Antiprotozoal assessment and phenolic acid profiling of five Fumaria (fumitory) species
2015-12-08IlkayErdoganOrhanNilgunOzturkBilgeSener
Ilkay Erdogan Orhan, Nilgun Ozturk, Bilge Sener
1Department of Pharmacognosy, Faculty of Pharmacy, Gazi University, 06330 Ankara, Turkey
2Department of Pharmacognosy, Faculty of Pharmacy, Anadolu University, 26470 Eskisehir, Turkey
Antiprotozoal assessment and phenolic acid profiling of five Fumaria (fumitory) species
Ilkay Erdogan Orhan1*, Nilgun Ozturk2, Bilge Sener1
1Department of Pharmacognosy, Faculty of Pharmacy, Gazi University, 06330 Ankara, Turkey
2Department of Pharmacognosy, Faculty of Pharmacy, Anadolu University, 26470 Eskisehir, Turkey
ARTICLE INFO
Article history:
Received 15 January 2015
Received in revised form 20 February 2015
Accepted 15 March 2015
Available online 20 April 2015
Fumaria
Fumitory
Antiprotozoal
Malaria
Trypanosomiasis
Phenolic acid
Objective: To explore some Fumaria species which were recorded to be traditionally used against malaria and other protozoal diseases. Methods: Consequently, in the current study, antiprotozoal effect of the ethanol extracts obtained from five Fumaria species (Fumaria densiflora, Fumaria cilicica, Fumaria rostellata, Fumaria kralikii, and Fumaria parviflora) was investigated against the parasites; Plasmodium falciparum (malaria) and Trypanosoma bruceirhodesiense (human African trypanosomiasis) at 0.81 and 4.85 μg/mL concentrations. Results: Among them, Fumaria densiflora extract exerted the highest antiplasmodial (93.80%) and antitrypanasomal effect (55.40%), while the ethanol extracts of Fumaria kralikii (43.45%) and Fumaria rostellata (41.65%) showed moderate activity against Plasmodium falciparum. Besides, phenolic acid contents of the extracts were analyzed using high performance liquid chromatography (HPLC) and trans-cinnamic (4.32 mg/g) and caffeic (3.71 mg/g) acids were found to be the dominant phenolic acids in Fumaria densiflora. Conclusions: According to our results, Fumaria densiflora deserve further study for its promising antiprotozoal activity.
1. Introduction
Fumaria species (Fumariaceae), known as “fumitory, earth smoke, beggary, fumus, vapor, fumittery or wax dolls”, consist of a total of 46 species of annual herbs and common weeds in the world and many of them have been reported to have traditional utilization against hepato-biliary diseases throughout the world[1-4]. The genus Fumaria, named locally “sahtere” in Turkish, is represented by 16 species in the flora of Turkey[5] and several Fumaria species are consumed in tea form in Bosnia and Herzegovina, Turkey, and Cyprus[6-8], while the leaves of the plant are used in salads and eaten by French, Italians, Spanish, and Arabs in rural areas[9]. Various species of the plant have been reported to be used in folk medicine in India, Iran, and Pakistan against malaria and other parasitic diseases[3,10,11]. Besides, Fumaria parviflora (F. parviflora) was found to exert anti-nematocidal activity against in ruminants[12], while a notable antimycobacterial effect was observed with Fumaria officinalis (F. officinalis)[13].
Malaria caused by Plasmodium falciparum is a vectoral disease frequently seen in sub-Saharan African and Asian countries. According to WHO statistics, around 34.8 million cases and 45 600 deaths attributable to malaria were reported in the Asia region during 2010, where over 85% of the cases happened in India, Indonesia, Myanmar, and Pakistan[14,15]. On the other hand, sleeping sickness, also known as human African trypanosomiasis (HAT), is another neglected vectoral disease caused by Trypanosomabrucei which is common in tropical regions of the world, particularly prevalent in Africa continent[16]. For instance; it was documented that more than 1 000 new cases per year was declared only in the Central African Republic and Congo in 2009[17]. Malaria and trypanosomiasis, therefore, still constitute a major health problem since the drugs used against these parasites seems to be often unsuccessful and possess high toxicity. Furthermore, another problem with the current antimalarial and antitrypanosomal drugs is occurrence of
quick resistance, which increases demand to development of more effective, less toxic, and inexpensive drugs.
Taking the traditional use of Fumaria species against malaria and other parasitic diseases into consideration, our goal was to test the ethanol extracts of five Fumaria species [Fumaria cilicica Hausskn. (FC), Fumaria densiflora DC. (syn. Fumaria micrantha Lag.) (FD), Fumaria kralikii Jordan (syn. Fumaria anatolica Boiss.) (FK), Fumaria parviflora Lam (FP), and Fumaria rostellata L (FR)] against the malaria vector; Plasmodium falciparum as well as Trypanosoma brucei rhodesiense (T. brucei rhodesiense); the factor of human African trypanosomiasis and identify their phenolic acid profile using HPLC.
2. Material and methods
2.1. Plant materials
The aerial parts of the Fumaria species were collected from various locations throughout Turkey and identified by Prof. Dr. Bilge Sener of Department of Pharmacognosy, Faculty of Pharmacy, Gazi University (Ankara, Turkey). Voucher specimensare preserved at the Herbarium of Faculty of Pharmacy of Gazi University (Ankara, Turkey).
2.2. Preparation of extracts
The plant materials were dried in the shade; powdered, and weighed accurately in a digital balance. Then, the plant samples were soaked in ethanol (85%) for 3 days and shaken by hand occasionally. Following filtration, the ethanol phase of each species was evaporated in vacuo until dryness and the crude ethanol extracts were obtained. The extract yields (w/w) were calculated as follows; FC: 30.86%, FD: 33.12%, FK: 35.97%, FP: 30.08%, and FR: 32.29%.
2.3. Antitrypanosomal activity test
Minimum essential medium supplemented according to Baltz et al[18] with 2-mercaptoethanol and 15% heat-activated horse serum was added to each well of a 96-well microtiter plate. Serial drug dilutions were prepared within a range between 90 μg/mL and 0.123 μg/mL. Afterward, 104bloodstream forms of T. brucei rhodesiense STIB 900 were added into each well and the plate incubated at 37 ℃ under a 5% CO2atmosphere for 72 hours. Alamar Blue dye was then added to each well again and incubation was applied for another 2-4 hours. Following this duration, the plate was read in a ELISA microplate reader (SpectraMax Gemini XS microplatefluorometer, Molecular Devices Cooperation, Sunnyvale, CA, USA) using an excitation wavelength of 536 nm and emission wavelength of 588 nm[19]. Fluorescence development was expressed as percentage of the control.
2.4. Antimalarial activity test
Antimalarial activity was tested against the K1 strain of Plasmodium falciparum (resistant to chloroquine and pyrimethamine) by a modified (3H)-hypoxanthine incorporation method[20]. To sum up, infected human red blood cells in RPMI 1640 medium with 5% Albumax were exposed to serial drug dilutions in microplates. Following 48 hours of incubation at 37 ℃ in a reduced oxygen atmosphere, 0.5 μCi3H-hypoxanthine was put into each well. Cultures were incubated for another 24 hs before they were harvested. The radioactivity was counted using a BetaplateTMliquid scintillation counter (Wallac, Zurich, Switzerland). The results were calculated as counts per minute (CPM) per well at each concentration and expressed as percentage of the untreated controls.

Table 1 Antiprotozoal activity (%) of Fumaria extracts.
2.5. HPLC analysis conditions for phenolic acids in the extracts
An HPLC system consisting of a quaterner G1311A HPLC pump, model 1100 Autosampler, model G 1315B photodiode array detector was used for the analysis [all from Agilent GL Sciences Inc (Waldbronn, Germany)]. Standards and the Fumaria extracts were analyzed on a reverse-phase Zorbax Eclipse XDB-C18 column (150 mm, 4.6 mm id. and particle size 3 μm) (Agilent, Waldbronn, Germany). Ultra-pure deionized water was purified by Synergy Water Purification System (Millipore, Rotterdam, Netherlands) to a specific resistance of 18 mO cm. Chromatographic analysis of the extracts was carried out by a gradient elution [solution A, methanol:water:formic acid (10:88:2 by volume); solution B, methanol: water: formic acid (90:8:2 by volume)] as reported in our previous publication[21]. The flow-rate was 1 mL/minute, and the injection volume was 10 μL. Signals were detected at 280 nm. The internal standard technique was applied to increase the repeatability. The relevant extracts were dissolved in a mixture of methanol and
water (1:1 vol/vol), and the mixture was injected into the HPLC apparatus (Figure 1).
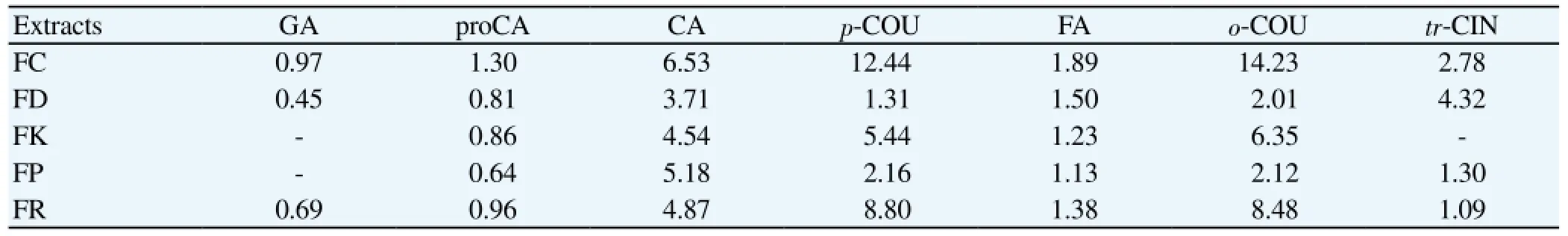
Table 2 Phenolic acid quantities in Fumaria extracts analyzed by HPLC.
3. Results
Among the ethanol extracts of five Fumaria species tested, FD extract showed the highest antiprotozoal effect (93.80%) at concentration of 4.85 μg/mL, followed by FK (43.45%) and FR (41.65%) (Table 1). Occurrence of the highest antitrypanasomal activity was also observed with the ethanol extract of FD (55.40%). Our HPLC analysis indicated that the richest extract considering phenolic acid quantity belonged to FC and FR (Table 2, Figure 1).
4. Discussion
Although some Fumaria species have been used in folk medicine against malaria in India, Iran, and Pakistan[3,10,11], we have not encountered any report about antimalarial and antitrypanasomal activities of Fumaria species up to date according to our literature survey. The genus Fumaria is well-known for its prosperous alkaloid content[22]. For instance; Fumaria densiflora, identified as the most active antimalarial extract herein, contains many isoquinolinealkaloids such as protopine, cryptopine, coptisine, palmatine, adlumidiceine, (+/-)-sinactine, fumafalorine, densiflorine, etc[23,24], while similar isoquinoline derivatives were also isolated from Fumaria kralikii and Fumaria parviflora[25]. In fact, several isoquinoline-type of alkaloids such as bidebiline E[26], 1-(4-hydroxybenzyl)-6,7-methylenedioxy-2-methylisoquinolinium trifluoroacetate[27], (+)-N-methylisococlaurine, atherosperminine, and 2-hydroxy-atherosperminine[28], and (-)-milonine were also reported from various other plant genera with antiprotozoal effect[29]. Therefore, it may be speculated that isoquinoline alkaloids seem to be the most responsible for the antimalarial effect of the Fumaria species.
According to our literature survey, only one report seems to be available on phenolic acid content of Fumaria species. In that early study by Sousek et al[30], presence of citric, coumaric, ferulic, fumaric, malic, S-hydroxybenzoic, protocatechuic, and caffeic acids were detected in several Fumaria species including FD and FP using gas chromatography-mass spectrometry (GC-MS), which is in accordance with our existent results. On the other hand, cinnamic acid derivatives have been stated to possess antiprotozoal activity presumably acting through inhibition of monocarboxylate transport and, therefore, blocking growth of intraerythrocytic Plasmodium falciparum[31]. p-Hydroxy-cinnamic acid also showed antiplasmodial effect against the multidrug-resistant W2mef strain of Plasmodium falciparum[32]. Since cinnamic acid is the most abundant phenolic acid in FD, the most active species in the current study, it might be contributing to the antiprotozoal effect of FD, at least in part. Consequently, the data obtained from this study confirms the antimalarial use of Fumaria species in folk medicine as its extracts displayed a notable profile in antimalarial assays.
In conclusion, the screening results suggest that the ethnopharmacological use of Fumaria species against malaria can be confirmed on a scientific base and the plant deserve further studies to be evaluated as promising antimalarial agent. To the best of our knowledge, this is the first study on antiprotozoal activity and phenolic acid profile of FC, FD, FK, FP, and FR.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgements
The authors would like to express their sincere thanks to Dr. Michael Adams from the Institute of Pharmaceutical Biology, University of Basel (Switzerland) as well as his collaborators for their kind assistance to provide antiprotozoal activity tests on the extracts of Fumaria sp.
[1] Aktay G, Deliorman D, Ergun E, Ergun F, Yesilada E, Cevik C. Hepatoprotective effects of Turkish folk remedies on experimental liver injury. J Ethnopharmacol 2000; 73: 121-129.
[2] Neves JM, Matos C, Moutinho C, Queiroz E, Gomes LR. Ethnopharmacological notes about ancient uses of medicinal plants in Tras-os-Montes (northern of Portugal). J Ethnopharmacol 2009; 124: 270-283.
[3] Gupta PC, Sharma N, Rao CV.A review on ethnobotany, phytochemistry and pharmacology of Fumariaindica (fumitory). Asian Pac J Trop Biomed 2012; 2: 665-669.
[4] Orhan IE, Sener B, Musharraf SG. Antioxidant and hepatoprotective activity appraisal of four selected Fumaria species and their total phenol and flavonoid quantities. Exp Toxicol Pathol 2012; 64: 205-209.
[5] Davis PH, Cullen J. Flora of Turkey and the East Aegean Islands. Edinburgh: Edinburgh University Press; 1984, p. 57-62.
[6] Šarić-Kundalić B, Dobeš C, Klatte-Asselmeyer V, Saukel J. Ethnobotanical study on medicinal use of wild and cultivated plants in middle, south and west Bosnia and Herzegovina. J Ethnopharmacol 2010; 131: 33-55.
[7] Cakilcioglu U, Turkoglu I. An ethnobotanical survey of medicinal plants in Sivrice (Elazig-Turkey). J Ethnopharmacol 2010; 132: 165-175.
[8] Karousou R, Deirmentzoglou S. The herbal market of Cyprus: Traditional links and cultural exchanges. J Ethnopharmacol 2011; 133: 191-203.
[9] Wright CA. Mediterranean vegetables: A cook's ABC of vegetables and their preparation. Massachusetts: The Harvard Common Press; 2001, p.163.
[10] Dwivedi A, Patel R, Jhade D, Sachan R, Argal A. Traditional phytotherapy used in the treatment of malaria by rural people of Bhopal, district of Madhya Pradesh, India. Ethnobot Leaflets 2009; 13: 475-479.
[11] Ghafari S, Esmaili S, Naghibi F, Mosaddegh M. Plants used to treat“taberebá” (malaria like fever) in Iranian traditional medicine. Int J Trad Herbal Med 2013; 1: 168-176.
[12] Hördegen P, Hertzberg H, Heilmann J, Langhans W, Maurer V. The anthelmintic efficacy of five plant products against gastrointestinal trichostrongylids in artificially infected lambs. Vet Parasitol 2003; 117: 51-60.
[13] Gautam R, Saklani A, Jachak SM. Indian medicinal plants as a source of antimycobacterial agents. J Ethnopharmacol 2007; 110: 200-234.
[14] Bhatia R, Rastogi RM, Ortega L. Malaria successes and challenges in Asia. J Vector Borne Dis 2013; 50: 239-247.
[15] Ghansah A, Amenga-Etego L, Amambua-Ngwa A, Andagalu B, Apinjoh T, Bouyou-Akotet M, et al. Monitoring parasite diversity for malaria elimination in sub-Saharan Africa. Science 2014; 345: 1297-1298.
[16] Franco JR, Simarro PP, Diarra A, Jannin JG. Epidemiology of human African trypanosomiasis. Clin Epidemiol 2014; 6: 257-275.
[17] Eperon G, Balasegaram M, Potet J, Mowbray C, Valverde O, Chappuis F. Treatment options for second-stage gambiense human African trypanosomiasis. Expert Rev Anti Infect Ther 2014; 12: 1407-1417.
[18] Baltz T, Baltz D, Giroud C, Crockett J. Cultivation in a semi-defined medium of animal infective forms of Trypanosoma brucei, T. equiperdum, T. evansi, T. rhodesiense and T. gambiense. EMBO J 1985; 4: 1273-1277.
[19] Räz B, Iten M, Grether-Bühler Y, Kaminsky R, Brun R. The Alamar Blue assay to determine drug sensitivity of African trypanosomes (T. b. rhodesiense and T. b. gambiense). Acta Trop 1997; 68: 139-147.
[20] Matile H, Pink JRL. Plasmodium falciparum malaria parasite cultures and their use in immunology. In: Lefkovits, I, Pernis, B. (Eds.). Immunological methods. San Diego: Academic Press; 1990, p. 221-234.
[21] Ozturk N, Tuncel M, Tuncel NB. Determination of phenolic acids by a modified HPLC: Its application to various plant materials. J Liq Chrom Rel Technol 2007; 30: 587-596.
[22] Suau R, Cabezudo B, Rico R, Nájera F, López-Romero JM. Direct determination of alkaloid contents in Fumaria species by GC-MS. Phytochem Anal 2002; 13: 363-367.
[23] Sener B.Densiflorine, a new alkaloid from Fumariadensi flora DC. Pharm Biol 1984; 22: 79-80.
[24] Táborská E, Bochořaková H, Soušek J, Sedmera P, Havlíček V, Šimánek V. Fumaflorine, a new 1-benzylisoquinoline alkaloid from Fumaria densiflora. Heterocycles 1997; 45: 817-821.
[25] Sener B.Turkish species of Fumaria L. and their alkaloids Ⅸ.Alkaloids of F. parviflora Lam., F. petteri Reichb.subsp. thuretii (Boiss.) Pugsley and F. kralikii Jordan. Pharm Biol 1988; 26: 61-62.
[26] Kanokmedhakul S, Kanokmedhakul K, Lekphrom R. Bioactive constituents of the roots of Polyalthia cerasoides. J Nat Prod 2007; 70: 1536-1538.
[27] Buchanan MS, Davis RA, Duffy S, Avery VM, Quinn RJ. Antimalarial benzylisoquinoline alkaloid from the rainforest tree Doryphora sassafras. J Nat Prod 2009; 72: 1541-1544.
[28] Nasrullah AA, Zahari A, Mohamad J, Awang K. Antiplasmodial alkaloids from the bark of Cryptocarya nigra (Lauraceae). Molecules 2013; 18: 8009-8017.
[29] Zahari A, Cheah FK, Mohamad J, Sulaiman SN, Litaudon M, Leong KH, et al. Antiplasmodial and antioxidant isoquinoline alkaloids from Dehaasia longipedicellata. Planta Med 2014; 80: 599-603.
[30] Soušek J, Guédon D, Adam T, Bochořáková H, Táborská E, Válka I, et al. Alkaloids and organic acids content of eight Fumaria species. Phytochem Anal 1999; 10: 6-11.
[31] Kanaani J, Ginsburg H. Effects of cinnamic acid derivatives on in vitro growth of Plasmodium falciparum and on the permeability of the membrane of malaria-infected erythrocytes. Antimicr Agents Chemother 1992; 36: 1102-1108
[32] Zofou D, Tene M, Tane P, Titanji VPK. Antimalarial drug interactions of compounds isolated from Kigelia africana (Bignoniaceae) and their synergism with artemether, against the multidrug-resistant W2mef Plasmodium falciparum strain. Parasitol Res 2012; 110: 539-544.
ment heading
10.1016/S1995-7645(14)60331-X
*Corresponding author: Ilkay Erdogan Orhan, Department of Pharmacognosy, Faculty of Pharmacy, Gazi University, 06330 Ankara, Turkey.
Tel.: +90 312 2023186
Fax: +90 312 2235018
E-mail: iorhan@gazi.edu.tr
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- A brief review on biomarkers and proteomic approach for malaria research
- Trigonelline protects the cardiocyte from hydrogen peroxide induced apoptosis in H9c2 cells
- In vitro cholinesterase inhibitory and antioxidant effect of selected coniferous tree species
- Monascus pilosus-fermented black soybean inhibits lipid accumulation in adipocytes and in high-fat diet-induced obese mice
- Profile and geographical distribution of reported cutaneous leishmaniasis cases in Northwestern Saudi Arabia, from 2010 to 2013
- Change of MicroRNA-134, CREB and p-CREB expression in epileptic rat
