions of Pakistan and their possible routes of transmission. BMC Infect Dis 2008; 8: 69.
[5] Butt S, Idrees M, Rehman IU, Akbar H, Shahid M, Afzal S, et al. Mixed genotype infections with hepatitis C virus, Pakistan. Emerg Infect Dis 2011; 17: 1565-1567.
[6] Aziz H, Raza A, Murtaza S, Waheed Y, Khalid A, Irfan J, et al.
Molecular epidemiology of hepatitis C virus genotypes in different geographical regions of Punjab Province in Pakistan and a phylogenetic analysis. Int J Infect Dis 2013; 17: e247-253.
[7] Strader DB, Wright T, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C. Hepatology 2004; 39:1147-1171.
[8] Hoofnagle JH, Mullen KD, Jones DB. Treatment of chronic non-A, non-B hepatitis with recombinant human alpha interferon: a preliminary report. N Engl J Med 1986; 315: 1575-1578.
[9] Duffy S, Shackelton LA, Holmes EC. Rates of evolutionary change in viruses: Patterns and determinants. Nat Rev Genet 2008; 9: 267-276.
[10] Feld JJ, Hoofnagle JH. Mechanism of action of interferon and ribavirin in treatment of hepatitis C. Nature 2005; 436: 967-972.
[11] Mansky LM, Bernard LC. 3′-azido-3′- deoxythymidine (AZT) and AZT-resistant reverse transcriptase can increase the in vivo mutation rate of human immunodeficiency virus type 1. J Virol 2000; 74: 9532-9539.
[12] Waheed Y, Bhatti A, Ashraf M. RNA dependent RNA polymerase of HCV: A potential target for the development of antiviral drugs. Infect Genet Evol 2013; 14: 247-257.
[13] Young KC, Lindsay KL, Lee KJ, Liu WC, He JW, Milstein SL, et al. Identification of a ribavirin resistant NS5B mutation of hepatitis C virus during ribavirin monotherapy. Hepatology 2003; 38: 869-878.
[14] Waheed Y, Saeed U, Tahir S, Afzal MS. Development of global consensus sequence and analysis of highly conserved domains of HCV NS5B protein. Hepat Mon 2012; 12: e6142.
[15] Aziz H, Ather MA, Murtaza S, Irafan J, Waheed Y, Bilal I, et al. Predictors of response to antiviral therapy in patients with chronic hepatitis C from Pakistani population. Chin Med J 2011; 124: 1333-1337.
[16] Ahmed F, Jacobson IM, Herrera JL, Brand M, Wasseman RB, Fixelle AM, et al. Seizures during pegylated interferon and ribavirin therapy for chronic hepatitis C: Obervations from the WIN-R trial. J Clin Gastroenterol 2011; 45: 286-292.
[17] Akram M, Idrees M, Zafar S, Hussain A, Butt S, Afzal S, et al. Effect of host and virus related factors on interferon alpha plus ribavirin and pegylated interferon plus ribavirin treatment outcomes in chronic hepatitis C patients. Virol J 2011; 8: 234.
[18] Aziz H, Gil ML, Waheed Y, Adeeb U, Raza A, Bilal I, et al. Evaluation of prognostic factors for peg interferon alfa-2b plus ribavirin treatment on HCV infected patients in Pakistan. Infect Genet Evol 2011; 11: 640-645.
[19] Aziz H, Raza A, Waheed Y, Gill U, Gil ML. Analysis of variables and interactions among variables associated with a sustained virological response to pegylated interferon alpha 2a plus ribavirin in hepatitis C virus genotype 3 infected patients. Int J Infect Dis 2012; 16: e597-602.
[20] Butt AS, Mumtaz K, Aqeel I, Shah HA, Hamid S, Jafri W. Sustained virological response to pegylated interferon and ribavirin in patients with genotype 3 HCV cirrhosis. Trop Gastroenterol 2009; 30: 207-212.
[21] Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, Urban TJ, et al. Genetic variation in IL28B predicts hepatitis C treatmentinduced viral clearance. Nature 2009; 461: 399-401.
[22] Thompson AJ, Muir AJ, Sulkowski MS, Ge D, Fellay J, Shianna KV, et al. Interleukin-28B polymorphism improves viral kinetics and is the strongest pre-treatment predictor of sustained virologic response in hepatitis C virus. Gastroenterology 2010; 139: 120-129.
[23] McHutchison JG, Lawitz EJ, Shiffman ML, Muir AJ, Galler GW, McCone J, et al. Peginterferon alfa-2b or alfa-2a with ribavirin for treatment of hepatitis C infection. N Engl J Med 2009; 361: 580-593.
[24] Abbas Z. IL-28B polymorphism and response to anti-hepatitis C therapy. J Pak Med Assoc 2011; 61: 621-622.
[25] Komatsu H, Fujisawa T, Inui A, Miyagawa Y, Onoue M. Mutations in the nonstructural protein 5A gene and response to interferon therapy in young patients with chronic hepatitis C virus 1b infection. J Med Virol 1997; 53: 361-365.
[26] Saiz JC, Lopez-Labrador FX, Ampurdanes S, Dopazo J, Forns X, Sanchez-Tapias JM, et al. The prognostic relevance of the nonstructural 5A gene interferon sensitivity determining region is different in infections with genotype 1b and 3a isolates of hepatitis C virus. J Infect Dis 1998; 177: 839-847.
[27] Sarrazin C, Berg T, Lee JH, Ruster B, Kronenberger B, Roth WK, et al. Mutations in the protein kinase-binding domain of the ns5a protein in patients infected with hepatitis C virus type 1a are associated with treatment response. J Infect Dis 2000; 181: 432-441.
[28] Ali I, Khan S, Attaullah S, Khan SN, Khan J, Siraj S, et al. Response to combination therapy of HCV 3a infected Pakistani patients and the role of NS5A protein. Virol J 2011; 8: 258.
[29] Frangeul L, Cresta P, Perrin M, Lunel F, Opolon P, Agut H, et al. Mutations in ns5a region of hepatitis C virus genome correlate with presence of ns5a antibodies and response to interferon therapy for most common European hepatitis C virus genotypes. Hepatology 1998; 28: 1674-1679.
[30] Afzal S, Idrees M, Akram M, Awan Z, Khubaib B, Aftab M, et al. Mutations in the E2-PePHD region of hepatitis C virus genotype 3a and correlation with response to interferon and ribavirin combination therapy in Pakistani patients. Virol J 2010; 7: 377.
[31] Kaufman RJ. The double-stranded RNA-activated protein kinase PKR. Translational control of gene expression. New York: Cold Spring Harbor Laboratory Press 2000; 2: 503-527.
[32] Le Guillou-Guillemette H, Vallet S, Gaudy-Graffin C, Payan C, Pivert A, Goudeau A, et al. Genetic diversity of the hepatitis C virus: Impact and issues in the antiviral therapy. World J Gastroenterol 2007; 13: 2416-2426.
[33] Nadeem A, Aslam M. Association of interferon-alpha and ribavirin-induced thyroid dysfunction with severity of diseases and response to treatment in Pakistani Asian patients of chronic hepatitis C. Hepat Res Treat 2012; 2012: 864315.
[34] Ghani MN, Masood N, Munir A, Baloch GH, Ghori RA. Haematological disorders during interferon and ribavirin therapy in chronic hepatitis C patients. Med Channel 2009; 15: 167-170.
[35] Sajjad SF, Ahmed WU, Arif A, Alam SE, Waquar J. Weight loss with interferon and ribavirin therapy in chronic hepatitis C patients. J Pak Med Assoc 2012; 62: 1229-1232.
[36] Mahmood K, Muhammad N. Side effects of combination of interferon plus ribavirin therapy in patients with chronic hepatitis C; An experience with 400 patients. J Postgrad Med Inst 2007; 21: 187-191.
[37] Ahmed WU, Arif A, Qureshi H, Alam SE, Ather R, Fariha S, et al. Factors influencing the response of interferon therapy in chronic hepatitis C patients. J Coll Physicians Surg Pak 2011; 21: 69-73.
[38] Alam MT, Sheikh SS, Aziz S, Masroor M. An unusual side effect of interferon alpha 2A: Digital clubbing. J Ayub Med Coll Abbottabad 2008; 20: 165-166.
[39] Hezode C. Boceprevir and Telaprevir for the treatment of chronic hepatitis C: Safety management in clinical practice. Liver Int 2012; 32: 32-38.
[40] Pierson R. Abbott hepatitis C drugs bring high cure rates in trial. New York: Reuters; 2012 [Accessed on 16 Jan 2013]. Available from: http://www.reuters.com/article/2012/11/10/us-liver-abbottidUSBRE8A90B020121110.
[41] Gane E, Stedman C, Hyland R, Sorenson R, Symonds W, Hindes R, et al. Once daily GS-7977 plus ribavirin in HCV genotypes 1-3: The electron trial. In: Levin J, editor. 47th Annual Meeting of the European Association for the Study of the Liver; 2012; Barcelona, Spain: European Association for the Study of the Liver; 2012.
[42] Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med 2013; 368: 1878-1887.
[43] Jacobson IM, Gordon SC, Kowdley KV, Yoshida EM, Rodriguez-Torres M, Sulkowski MS, et al. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment option. N Engl J Med 2013; 368: 1867-1877.
ment heading
10.1016/S1995-7645(14)60193-0
*Corresponding author: Dr. Yasir Waheed, Atta ur Rahman School of Applied Biosciences, National University of Sciences & Technology, Islamabad 44000, Pakistan.
Tel: 0092-300-5338171
E-mail: yasir_waheed_199@hotmail.com
Interferon therapy
Genotype 3
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Imported cases of dengue fever in Russia during 2010-2013
- Detection and characterization of Chlamydophila psittaci in asymptomatic feral pigeons (Columba livia domestica) in central Thailand
- Chemical composition of Rosmarinus and Lavandula essential oils and their insecticidal effects on Orgyia trigotephras (Lepidoptera, Lymantriidae)
- Total phenolic content, in vitro antioxidant activity and chemical composition of plant extracts from semiarid Mexican region
- Cytoprotective and anti-inflammatory effects of kernel extract from Adenanthera pavonina on lipopolysaccharide-stimulated rat peritoneal macrophages
- Cattle toxoplasmosis in Iran: a systematic review and meta-analysis
