Effect of G-CSF and TPO on HIBD in neonatal rats
2015-12-08XueMeiLiuYiFengAiMinLi
Xue-Mei Liu, Yi Feng, Ai-Min Li
1Department of Pediatrics, Affiliated Yantai Yuhuangding Hospital of Qingdao University Medical College, Yantai 264000, Shandong Province, China
2Medical Insurance Office of Yantai Zhifu Hospital, Yantai 264000, Shandong Province, China
Effect of G-CSF and TPO on HIBD in neonatal rats
Xue-Mei Liu1, Yi Feng2*, Ai-Min Li1
1Department of Pediatrics, Affiliated Yantai Yuhuangding Hospital of Qingdao University Medical College, Yantai 264000, Shandong Province, China
2Medical Insurance Office of Yantai Zhifu Hospital, Yantai 264000, Shandong Province, China
ARTICLE INFO
Article history:
Received 15 November 2014
Received in revised form 20 December 2014
Accepted 15 January 2015
Available online 20 February 2015
G-CSF
TPO
BHID
Neonatal rats
Nestin
Objective: To observe effect of granulocyte colony-stimulating factor (G-CSF) and restructure human thrombopoietin on hypoxic-ischemic brain damage (HIBD) in new born rats. Methods: A total of 60 neonatal SD rats were selected and divided into 4 groups, with 15 in each group. Group A served as control group. Rats of Groups B-D were prepared for HIBD model by ligation of left common carotid artery combined with hypoxia method. Rats of Group A were only completed with free left common carotid artery without ligation and hypoxia operation. After HIBD model preparation, Group B was administrated with subcutaneous injection of normal saline for placebo treatment; Group C was administrated with cervical subcutaneous injection of 0.5 μg/10 g granulocyte colony stimulating factor (G-CSF) for 5 d (Once a day); Group D was administrated with intraperitoneal injection of 15 U/10 g recombinant human thromobopoietin (rhTPO) for treatment. After modeling for 7, 14 and 21 d, 5 rats were sacrificed in each group, respectively. Brain quality damage (%) conditions of experimental animals in each group were compared in different time points, and cerebral histopathological changes of each group were observed. Expression of nestin in rats of each group was detected by immunohistochemical method. Results: After modeling for 7, 14 and 21 d, brain quality damages (%) of Groups B, C and D were significant higher than that of in Group A (P<0.05), while brain quality damage (%) degree of Group B was the highest in different time points, followed by Groups D and C, respectively. It was significant different compared among groups (P<0.05). The histopathological observation showed that degrees of brain damages in Groups C and D were significant lower than that of in Group B. After modeling for 7, 14 and 21 d, nestin positive cell populations in Groups B, C, and D were significant higher than Group A (P<0.05). Nestin cell populations of Group C in different time points were significant higher than Groups B and D (P<0.05). There was no significant difference in nestin positive cell populations in the above time points between Groups B and D (P>0.05). Conclusions: Both G-CSF and TPO can protect the nervous system of HIBD neonatal rats. G-CSF can promote the proliferation and differentiation of neural precursor cells to decrease the degeneration and necrosis of nerve cell. TPO can obviously ameliorate morphology index of HIBD rats. Through regulating ratio of TIMP-1 and MMP-9, TPO can maintain the integrity of blood brain barrier to relieve the occurrence of brain damage.
1. Introduction
Neonatal hypoxic-ischemic brain damage (HIBD) is caused by various factors of the neonatal hypoxia and asphyxia in the perinatal period, which is common in clinic[1-3]. It is reported that about 4 million newborns die each year worldwide, among which 23% death is caused by asphyxia[4]. Cerebral neuronal apoptosis was the main pathological change during the course of the disease, which can lead to permanent neurological damage or death in newborns. With the advance of medical technology, gratifying achievements were made in neonatal asphyxia recovery technology in clinic, but there is no significant improvement in HIBD prognosis. The main reason of tardive cerebral neuronal apoptosis caused by hypoxia-ischemia is nerve cell apoptosis. In the early period, nerve injury can be reduced if patients receive interventional treatment timely and effectively and cascade reaction of nerve cell apoptosis is blocked[5].
Granulocyte colony stimulating factor (G-CSF), produced by macrophage and fibroblast, can stimulate proliferation and differentiation in marrow neutrophil precursor cells[6]. It is confirmed by an animal experiment that in HIBD rat's model, after treatment with G-CSF, it can promote the regeneration of neurons in brain damage area of HIBD rats and increase the nerve cell numbers. Hence, it is speculated that G-CSF is protective for HIBD rat's nerve. Thrombopojetin (TPO), which is the hematopoietic growth factor secreted by liver, can promote hematopoietic stem cell to the differentiation of colony-forming unitmegakaryocyte and stimulate maturity of megakaryocyte and release of platelets[7]. It has been confirmed that TPO can improve sensorimotor function of post-HIBD and reduce brain damage[8]. The present study aims to discuss the protective effect and mechanism of G-CSF and TPO for nerve of neonatal HIBD rats. Neonatal SD rats were selected to prepare for HIBD model. G-CS and recombinant human thromobopoietin (rhTPO) were injected to observe the nerve protective effect of HIBD rats.
2. Materials and methods
2.1. Experimental animal
A total of 60 SD rats of clean grade of both sexes, aged 7 days old, weighing (14.2±2.5) g were selected and purchased from Medical Experimental Animal Center of Guangdong Province. Experimental rats were fed with food and water ad libitum by ClassⅡ breeding. Experimental process on animals was strictly followed according to regulations of Guide for the Care of Laboratory Animals implemented in 2006.
2.2. Instruments and reagents
Systematic microscope BX40-12J02 (Olympus, Japan), CY-12C Oxygen Concentration Tester (Jiande Meicheng Electrochemical Analysis Instrument Plant), and BI-2000 Medical Image Analysis System (Westernization Instrument Technology Co., Ltd) were used. Sevoflurane (inhaled) (Jiangsu Hengrui Medicine Co. Ltd.), TPO injection (Drug approved by S20010062, Kirin Kunpeng Bio-pharmaceutical Co. Ltd), G-CSF injection (Drug approved by S20050048, Shenyang Sunshine Pharmaceutical Co.,Ltd.) were used in the present study. Hematoxylin and Eosin staining kit, DAB chromogenic reagent kit, HistostainTM-Plus Kits and white blood cells diluents were provided by Nanjing Jiancheng Bioengineering Institute.
2.3. Modeling approach
HIBD model was prepared by ligation of left common carotid artery combined with hypoxia method[9]. Experimental animals were anesthetized by sevoflurane inhalation and fixed in supine position. After regular disinfection, a longitudinal incision was cut in 2 mm to the left of centre of neck. The tissue was isolated and common carotid artery was exposed. The left carotid artery was ligated by aseptic ligature. After 2 h of recovery of closed incision, animals were put in homemade seal box, and mixed gas including 8% O2and 92% N2was furnished for 2 h (1.5-2.5 L/min). After modeling of 30 min, brain damage symptoms including disequilibrium, polypnea, dystonia, accidie and drowsiness were found in experimental animals. After reoxygenation, behavior of rats was rotated to ligation side. That modeling was considered as successful.
2.4. Animal groups
A total of 60 SD rats were divided into 4 groups, with 15 in each group. Group A served as control group in which rats ubderwent only free left common carotid artery without ligation and hypoxia operation. Rats of Groups B-D were prepared for HIBD model by ligation of left common carotid artery combined with hypoxia method. After HIBD model preparation, Group B was administrated with subcutaneous injection of normal saline for placebo treatment; Group C was administrated with cervical subcutaneous injection of 0.5 μg/10 g G-CSF for 5 d (Once a day); Group D was administrated with intraperitoneal injection of 15 U/10 g rhTPO for treatment.
2.5. Observation of experiment
After modeling for 7, 14 and 21 d, 5 rats were sacrificed in each group, respectively. Brain quality damage (%) conditions of rats in each group were compared after modeling for 7, 14 and 21 d, and cerebral histopathological changes of rats in each group were observed on Day 21 modeling. Expression of nestin in rats of each group was detected by immunohistochemical method.
2.5.1. Histological observation
Experimental animals were anesthetized by sevoflurane inhalation, and then thoracotomy was done to expose heart. After cutting bilateral jugular vein, normal saline was infused to ventriculus sinister. When lung became white and effluent from broken ends of jugular vein, brain tissue was taken to wash with distilled water, dried and
placed in 4% paraformaldehyde for fixation at 4 ℃ for 48 h. After conventional gradient ethanol dehydration, xylene transparent, and paraffin embedding (4 μm), brain tissue was sliced to coronary shape and stained with HE staining. The histological changes were observed under microscope.
2.5.2. Nestin detection
Tissue slice was dehydrated by conventional gradient ethanol dehydration, washed with distilled water for three times, and placed in 3% H2O2for 10 min at room temperature. After that, the slice was washed with distilled water for three times and placed in 0.01 M sodium citrate buffer (pH 6.0) with oven heating for 10-20 min. After kept for 20 min, it was washed with PBS for three times, and then normal goat serum was added to sealing for 20 min at room temperature, after which raffinate was removed. A total of 50 μ L of nestin antibodies were added for dilution, incubated for 2 h at room temperature, and washed with PBS for three times. Then 50 μL of biotinylated secondary antibodies were added and incubated in an incubator of 37 ℃, and then washed with PBS for three times. The color development was performed DAB solution. After staining, 5 views were selected randomly to shoot under the 40× objective lens, which was counted by Image Analysis Software. The average number of unit area of nestin positive cells in each group was calculated.
2.6. Statistics analysis
Data were analyzed by SPSS 13.0 statistical software and expressed as (mean±sd). S-N-K tests (Student-Newman-Keuls, q test) were used to compare with the data among groups. P<0.05 was considered as statistically significantly different.
3. Results
3.1. Comparison of brain quality damage (%) in different time points
After modeling for 7, 14 and 21 d, brain quality damages (%) of Groups B, C and D were significant higher than that of in Group A (P<0.05), while brain quality damage (%) degree of Group B was the highest in different time points, followed by Groups D and C, respectively. It was significant different compared among groups (P<0.05) (Table 1).
3.2. Histopathological changes of brain tissues in each group after modeling for 21 d
The microscopic displayed that in Group A, neurogliocyte and nerve cell of cerebral cortex were in alignment with normal morphology. Cell nucleus of nerve cell was in centre, with abundant cytoplasm and large counts, and there was no lack of nerve cell. In Group B, cerebral cortical neuron of damage side was in disordered arrangement, and nerve cell was shrinking obviously with dissolution of cell body. Axis and dendrite could not be recognized under microscope, and some were of nuclear cracking phenomenon. In rats of Group C, nerve cell was basically regularly arranged and survival numbers were increased. The typical structures such as axis and dendrite could be recognized. The damage of Group C was obviously lower than Group B. The observed result of Group D under the microscope was similar with Group C, but the damage degree was lower than Group B (Figure 1).
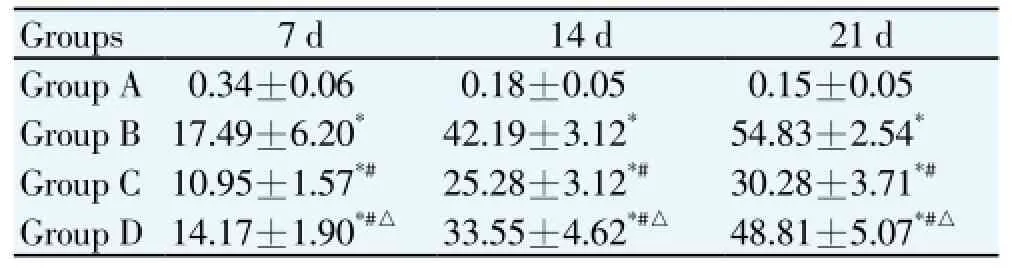
Table 1 Comparison of the treatment of brain quality damage (%) in different time points.
3.3. Comparison of nestin cell population in different time points
After modeling for 7, 14 and 21 d, nestin positive cell populations in Groups B, C, and D were significant higher than Group A (P<0.05). Nestin cell populations of Group C in
different time points were significant higher than Groups B and D (P<0.05). There was no significant difference in nestin positive cell populations in the above time points between Groups B and D (P>0.05) (Table 2).

Table 2 Comparison of nestin positive cell populations after modeling for 7, 14 and 21 d.
3.4. Nestin expression in different time points
The microscopic observation showed that nestin positive protein was mainly located in axon, cytoplasm and dendrite of nerve cell, and was largely expressed in hippocampus and cerebral tissue where the expression was claybank. In Group A, only a few nestin protein positive expressions were in cerebral cortex and hippocampus with little change of counts in different time points. After modeling of Groups B-D for 7 d, little nestin positive protein expression could be seen in cytoplasm, axon and dendrite of nerve cell. On Day 14, nestin positive protein expression was slightly increased than that of on Day 7, while on Day 21 it was decreased compared with that on Day 7 (Figure 2).
4. Discussion
Neonatal HIBD is often caused by asphyxia in the perinatal period, which can create permanent damage of nervous system in newborns, and severe case can lead to death in children. The pathogenesis is a multistage pathological process with main manifestation of neuronal apoptosis which is caused by anoxia and ischemia of brain cell[10]. Ligation of arteria carotis communis combined with hypoxia method is a common used way for model preparation in HIBD research. Since blood supply system in rat's brain is similar with that of in human's that circle of Willis was formed by vertebral artery and internal carotid. Hence, a certain degree of anoxia should be combined in the process of model preparation so that brain ligation side can form HIBD. In the present study, HIBD rat model was prepared successfully to confirm that this method can prepare for HIBD rat model rapidly and effectively.
TPO, which is secreted by liver, is a hematopoietic growth factor. The animal experiment found that TPO receptors were existed in cerebral hemisphere, cerebellum and neural stem cell of mice which indicated that there were a large number of TPO receptors in central nervous system[11]. Another study confirmed that TPO could promote the growth of C17.2 cell and activate PI3-K/AKT signal path to reduce the nerve cell apoptosis. Nestin is an antigen sign of undifferentiated pluripotent neural stem cells, which can up-regulate nestin expression by proliferation activity of the nervous system under the stress of brain damage. Nestin positive counts can reflect the change of precursor cell of rat brain nerve[12]. In this study, after modeling of 7, 14 and 21 d, nestin cell populations in Groups B-D were all higher than that of in Group A (P<0.05), which showed that Hypoxia-ischemia stimulation could cause the up-regulation of nestin expression. Nestin cell populations in Group C in different time points were higher than Groups B and D (P<0.05), which suggested that the role of TPO in protecting nerve was probably different from G-CSF. The results indicated that brain quality damage (%) of rats in Group D is significant lower than Group B, and histopathological observation showed that damage of brain tissue was significant lower than Group B, which was similar to Group C. This confirms that TPO can relieve HIBD, which is in agreement with literature[13]. Through regulating ratio of TIMP-1 and MMP-9, TPO can maintain the integrity of blood brain barrier to avoid the occurrence of secondary brain edema and brain damage[11]. It was confirmed that after G-CSF injection, CD34+bone marrow stem cell can migrate to the diseased region of nervous system through BBB and differentiate into
gitter cell and nerve cell[14]. Besides, G-CSF can regulate and control the apoptosis gene in the cell nucleus to make an effect on the apoptosis process of nerve cell and play a role of anti-apoptosis. Another study reported that G-CSF can significantly ameliorate the nervous system function of HIBD children[15]. In this study, the brain damage degree of Group C was lower than Group B in different time points, and the brain quality damage (%) was also less than Group B (P<0.05), which indicated that G-CSF had a significant protective effect on the nervous system of HIBD neonatal rats. Histological observation showed that HIBD neonatal rats treated by G-CSF, nerve cells were basically regularly arranged and survival numbers were increased, the typical structures such as axis and dendrite could be recognized, and the damage was significantly reduced, which indicated that G-CSF had the effects of repairing morphology of brain tissue and inhibiting neuronal apoptosis. After modeling in different time point, nestin cell population of rats in Group C was significantly higher than Group D (P<0.05). The reason for this may be that after anoxia and ischemia of brain tissue, the stimulation of local inflammation and blood-brain barrier damage promoted bone marrow stem cell to migrate to brain damage lesions and differentiate into astrocyte and nerve cell so as to promote the restoration of the injury of cerebral ischemia, which showed that G-CSF is a CVNPF that has a good application prospect.
The results of this study showed that both G-CSF and TPO have a protective role on the nervous system of HIBD neonatal rats. G-CSF can promote proliferation and differentiation of neural precursor cells so as to reduce the degeneration and necrosis of nerve cell. TPO can obviously ameliorate the morphology index of HIBD rats. Through regulating ratio of TIMP-1 and MMP-9, TPO can maintain the integrity of blood brain barrier to relieve the occurrence of brain damage.
Conflict of interests
We declare that we have no conflict of interest.
[1] Guo MF, Yu JZ, Ma CG.Mechanisms related to neuron injury and death in cerebral hypoxic ischaemia. Folia Neuropathol 2011; 49(2): 79-87.
[2] Northington FJ, Chavez-Valdez R, Martin LJ. Neuronal cell death in neonatal hypoxia-ischemia. Ann Neurol 2011; 69(5): 743-758.
[3] Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, et al. Global, regional, and national causes of child mortality:an updated systematic analysis for 2010 with time trends since 2000. Lancet 2012.[Epub ahead of print].
[4] Chen HJ, Wei KL, Zhou CL, Yao YJ, Yang YJ, Fan XF, et al. Incidences of brain injuries in premature infants in seven large cities of China. J Chin Pediatr 2011; 29(11): 1001-1011.
[5] Zhao H, Zhang QR, Lu XM, Zhang HP, Chen XX. Effects of hyperbaric oxygen on caveolin-1 and matrix metalloproteinase-9 in brain tissues after cerebral focal ischemia-reperfusion in rats. Chin J Reh Med 2011; 26(6): 550-552.
[6] Lin XJ, Yang YJ, Qi BX, Wang X, Song JH. Protective effects of baicalin pretreatment on hypoxic-ischemic brain damage in neonatal rats. Chin J Contemp Pediatr 2006; 8(3): 221-224.
[7] Lin X, Li M, Hu Y, Han Z, Zhang H, Shang A, et al. An experimental research of neuroglobin expression changes and neural apoptosis after traumatic brain injury. Chin J Appl Physiol 2010; 26(1): 39-44.
[8] Li W, Wu Y, Ren C, Zheng X, Zhang C. The activity of recombinant human neuroglobin as an antioxidant and free radical scavenger. Proteins 2011; 79(1): 115-125.
[9] Zhou J, Li J, Rosenbaum DM, Barone FC. Thrombopoietin protects the brain and improves sensorimotor functions: reduction of stroke-induced MMP-9 upregulation and blood-brain barrier injury. J Cerebral Blood Flow Metabolism 2011; 31(3): 924-933.
[10] Jia J, Hu Y, Wu Y, Liu G, Yu H. Effectiveness of nerve growth factor in treatment of acute cerebral ischemia in rats. Neural Injury Functional Reconstruction 2011; 6(1): 1-5.
[11] Acioly MA, Carvalho CH, Koerbel A, Löwenheim H, Tatagiba M, Gharabaghi A. Intraoperative brainstem auditory evoked potential observations after trigeminocardiac reflex during cerebellopontine angle surgery. J Neurosurg Anesthesiol 2010; 22(4): 347-353.
[12] Lavrik IN, Krammer PH. Regulation of CD95/Fas signaling at the DISC. Cell Death Differ 2012; 19(1): 36-41.
[13] Zhang Z, Zhou W, Lu W, Rong X, Chen X. Effects of early therapeutic intervention with nerve growth factor on brain damage following severe asphyxia in neonates. Chin J Pract Pediatrics 2011; 26(1): 33-36.
[14] Jing ZW, Yan ZL, Zhou CX. Comparison of the network structural characteristics ofcalcium signaling pathway in cerebral ischemia after intervention by different components of Chinese medicine. J Tradit Chin Med 2011; 31(3): 251-255.
[15] Cao Y, Mao X, Sun C, Zheng P, Gao J, Wang X, Min D, et al. Baicalin attenuates global cerebral ischemia/reperfusion injury in gerbils via anti-oxidative and anti-apoptotic pathways. Brain Res Bull 2011; 85(6): 396-402.
ment heading
10.1016/S1995-7645(14)60303-5
*Corresponding author: Yi Feng, Medical Insurance Office of Yantai Zhifu Hospital, Yantai 264000, Shandong Province, China.
Tel: +86-13606380586
E-mail: liuxmtg@163.com
Foundation project: It is supported by Science and Technology Plan Projects of Yantai (No. 2004221).
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Effect of interferon plus ribavirin therapy on hepatitis C virus genotype 3 patients from Pakistan: Treatment response, side effects and future prospective
- Imported cases of dengue fever in Russia during 2010-2013
- Detection and characterization of Chlamydophila psittaci in asymptomatic feral pigeons (Columba livia domestica) in central Thailand
- Chemical composition of Rosmarinus and Lavandula essential oils and their insecticidal effects on Orgyia trigotephras (Lepidoptera, Lymantriidae)
- Total phenolic content, in vitro antioxidant activity and chemical composition of plant extracts from semiarid Mexican region
- Cytoprotective and anti-inflammatory effects of kernel extract from Adenanthera pavonina on lipopolysaccharide-stimulated rat peritoneal macrophages
