Suppressive effect of pectic polysaccharides extracted from Rauwolfia verticillata (Lour.) Baill.var.hainanensis Tsiang on inflammation by regulation of NF-κB pathway and interleukin-17 in mice with dextran sulphatesodium-induced ulcerative colitis
2015-12-08XinPuMiaoXiaoNingSunLuJiaCuiQinFangCaoGuiFengZhuangTaoZhiDengDongYanZhang
Xin-Pu Miao, Xiao-Ning Sun, Lu-Jia Cui, Qin-Fang Cao, Gui-Feng Zhuang, Tao-Zhi Deng, Dong-Yan Zhang
1Department of Gastroenterology, Hainan Provincial People's Hospital, Haikou City 570311, Hainan Province, China
2Department of Gastroenterology, The Affiliated Hospital of Hainan Medical College, Haikou City 570103, Hainan Province, China
Suppressive effect of pectic polysaccharides extracted from Rauwolfia verticillata (Lour.) Baill.var.hainanensis Tsiang on inflammation by regulation of NF-κB pathway and interleukin-17 in mice with dextran sulphatesodium-induced ulcerative colitis
Xin-Pu Miao1*, Xiao-Ning Sun1, Lu-Jia Cui1, Qin-Fang Cao1, Gui-Feng Zhuang2, Tao-Zhi Deng1, Dong-Yan Zhang1
1Department of Gastroenterology, Hainan Provincial People's Hospital, Haikou City 570311, Hainan Province, China
2Department of Gastroenterology, The Affiliated Hospital of Hainan Medical College, Haikou City 570103, Hainan Province, China
ARTICLE INFO
Article history:
Received 15 November 2014
Received in revised form 20 December 2014
Accepted 15 January 2015
Available online 20 February 2015
Pectic polysaccharides
Ulcerative colitis
Nuclear factor
Dextran sulfate sodium-induced colitis
Interleukin-17
Objective: To investigate the effects of pectic polysaccharides extracted from Rauwolfia verticillata (Lour.) Baill.var.hainanensis Tsiang on an experimental murine colitis model. Methods: Experimental colitis was induced by dextran sulfate sodium (DSS), and mice were divided into 4 groups: control, DSS alone, DSS plus SASP, DSS plus pectic polysaccharides. The disease activity index (DAI) and histological score were observed. The tumor necrosis factor TNF)-α and interleukin (IL)-17 levels were measured by enzyme-linked immunosorbent assay. IκB and NF-κB p65 expression were assessed by western blot analysis. Myeloperoxidase MPO) activity was determined by using MPO assay kit. Results: Administration of pectic polysaccharides significantly reduced the severity of DSS-induced colitis as assessed by DAI and histological score, and resulted in down regulation of MPO activity and NF-κB p65 expression and subsequent degradation of IκB protein, strikingly reduced the production of TNF-α and IL-17. Conclusions: Pectic polysaccharides extracted from Rauvolfia verticillata (Lour.)Baill.var. hainanensis Tsiang exerts beneficial effects in experimental colitis and may therefore provide a useful therapeutic approach for the treatment of UC.
1. Introduction
Ulcerative colitis (UC) is one of the idiopathic forms of inflammatory bowel disease (IBD). It is a chronic, relapsing inflammatory disease of the colon, characterized by ulcers in the colon and rectum. The etiology is still largely unknown. Environmental and genetic factors in combination with the microbial flora or specific microorganisms trigger an event, leading to the activation of an intestinal immune response[1]. Immune and non-immune cells create a cross talk via the secretion of soluble mediators and expression of cell adhesion molecules, resulting in further cell activation[1,2]. Mediators such as cytokines and chemokines play a role in cell recruitment and polarization, intercellular signal amplification or activation and differentiation. Considering these aspects, medical management of inflammatory bowel disease has changed considerably over the past decade[3].
Although much progress has been made in the management of the disease, definitive causal therapies for human UC until now are not available. In fact, 5-aminosalicyclic acid and its derivatives are still the drugs of choice for current medical treatment; corticosteroids, azathioprine, mercaptopurines and cyclosporine are used in more severe forms of the disease. However, the therapies not only show limited benefits but also have serious side effects[4]. Plant-sourced formulations purportedly possess anti-inflammatory and radical scavenging properties. Despite the lack of sufficient information on
the safety of herbal products, their use as alternative and/or complementary medicine is globally popular.
Rauwolfia verticillata (Lour.) Baill.var.hainanensis Tsiang is an erect evergreen shrub growing wild in the islands of Hainan. The dried root of Rauwolfia verticillata (Lour.) Baill. var. hainanensis Tsiang, according to the Pharmacopeia of the People's Republic of China (2010 ed), is a traditional Chinese herbal medicine. It has been earlier shown to possess an anti-inflammatory effect. In China, Rauwolfia verticillata (Lour.) Baill. var. hainanensis Tsiang has been extensively used for more than 1 000 years in the treatment for lowering blood pressure, treating arrhythmia and tumors. However, rauwolfia is rarely used to cure UC. Several researches found the pectin obtained from Rauvolfia serpentina L. could inhibit colitis in mice[5]. However, no systematic studies on its anti-inflammatory activity and mechanisms have been reported.
It is widely accepted that activation of the nuclear factorκB (NF-κB) signaling pathway and overexpression of associated cytokines such as tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), IL-10, and interferon-γ (IFN-γ) play key roles in the development of UC[6,7]. NF-κB regulates the expression of multiple pro-inflammation genes and is thus a key player in maintaining immune system homeostasis. Thus, inhibition of NF-κB and its associated molecules may be a novel therapeutic tool [8].
An increasing number of studies have focused upon the role of Th17 cells in the pathogenesis of UC. Th17 cells develop from naive T lymphocytes through distinct pathways from classical Th1 and Th2 cells. Th17 cells secrete IL-17, which promotes the recruitment of inflammatory cells into the intestinal mucosa via its ability to enhance the synthesis of chemoattractants and adhesion molecules on epithelial, endothelial and mesenchymal cells. It has been generally accepted that Th-17 probably playa key role in the occurrence and development of UC[9,10].
Therefore, we hypothesized pectic polysaccharides from Rauvolfia verticillata (Lour.)Baill.var.hainanensis Tsiang could exert its anti-inflammatory effect on UC by inhibiting the activation of NF-κB signaling pathway. In this study, we extracted pectic polysaccharides from Rauvolfia verticillata (Lour.) Baill. var. hainanensis Tsiang and elucidated the effects of anti-inflammatory in a murine model of dextran sulfate sodium (DSS)-induced colitis, which resembles human UC, in order to provide experimental evidence that pectic polysaccharides serves as a possible treatment for patients with UC.
2. Material and methods
2.1. Pectic polysaccharide extracted from Rauwolfia verticillata (Lour.) Baill. var. hainanensis Tsiang
Rauwolfia verticillata (Lour.) Baill. var. hainanensis Tsiang was obtained from the Jianfengling National Forest Park of Ledong li autonomous county, Hainan province, China. The extract method of pectic polysaccharide was in accord with Popov et al[5]. Briefly, the rootstock (5 g) dried at 40 ℃ was treated with diluted HCl (up to pH 4.4 ) at 50 ℃for 3 h and the material obtained was extracted with 0.7% aqueous ammonium oxalate. A polysaccharide fraction was precipitated with four volumes of 96% ethanol. After centrifugation at 8 000 g for 10 min, precipitate was collected and dried at 40 ℃. Subsequently, the precipitate was dissolved in distilled water and stored at -20 ℃.
2.2. Animals
A total of 40 female BALB/c mice (6-8 weeks old, 17-22 g) were purchased from the Huaxi Medical Animal Center of Sichuang University (Sichuang, China). All animals were acclimated under standard conditions and maintained in a 12-h light/12-h dark cycle at a temperature of 23 ℃± 1 ℃ with 55%±5% humidity. The animals were maintained with free access to a standard diet and tap water. All experimental procedures were approved by the local Animal Care and Use Committee, and were conducted following the guidelines of the animal care policy.
2.3. Drugs and reagents
DSS was purchased from Beijing Bitab Biotechnology Co. Ltd. (Beijing, China). Salicylazosulfa-pyridine (SASP, used as a positive control drug) was purchased from
Fuda Pharmaceutical Co. Ltd (China). The mice TNF-α and IL-17 enzyme-linked immunosorbent assay (ELISA) Kit, a product of R and D Systems (United States), was obtained from Shanghai Chuanxiang Biotechnology Co. Ltd. (Shanghai, China). The antibodies used in this study were anti-NF-κ B p65 (ab16502, Abcam), anti-inhibitor of NF-κB (IκB) α (ab32518, Abcam). Goat anti-rabbit IgG were purchased from Boster Biotechnology (Wuhan, Hubei province, China).
2.4. Induction of colitis
BALB/c mice were randomly assigned to the following groups (n=10 per group): control, DSS alone (DSS), DSS plus SASP (100 mg/kg), DSS plus pectic polysaccharides (200 μL). Colitis was induced in BALB/c mice by adding DSS (molecular weight: 36-50 kDa; MP Biomedicals) to drinking water at a level of 4% for 7 days. Control animals were given water only. After 7 d, DSS was then removed from the drinking water and the animals received 200 μL of normal saline (control group and UC group) or SASP (100 mg/ kg, UC plus SASP group) or 200 μ of pectic polysaccharides (UC plus pectic polysaccharides group) orally each day. All of the mice were sacrificed by cervical dislocation after 14 days.
2.5. Evaluation of disease activity index
The mice were checked daily for colitis based on body weight monitoring, gross rectal bleeding, and stool consistency. The disease activity index (DAI) was assessed in accordance with the method described by Murano et al[11]. The DAI was assessed by an investigator who was blind to the experimental groups.
2.6. Sample collection and histopathological examination
Mice were then sacrificed, and the colon and rectum were dissociated. After removal of the entire colon and rectum, the total length was measured, and the colon was then opened longitudinally, gently washed with ice-cold saline and blotted dry with filter paper. The colon tissue was then weighed and cut into several segments. A 1-cm colon segment 2 cm from the anus was fully expanded, affixed to filter paper, and then fixed in 10% neutral formalin. The tissues were then embedded in paraffin, stained with hematoxylin and eosin, and assessed under light microscopy. Colonic damage was scored as described previously. The remaining colon tissue was stored at -80 ℃ for biochemical measurements, Western blot analysis and ELISA analysis.
2.7. Determination of IL-17 and TNF-α level
The murine colon tissues were used for cytokines ELISA analysis. In addition, the murine colon homogenates were obtained by handwork, and centrifuged at 3 000 rpm, 10 min. The amounts of cytokines in the supernatants for TNF-α and IL-17 were determined by ELISA according to the manufacturer's instructions.
2.8. Western blot analysis
Nuclear and cytoplasmic extracts were prepared using a nuclear and cytoplasmic protein extraction kit (Beyotime, China). Briefly, colon tissues (approximately 80 mg) were homogenized in cytoplasmic extraction buffer. Tissue homogenates were rapidly lysed by vortexing. The homogenate was centrifuged at 12 000 g for 10 min at 4 ℃and proteins were collected from the supernatant. The pellets were re-suspended in 50 μL of nuclear protein extraction buffer, lysed by ultrasound in an ice water bath, and then centrifuged to yield the nuclear fraction. Protein concentration was measured with a BCA protein assay kit. Protein samples (50-80 μg) were separated using 8%-12% SDS-PAGE gels and transferred onto PVDF membranes. The membranes were blocked with 5% skim milk in Trisbuffered saline with Tween 20 for 2 h at room temperature and then incubated with primary antibodies to anti-NF-κB p65 and anti-IκBα, at a 1:1 000-1:10 000 dilution overnight at 4 ℃. Then, the membrane was washed 3 times for 10 min each and incubated with a horseradish peroxidase- conjugated anti-rabbit IgG antibody. Protein bands were detected using enhanced chemiluminescence detection kit (Beyotime Institute of Biotechnology) and visualized by exposure to photographic film. Finally, the densitometry values of each protein analyte normalized to GADPH were compared.
2.9. Myeloperoxidase activity
Myeloperoxidase (MPO) activity, an index of leukocyte recruitment, was measured with a myeloperoxidase assay kit according to the manufacturer's instructions (CytoStore, Alberta, Canada).
2.10. Statistical analysis
Statistical analyses were performed using SPSS software 11.5 for Windows. All results are expressed as mean±SD. Quantitative data were tested for homogeneity of variance. If the variance was homogeneous, one-way ANOVA followed by the Bonferroni post hoc test was used. Comparisons between groups of nonparametric data were made using the Kruskal-Wallis test followed by the Mann-Whitney U test. P< 0.05 was considered significant
3. Results
3.1. Effects of pectic polysaccharides on colonic mucosal damage in DSS-induced colitis
After induction of colitis with DSS, the colonic mucosa showed congestion, erosion, and hemorrhagic ulcerations. DAI score were monitored each day. Mice given 4% DSS in their drinking water for 7 d developed symptoms of colitis. Compared with control mice, the DAI value was significantly greater from day 9 to 14 in mice treated with DSS (P<0.05, Table 1).Compared to the UC group, the colitis symptoms were relieved from day 12 to 14 in mice of the UC plus pectic polysaccharides group and UC plus SASP group
Treatment of mice with pectic polysaccharides and SASP significantly meliorated experimental colitis as assessed by DAI and histological injury scores. As shown in Table 1 and Figure 2. Compared with normal control group, the DAI scores were increased markedly in mice with DSS-induced colitis (P<0.05, Table 1). There are no statistically significant between the two drug treatment groups.
After induction of colitis with DSS, Histological findings demonstrated marked epithelial destruction, inflammatory infiltration, crypt distortion, and submucosal edema (Figure 1). The control group given normal saline showed no histological alterations. Similarly, mice given SASP exhibited virtually the same normal histology with no inflammatory cell infiltration, oedema or crypt abscesses. Severe submucosal oedema, erosion, ulceration, inflammatory
cell infiltration and extensive destruction of mucosal layer were observed in the mucosa` of UC group animals. UC plus pectic polysaccharides group revealed attenuation in inflammation, characterized by suppression of erosion, ulceration, reduction in inflammatory cellular infiltrate, and protection against epithelium damage, although oedema was still existed (Table 2).
3.2. Effects of pectic polysaccharides on expression of NF-κ B p65 and IκB-α
When mice were treated with 4% DSS, the levels of NF-κB p65 were significantly increased, otherwise IκB-α protein was decreased in colonic tissue (Figure 2). In the case of NF-κB p65, treatments with Pectic polysaccharides and SASP resulted in a decrease of expression. However, IκB-α protein was increased in UC plus pectic polysaccharides group and UC plus SASP group, when compared with UC group in murine colon tissues by Western blot (P<0.05, Table 3).
3.3. Effects of pectic polysaccharides on TNF-α and interleukin-6 serum levels
IL-17 and TNF-α are considered important inflammatory mediators that play a key role in the pathogenesis of UC. In normal stimulation, colon cells release of pro-inflammatory cytokines, including IL-17 and TNF-α is protective in fighting off pathogens like bacteria, for example. To determine the effect of Pectic polysaccharides on major inflammatory cytokines in the colon, we determined the levels of IL-17and TNF-α (Figure 3). After 7 d of DSS administration, the levels of IL-17and TNF-α increased significantly. Pectic polysaccharides and SASP group; Likewise, IL-17 and TNF-α were down regulated in colon culture supernatants of UC patients by cultured with pectic polysaccharides and SASP group(Figure 4B).These results indicated that pectic polysaccharides have antiinflammatory effects in the DSS-induced colitis mouse model.
3.4. Effects of pectic polysaccharides on myeloperoxidase activity
Evaluation of leukocyte recruitment was assessed by the measurement of myeloperoxidase activity. As shown in Table 2, MPO activity levels were low in the colonic tissues of normal control mice and markedly increased in mice with DSS-induced colitis. However, MPO activity was significantly lower in mice with pectic polysaccharides and SASP than those with DSS-induced colitis (P<0.05).
Compared with that of normal controls, colon of DSS-treated mice showed complete destruction of epithelial architecture with loss of crypts and epithelial integrity, submucosal edema, and intense inflammatory cellular in all layers. Pectic polysaccharides and SASP treatment attenuated morphological damage but showed mild cellular infiltrate.
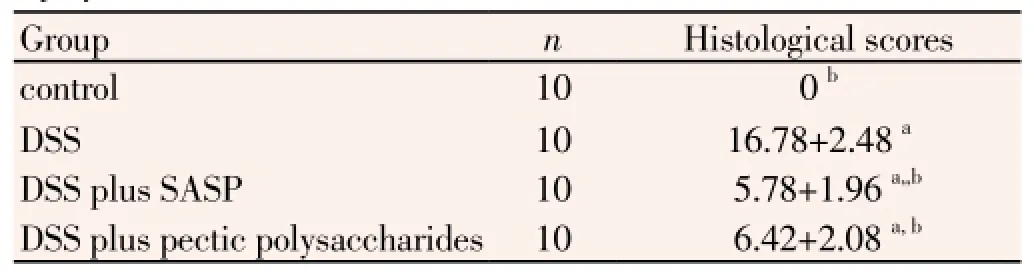
Table 2 Effect of pectic polysaccharide on clinical indices and histological injury scores.
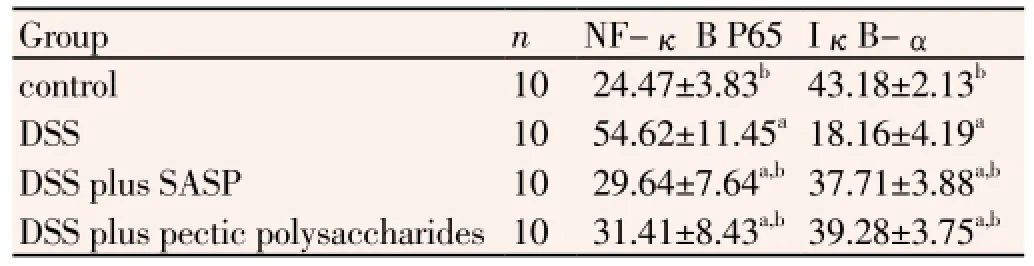
Table 3 Western blot results display protein expression of NF-kB P65/IκBα in the colonic tissues.
4. Discussion
UC is a chronic, relapsing disease that causes inflammation and ulcerations of the colonic mucosa with a variable extent and severity. The etiology of UC remains essentially unknown but the results from many studies in humans and animal models suggest that it is related to an abnormal immune response in the gastrointestinal tract, possibly associated with genetic and environmental - mainly microbial - factors[12]. Aminosalicylates, glucocorticoids and immunosuppressive drugs have been mainly used for the treatment and maintenance of remission of UC, but the side effects or toxicity of these drugs represents a major clinical problem[13,14]. For these reasons, natural medicine has become an alternative therapy in addition to the conventional therapies that are used to treat UC.
In the present study, we demonstrated that pectic polysaccharides extracted from Rauvolfia verticillata (Lour.) Baill.var.hainanensis Tsiang has an anti-inflammatory effect on colonic injury provoked by oral supplementation with DSS in mice. DSS-induced colitis is a well-established model that is phenotypically similar to UC in humans[15]. Oral administration of DSS for several days, leads to colonic epithelial lesions and acute inflammation characterized by the presence of neutrophils and macrophages within damaged segments. The reason for the deleterious effects of DSS is not well understood, however, epithelial cell permeability and macrophage activation have been proposed as potential mechanisms[16]. We administered pectic polysaccharides to evaluate the role of treatment with this product. The results presented herein clearly indicated that pectic polysaccharides efficiently relieved the symptoms of DSS-induced colitis in mice. In this study, we showed that pectic polysaccharides decreased DAI scores in mice with DSS-induced colitis, mitigated colitis-induced histological damage by suppression of erosion, ulceration, reduction in inflammatory cellular infiltrate.
To further characterize the nature of the inhibitory effect of pectic polysaccharides on pro-inflammatory proteins production, the NF-κB signal transduction pathway was examined. NF-κB is the major transcription factor which mediates inflammatory signaling[17,18]. A large number of studies showed that NF-κB played a broad regulatory role in ulcerative colitis, promoted expression of various proinflammatory cytokines including IL-17 and TNF-α. NF-κB exists mainly as a heterodimer composed of subunits of the Rel family, p50 and p65. In resting stage, NF-κB normally localizes to the cytoplasm, where it is bound by I κB-α protein. During inflammatory stimulus, IκB-α is phosphorylated by IκB kinase, subsequently degraded by proteasome, then NF-κB gets released and translocates into the nucleus, where it triggers the transcription of multiple genes involved in inflammatory cascade[19]. The results of this study suggested that pectic polysaccharides increased the production of IκB-α protein and decreased the expression of NF-κB p65.These indicated that pectic polysaccharides exhibited anti-inflammatory effects on ulcerative colitis which might be due to the inhibition of NF-κB signal transduction pathways[20].
In pathological conditions, including oxidative stress, toxicity, colon cells can be over stimulated and produce excess pro-inflammatory cytokines which result in inflammatory bowel disease like UC. IL-17 and TNF-α have been described as a key molecule in UC pathogenesis[21]. These cytokine, recruits leukocytes to inflammatory sites, stimulates monocytes and vascular endothelial cells to express cytokines, induces the cascade effects for other cytokines, and finally results in inflammatory lesions in tissues. Our data showed that pectic polysaccharides
reduced protein expression of IL-17 and TNF-α in mice with DSS-induced colitis. These results suggested that pectics exerted anti-inflammatory effects in UC. The results consisted with previous studies.
The reduction of inflammatory mediators IL-17 and TNF-α expression induced by pectic polysaccharides can be correlated with its antioxidant properties. The effects of antioxidant agents have been ascribed by some authors to inhibition of activation of the NF-κ B, which is activated by ROS with the subsequent induction and expression of various cytokines (such as IL-17) that are involved in the induction and development of UC.
A previous study has demonstrated the ability of pectic polysaccharides to modulate macrophage function through inhibition of chemotaxis and phagocytosis. Macrophages are one of the main sources of cytokines (ie. IL-17 and TNF-α), therefore, a possible modulation of macrophage activity by pectic polysaccharides could influence the decrease in cytokine production. This result suggests an important role for pectic polysaccharides as a modulator of the immune system and should be taken into account for future investigations.
In conclusion, the results showed that pectic polysaccharides extracted from Rauvolfia verticillata (Lour.) Baill.var.hainanensis Tsiang is able to prevent body weight loss and colon shortness, as well as increased the production of IκB-α protein, decreased the expression of NF-κB p65, inhibited the level of IL-17 and TNF-α . Rauvolfia verticillata (Lour.) Baill. var. hainanensis Tsiang might ameliorate ulcerative colitis and exhibit its antiinflammatory effects via increased expression of IκB-α proteins and suppressing NF-κB translocation.
Conflict of interest statement
We declare that we have no conflict of interest.
[1] Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature 2011; 474(7351): 307-317.
[2] Ordás I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ. Ulcerative colitis. Lancet 2012; 380(9853): 1606-1619.
[3] Katz JA. Advances in the medical therapy of in inflammatory bowel disease. Curr Opin Gastroenterol 2002; 18: 435-440.
[4] Reddy JG, Loftus Jr EV. Safety of in iximab and other biologic agents in the in inflammatory bowel diseases. Gastroenterol Clin North Am 2006; 35: 837-855.
[5] Popov SV,Vinter VG,Patova OA, Markov PA, Nikitina IR, Ovodova RG, et al. Chemical characterization and antiinflammatory effect of rauvolfian, a pectic polysaccharideof Rauvolfia callus. Biochemistry (Mosc) 2007; 72: 778-784.
[6] Imanifooladi AA, Yazdani S, Nourani MR. The role of nuclear factor-kappaB in inflammatory lung disease. Inflamm Allergy Drug Targets 2010; 9(3): 197-205.
[7] Papadakis KA, Targan SR. Role of cytokines in the pathogenesis of in inflammatory bowel disease. Annu Rev Med 2000; 51: 289-298.
[8] Pasparakis M. Role of NF-κB in epithelial biology. Immunol Rev 2012; 246(1): 346-358.
[9] Kobayashi T, Okamoto S, Hisamatsu T, Kamada N, Chinen H, Saito R, et al. IL 23 differentially regulates the Th1/Th17 balance in ulcerative colitis and Crohn's disease. Gut 2008; 57(12): 1682-1689
[10] Geremia A, Jewell DP. The IL-23/IL-17 pathway in inflammatory bowel disease. Expert Rev Gastroenterol Hepatol 2012; 6(2): 223-237.
[11] Murano M,Maemura K,Hirata I, Toshina K, Nishikawa T, Hamamoto N, et al. Therapeutic effect of intracolonically administered nuclear factor kappa B (p65) antisenseoligonucleotide on mouse dextran sulphate sodium (DSS)-induced colitis. Clin Exp Immunol 2000; 120: 51-58.
[12] Kaser A, Zeissig S, Blumberg RS.Inflammatory bowel disease. Annu Rev Immunol 2010; 28: 573-621.
[13] Burger D, Travis S. Conventional medical management of inflammatory bowel disease. Gastroenterology 2011; 140(6): 1827-1837.
[14] Cario E.Commensal-innate immune miscommunication in IBD pathogenesis. Dig Dis 2012; 30(4): 334-340.
[15] Goyal N, Rana A, Ahlawat A, Bijjem KRV, Kumar P. Animal models of inflammatory bowel disease: a review. Inflammopharmacology 2014; 22: 219-233.
[16] O'Connor PM, Lapointe TK, Beck PL, Buret AG. Mechanisms by which inflammation may increase intestinal cancer risk in inflammatory bowel disease. Inflamm Bowel Dis 2010; 16(8): 1411-1420.
[17] Sweeney CJ, Mehrotra S, Sadaria MR, Kumar S, Shortle NH, Roman Y, et al. These squiterpene lactone parthenolide in combination with docetaxel reduces metastasis and improves survival in a xenograft model of breast cancer. Mol Cancer Ther 2005; 4: 1004-1012
[18] Hehner SP, Heinrich M, Bork PM, Vogt M, Ratter F, Lehmann V, et al. Sesquiterpenelactones specially inhibit activation of NF-κ B by preventing the degradation of IκB-α and IκB-β. J Biol Chem 1998; 273: 1288-1297.
[19] Tanaka K, Hasegawa J, Asamitsu K, Okamoto T. Prevention of the ultraviolet B-mediated skin photoaging by a nuclear factor-κ B inhibitor, parthenolide. J Pharmacol Exp Ther 2005; 315: 624-630.
[20] Weber CK, Liptay S, Wirth T, Adler G, Schmid RM. Suppression of NF-kappa activity by sulfasalazine is mediated by direct inhibition of Kappa kinasesalpha and beta. Gastroenterology 2000; 119: 1209-1218.
[21] Chabaud M, Page G, Miossec P. Enhancing effect of IL-1, IL-17, and TNF-alpha on macrophage inflammatory protein-3alpha production in rheumatoid arthritis: regulation by soluble receptors and Th2 cytokines. J Immunol 2001; 167(10): 6015-6020.
ment heading
10.1016/S1995-7645(14)60306-0
*Corresponding author: Xin-Pu Miao, MD, Department of Gastroenterology, Hainan Provincial People's Hospital, Haikou City 570311, Hainan Province, China.
Tel and Fax: +86-0898-68622157
E-mail: miaoxinpu@163.com
Foundation project: It is supported by National Natural Science Foundation of China (Grant No. 81360603) and Natural Science Foundation of Hainan Province (Grant No. 813215).
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Effect of interferon plus ribavirin therapy on hepatitis C virus genotype 3 patients from Pakistan: Treatment response, side effects and future prospective
- Imported cases of dengue fever in Russia during 2010-2013
- Detection and characterization of Chlamydophila psittaci in asymptomatic feral pigeons (Columba livia domestica) in central Thailand
- Chemical composition of Rosmarinus and Lavandula essential oils and their insecticidal effects on Orgyia trigotephras (Lepidoptera, Lymantriidae)
- Total phenolic content, in vitro antioxidant activity and chemical composition of plant extracts from semiarid Mexican region
- Cytoprotective and anti-inflammatory effects of kernel extract from Adenanthera pavonina on lipopolysaccharide-stimulated rat peritoneal macrophages
