新型酰胺功能化铱[III]配合物的光电性能
2015-12-07田雯雯祁争健孙岳明
沈 伟 田雯雯 祁争健 孙岳明
(东南大学化学化工学院, 南京 211189)
新型酰胺功能化铱[III]配合物的光电性能
沈 伟 田雯雯 祁争健*孙岳明*
(东南大学化学化工学院, 南京 211189)
设计并制备三种具不同官能团的铱[III]邻菲啰啉配合物: [Ir(ppy)2phen-Br]Cl, [Ir(ppy)2phen-COOH]Cl, [Ir(ppy)2phen-Si]Cl, 以及对比参照物[Ir(ppy)2phen-NH2]Cl. 其中, ppy为2-苯基吡啶, phen-Br为2-溴-2甲基-N-(1,10-菲啰啉-5-基)丙酰胺, phen-COOH为4-[(1,10-菲啰啉-5-基)氨基]-4-酰基丁烯酸, phen-Si为5-[N,N-二-3-(三乙氧硅)基]酰亚胺-1,10-菲啰啉, phen-NH2为5-氨基-邻菲啰啉, 并采用核磁共振(NMR)、质谱(MS)、紫外-可见(UV-Vis)吸收光谱、荧光(PL)光谱法和循环伏安法(CV)等对上述配合物进行了分析和表征. 光物理性能研究结果表明: 这些配合物在蓝-紫色可见光区域有较强吸收, 可发射出明亮的黄色到橙红色荧光, 量子效率达到12%以上. 相比较于参照物[Ir(ppy)2phen-NH2]Cl (5.78 μs), 三种新型配合物在量子效率未明显降低甚至提高的前提下, 荧光寿命有了显著的提高(9.18–12.00 μs). 其中, [Ir(ppy)2phen-Br]Cl (1) 不但有最高的荧光量子产率(32%)和最长的荧光寿命(12.00 μs), 而且也具有最好的氧传感性能, I0/I (无氧与纯氧条件下的荧光强度比值)可达到10.91. 这使得[Ir(ppy)2phen-Br]Cl有望成为接枝型, 较高性能的光学氧传感器的候选氧敏指示剂. 除此之外, 还通过含时密度泛函理论(TD-DFT)计算对配合物光电性能进行补充说明, 理论计算表明: 这些配合物是以铱为中心的近似八面体结构, 理论计算结果与实际实验数据相一致.
光电性能; 铱[III]配合物; 邻菲啰啉; 氧淬灭; 荧光传感
1 Introduction
One of the most attractive advantages of luminescent transition-metal complexes is their high sensitivity to surroundings, which makes them find applications in a variety of sensors or probes such as the monitoring of oxygen partial pressure,1–7biological measurements,8,9and flow visualization in the industries.10,11Among them, luminescent cyclometalated Ir(III) complexes have been frequently utilized as oxygen sensing materials, due to their excellent photophysical properties and efficient luminescence-quenching parameters such as: high quantum yield (Φ), large Stern-Volmer constant for oxygen, long excited state lifetime (τ), and large Stokes shift.10–23
As is well known that luminescent molecules of sensors or probes for oxygen are usually incorporated in a gas-permeable polymer host matrix,24–27and applied in various formats such as foils and fibers.28–33However, many early studies which physically incorporated Ir(III) complexes into matrices were proved unsatisfying.34–36The weak interactions between the probe molecules and the matrix would give little control over the clustering of emitting centers and frequently lead to inhomogeneous dispersion of the organic components within the matrices on a microscopic scale and unexpected sensing behaviors.37–39Therefore, a practical strategy to overcome the above problems is to covalently link the luminescent molecules to the matrix.40–43Herein, we present the synthesis, characterization, optical and oxygen sensing properties of three novel Ir(III) complexes with N^N ligands functionalized by amide derivatives (-NHCO-): [Ir(ppy)2phen-Br]Cl (1), [Ir(ppy)2phen-COOH]Cl (2), [Ir(ppy)2phen-Si]Cl (3). Ir(ppy)2phen-NH2]Cl (4) was also prepared as a contrast.
2 Experimental
2.1 Materials
Tetrahydrofuran (THF, AR), triethylamine (TEA, AR), dichloromethane (CH2Cl2, AR), N,N-dimethyl formamide (DMF, AR), acetonitrile (CH3CN, AR), methanol (CH3OH, AR), sodium bicarbonateare (NaHCO3, AR), hexane (AR), pyridine (AR), maleic anhydride (AR), ferrocene (98%), and tetrabutylammonium perchlorate (98%) were purchased from Sinopharm Shanghai Medical Instrument Co. Ltd. 2-Bromo-2-methylpropanoyl bromide (98%) and 3-(tri-ethoxysilyl)propyl isocyanate (95%) were purchased from Aladdin Chemistry Co. Ltd. THF, TEA, and pyridine were dried and distilled before use, and other reagents were used as purchased without further purification. Purity water was prepared by our department. [Ir(ppy)2Cl]2and phen-NH2were prepared by our research group according to literature methods.44,45
2.2 Apparatus
The absorption spectra of the complexes were characterized by using a UV-visible absorption spectroscopy (UV-2450, Shimadzu, Japan). Photoluminescence (PL) spectra were mensured using a FluoroMaxR-4 fluorescence spectrophotometer (HORIBA Jobin Yvon, France), and excited state lifetimes were taken on time-resolved fluorescence spectrometer (FLS920, Edinburgh Instruments, England). NMR spectra were acquired on a Bruker AV-500 (Bruker, US). Mass spectra (MS) were recorded on Q-TOF Micro System Mass Spectrome (Waters Micromass, US). Cyclic voltammetry (CV) was performed on CHI750C voltammetric analyzer.
2.3 Synthesis
2.3.1 Synthesis of N^N ligands
2-Bromo-2-methyl-N-(1,10-phenanthrolin-5-yl)propanamide (phen-Br): 5-amino-[1,10]-phenanthroline (0.30 g, 1.54 mmol) was placed in a round bottomed flask under N2. THF (dry, 55mL) and triethylamine (0.16 g, 1.58 mmol) were added and the suspension was stirred for 30 min. The mixture was cooled to 273 K before 2-bromo-2-methylpropanoyl bromide (0.43 g, 1.89 mmol) in THF (5 mL) was added dropwise. The mixture was left stirring overnight at room temperature. The solution was washed with 5% NaHCO3aqueous solution. After the collection and concentration of the organic layer, the residue was washed with water and ether to yield a red solid.1H-NMR (DMSO-d6), δ: 10.29 (s, 1H), 9.15–9.06 (m, 2H), 8.51 (dd, J = 8.1, 1.6 Hz, 1H), 8.41 (dd, J = 8.3, 1.6 Hz, 1H), 7.92 (s, 1H), 7.79 (m, 2H), 2.11 (s, 6H).
4-[(1,10-phenanthrolin-5-yl)amino]-4-oxobut-2-enoic acid (phen-COOH): 5-amino-1,10-phenanthroline (0.40 g, 2.05mmol) and maleic anhydride (1.00 g, 10.20 mmol) were suspended in CH2Cl2, and then the above mixture was heated to re-flux for 4 h. After cooling to room temperature, the mixture was filtered off and washed with CH2Cl2to give a yellow solid.461HNMR (DMSO-d6), δ: 10.65 (s, 1H), 9.24–9.12 (m, 1H), 9.07 (dd, J = 4.3, 1.6 Hz, 1H), 8.73 (dd, J = 4.8, 3.3 Hz, 1H), 8.49 (t, J = 9.0 Hz, 1H), 8.22 (s, 1H), 7.93-7.73 (m, 2H), 6.74 (d, J = 12.0 Hz, 1H), 6.39 (d, J = 12.1 Hz, 1H), 6.10 (s, 1H).
5-[N,N-bis-3-(triethoxysilyl)propyl]ureyl-1,10-phenanthroline (phen-Si): 5-amino-1,10-phenanthroline (0.20 g, 1.02mmol) was dissolved in 5 mL of dry pyridine. 3-(tri-ethoxysilyl)propyl isocyanate (1.7 mL, 6.37 mmol) was added, and then the mixture was stirred overnight at 353 K under N2. Cold hexane was added to the mixture and a white precipitate was obtained. The precipitate was filtered off, washed with cold hexane, and then dissolved in methanol. The solution was gravity filtered and the methanol was removed by rotary evaporation. Cold hexane was added to reprecipitate the compound. The purified product was filtered off and dried in vacuo as a white powder.471H-NMR (CHCl3-d), δ: 9.25 (s, 2H), 8.28 (t, J = 9.0 Hz, 2H), 7.89 (s, 1H), 7.71 (dd, J = 7.9, 4.4 Hz, 2H), 7.17(s, 2H), 3.71 (p, J = 7.0 Hz, 12H), 3.23 (dd, J = 12.8, 6.6 Hz, 4H), 1.63 (s, 4H), 1.36–0.90 (m, 18H), 0.60–0.44 (m, 4H).
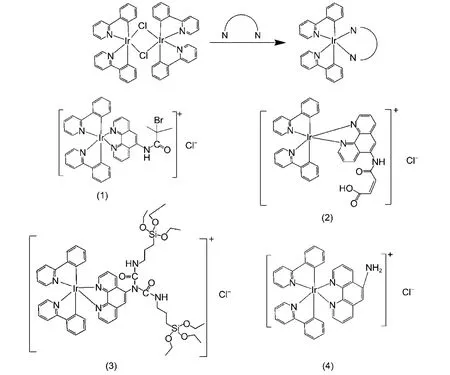
Scheme1 Synthetic procedure of Ir(III) complexes
2.3.2 Synthesis of Ir(III) complexes
[Ir(ppy)2phen-Br]Cl (1): complex (1) was prepared according to a procedure (shown in Scheme 1).48[Ir(ppy)2Cl]2(0.10 g, 0.09 mmol) and 2-bromo-2-methyl-N-(1,10-phenanthrol-in-5-yl)propanamide (0.08 g, 0.24 mmol) were dissolved in the mixture of CH2Cl2(15 mL) and CH3OH (15 mL) and then refluxed under N2for 4 h. After concentrating, the crude product was purified by column chromatography (silicagel; eluent: CH2Cl2/CH3OH) to give complex (1) as a red powder in 70% yield.1H-NMR (DMSO-d6), δ: 10.65 (d, J = 5.5 Hz, 1H), 8.93 (d, J = 7.1 Hz, 1H), 8.70 (t, J = 8.1 Hz, 1H), 8.40 (d, J = 9.1 Hz, 1H), 8.33–7.84 (m, 12H), 7.48 (s, 2H), 7.09–6.92 (m, 7H), 6.31 (d, J = 7.6 Hz, 2H), 2.13 (d, J = 2.9 Hz, 6H). Calculated for C38H20BrIrN5O ([M-Cl]): m/z = 844.2; found, 844.2.
[Ir(ppy)2phen-COOH]Cl (2): complex (2) was prepared similarly as for complex (1) by using [Ir(ppy)2Cl]2(0.10 g, 0.09 mmol) and 4-((1,10-phenanthrolin-5-yl)-amino)-4-oxobut-2-enoic acid (0.07 g, 0.24 mmol), and it was isolated as a yellow powder. Yield: 60%.1H-NMR (DMSO-d6), δ: 9.50 (d, J = 8.3 Hz, 1H), 9.13 (s, 1H), 8.79 (d, J = 8.1 Hz, 1H), 8.38–7.82 (m, 11H), 7.48 (d, J = 5.1 Hz, 2H), 7.18–6.87 (m, 6H), 6.41–6.24 (m, 3H), 5.98 (d, J = 13.1 Hz, 1H), 5.75 (s, 1H). Calculated for C38H27IrN5O3([M-Cl]): m/z = 794.2; found, 794.2.
[Ir(ppy)2phen-Si]Cl (3): the same procedure as for complex (1) was adopted for the preparation of [Ir(ppy)2Cl]2(0.10 g, 0.09 mmol) and 5-(N,N-bis-3-(triethoxysilyl)propyl)ureyl-1,10-phenanthroline (0.17 g, 0.24 mmol). It was isolated as a yellow powder. Yield: 70%.1H-NMR (DMSO-d6), δ: 8.90 (s, 1H), 8.50(t, J = 22.0 Hz, 3H), 8.25 (s, 4H), 8.01 (d, J = 44.2 Hz, 6H), 7.43 (d, J = 36.5 Hz, 3H), 7.01 (d, J = 27.0 Hz, 6H), 6.26 (s, 2H), 3.69 (d, J = 6.7 Hz, 12H), 3.07 (s, 4H), 1.43 (s, 4H), 1.16(d, J = 40.0 Hz, 18H), 0.42 (d, J = 30.8 Hz, 4H). Calculated for C54H67IrN7O8Si2([M-Cl]): m/z = 1190.4; found, 1190.4.
[Ir(ppy)2phen-NH2]Cl (4): complex (4) was prepared from [Ir(ppy)2Cl]2(0.1 g, 0.09 mmol) and 5-amino-1,10-phenan-throline (0.05 g, 0.24 mmol) with a orange yellow powder in 40% yield.1H-NMR (DMSO-d6), δ: 8.99 (d, J = 8.8 Hz, 1H), 8.38 (d, J = 8.5 Hz, 1H), 8.22 (d, J = 8.3 Hz, 2H), 8.13 (d, J = 5.0 Hz, 1H), 7.98―7.82 (m, 5H), 7.71 (dt, J = 13.2, 4.2 Hz, 2H), 7.48–7.38 (m, 2H), 7.27–6.81 (m, 10H), 6.26 (dd, J = 14.4, 7.4 Hz, 2H). Calculated for C34H25IrN5([M-Cl]): m/z = 696.2; found, 696.2.
3 Results and discussion
3.1 UV-Vis absorption spectra
The absorption spectra of the four complexes were measured in CH2Cl2, DMF, and CH3CN solutions (Fig.1). The intense bands observed in the range 287–300 nm are ascribed to spinallowed ligand-centered (LC)1π–π*transitions of C^N ligand of ppy, while the weak bands observed between 334 and 468 nm are arised from spin allowed [dπ(Ir)–π*(ligand)]1MLCT (metalligand charge transfer) transition. Somewhat extremely unconspicuous bands are observed at lower energies (longer than 468 nm), and these bands are ascribed to spin forbidden3MLCT transition.

3.2 Photoluminescence
PL emission spectra were recorded by excitation at the excited maximum. As shown in Fig.2 and Table 1, the emission maxima of the four complexes in CH2Cl2solutions lied at 599, 565, 602, and 574 nm, emitting bright yellow to orange light. With the increasement of solvent polarity, there were bathochromic shifts occuring in the emission spectra of the three novel complexes. The excitation spectra corresponded with the UV-Vis absorption spectra, and the maximum excitation wavelengths were all in the region of MLCT absorption bands. The Stokes shifts of complexes were longer than 130 nm, beneficial for the supression of the PL self-quenching.
PL quantum yield (Φ) is another important factor for oxygensensitive dyes. According to the optical dilute method49with a standard of quinine sulfate (Φ = 55%, quinine in 0.05 molL–1sulfuric acid), the PL quantum yields of complexes were measured and summarized in Table 2. The Φ value of complex is higher than 12%. Of them all, complex (1) has the highest quantum yield of 32%, and it may be contributed to the incorporation of Br which induces the internal heavy atom effect.50The average decay lifetimes (τ) of the four Ir(III) complexes in CH2Cl2solution (1 × 10–5molL–1) were also measured and listed in Table 2. All of the complexes possess long lifetimes, achieving microsecond level. The lifetimes of the three novel complexes are increased by 108%, 94%, and 59% in comparison to complex (4). As is well-known, a longer lifetime renders a higher probability of the collision between molecular oxygen and the excited states of the complexes. Therefore, the novel complexes with longer luminescence lifetime may possess better oxygen quenching properties.
3.3 Influence of solvent polarity on the luminescent properties of Ir(III) complexes
To explore the influence of solvent polarity on the fluorescent properties of the three novel complexes, the UV-Vis absorption spectra and PL spectra in different solutions were performed (Fig.1), and the Lippert-Mataga equation50,51was employed to investigate the solvent dependent spectral shifts:

where h is Planck's constant, c is the velocity of light in vacuum, υabsand υemare wavenumbers (cm–1) of the absorbance and fluorescence emissions, μeand μgare the dipole moments in the excited and ground state, α is the solute cavity radius, and C is constant. Δf is the solvent polarity obtained by Eq.(2) where ε is the dielectric constant and n is the optical refractive index. With the increasement of solvent polarity (CH2Cl2, DMF, and CH3CN), the three novel complexes showed a positive solvato-chromism and the Lippert-Mataga plots exhibited a linear behavior. These results might indicate that the general solvent effects were dominant in the spectral shifts.52

Fig.2 Luminescence spectra of the complexes in CH2Cl2solutions (1 × 10–4molL–1) under different oxygen concentrations
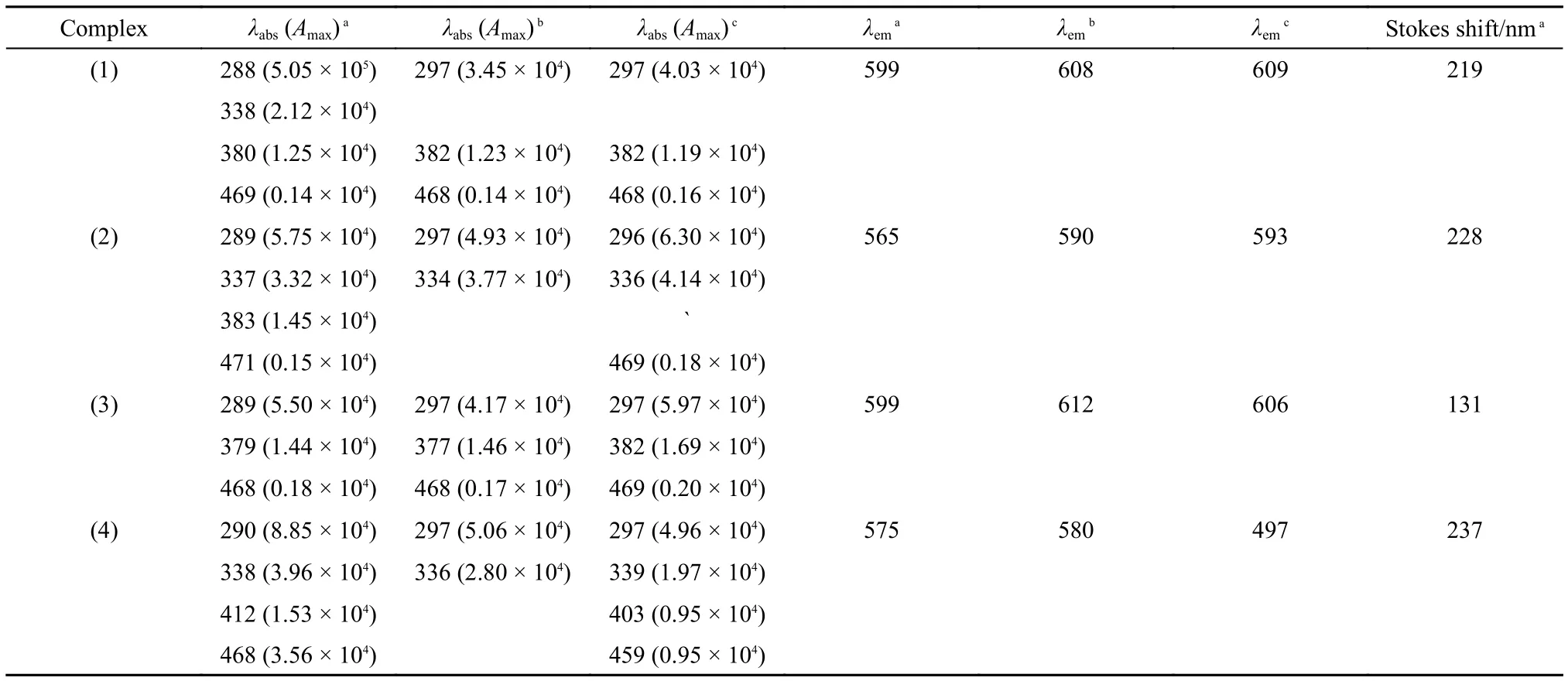
Table1 PL and UV-Vis absorption spectral parameters of the complexes in different solvents

Table2 PL parameters of the complexes in different solutions
3.4 PL quenching by oxygen
In order to investigate the oxygen sensing properties of Ir(III) complexes, the PL spectra of Ir(III) complexes in varied gas ambience were measured in CH2Cl2solutions (Fig.2). As we all know, the reduction of luminescence intensity of complexes induced by triplet oxygen should belong to collision-dependent quenching. The quenching process can be described by the Stern-Volmer equation as follow:53

where I0and ISare the PL intensities of the complex in the ab sence and presence of O2, and I is the PL intensity of the com plex under pure oxygen. KSVis the Stern-Volmer quenchin constant. The Stern-Volmer curves are shown in Fig.3, and th relative data are listed in Table 3.
As shown in Fig.2, the PL intensity dramatically decrease with the growth of oxygen percentage. When the solutions ar under pure oxygen, the emission intensities of the complexe drop by 86%–91%. These results were much better than thos of Ru(II) complexes in our previous research.54Among them complex (1) displayed the best linearity of quenching Stern Volmer plot, the highest KSVand the biggest I0/I factor of 10.9 with a Stern-Volmer equation as: I0/IS= 1.16 + 9.84[O2]. Thes results are in accord with the fact that complex (1) owns th highest quantum yield and the longest lifetime. Other two novel complexes also showed good oxygen quenching performances, and the I0/I values of complexes (2) and (3) were close to that of complex (4). These facts may suggest that these complexes still own good and even better oxygen quenching properties after the insertion of different appended groups on polycyclic aromatic hydrocarbon of 1,10-phenanthroline.

Fig.3 Stern-Volmer plots of the four complexes in CH2Cl2solution (1 × 10–4molL–1)?
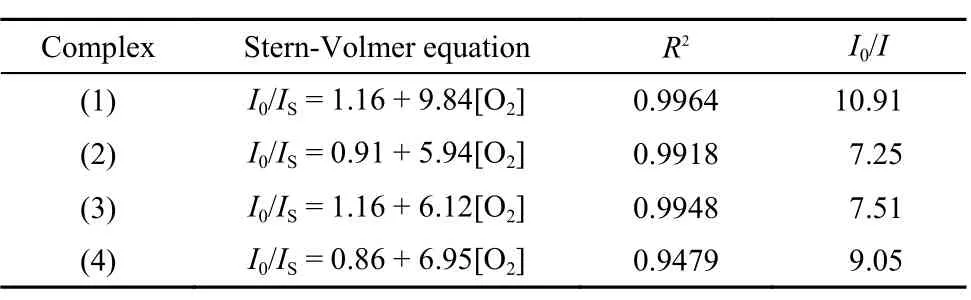
Table3 The data of the Stern-Volmer plots of four complexes in CH2Cl2solutions.
3.5 Electrochemical data
Cyclic voltammetry (CV) was performed in acetonitrile solutions at a scan rate of 100 mVs–1with a platinum disk as the working electrode, a Ag/AgCl electrode as the pseudo-reference electrode, and a platinum disk as the counter electrode. The supporting electrolyte was tetrabutylammonium perchlorate (0.1 molL–1) and ferrocene was selected as the internal standard. The solutions were bubbled with a constant nitrogen flow for 10 min before measurements. The relative data of electrochemistry are listed in Table 4. The values of the initial oxidation potentialsof the four complexes are close to each other in the range of 1.16–1.19 V, which could be assigned to metal-centered Ir(IV/III) oxidation. With regard to the initial reduction potentialsthey changed more obviously than oxidation potentials. This indicates that synthesized N^N ligands might have little effect on the highest occupied molecular orbital (HOMO) but more function on the lowest unoccupied molecular orbital (LUMO). Furthermore, the error between the electrochemical energy gaps obtained by cyclic voltammetryand the optical energy gaps calculated by band edge absorption of UV-Visis lower than 0.46 eV, which coincides with the literature.55

Table4 Orbit energy level and bandgap parameters of the complexes
3.6 Theoretical calculation
DFT calculations were performed using the Gaussian 03 software package.56The optimized ground-state geometries of these complexes at the B3LYP/6-31G level are shown in Fig.4 and the relative data are summarized in Table S1 (Supporting Information). The bond angles between central metal atoms and ligating atoms were about 76.32°–99.10° or 172.47°–174.32°, which indicated that all the mononuclear complexes were approximate octahedron structures with Ir(III) being coordination center. The data of electronic absorptions of complexes based on TD-DFT at the B3LYP/6-31G level were calculated and listed in Table S2 (Supporting Information). The relative frontiermolecular orbitals (FMOs) of Ir(III) complexes are shown in Fig.5, and the frontier molecular orbital compositions were analyzed and listed in Table S3 (Supporting Information). The electronic densities of the complexes for HOMOs were mainly based on the d-orbital of the centre metal (28%–54%) and the C^N ligand ppy (36%–59%), while the electronic densities for LUMOs were mainly distributed over the condensed nucleus part of N^N ligands (over 89%). These results were in good agreement with the electrochemical data. The excited states 4, 11, 24, and 35 of complex (1) have absorption bands at 456, 365, 318, and 298 nm in good agreement with the experimental results (Fig.1). The calculated electronic absorption spectra of the other three complexes are similar to complex (1), and they are also in accord with the experimental data.

Fig.4 Optimized geometry structures of the four complexes

Fig.5 Frontier molecular orbitals of the four complexes
4 Conclusions
Three novel luminescent complexes with functionalized lingand were designed, synthesized, and investigated. Complex[Ir(ppy)2phen-NH2]Cl was also prepared for comparison study meanwhile. Four complexes can emit bright yellow to orange light and the PL maxia are ranging from 565 to 612 nm. Among them, complex (1) obtains the highest I0/I value (10.91) and KSV(9.84), which is in aggreement with the highest quantum yield and the longest lifetime. The other two novel complexes also show good oxygen luminescence quenching performances. The energy gaps of the complexes from electrochemical method are ranging from 2.46 to 2.88 eV in accord with the optical energy gaps. Theoretical calculations based on B3LYP/6-31G level indicate that the novel complexes are approximate octahedron structures with Ir(III) being coordination center, and the calculated electronic absorptions are in good agreement with the UVVis data. The above results might provide valuable data for designing grafting and luminescent oxygen sensor with good performance.
Supporting Information: available free of charge via the internet at http://www.whxb.pku.edu.cn.
(1)Zelelow, B.; Khalil, G. E.; Phelan, G.; Carlson, B.; Gouterman, M.; Callis, J. B.; Dalton, L. R. Sensor Actuat. B-Chem. 2003, 96, 304. doi: 10.1016/S0925-4005(03)00547-1
(2)Borisov, S. M.; Vasylevska, A. S.; Krause, C.; Wolfbeis, O. S. Adv. Funct. Mater. 2006, 16, 1536. doi: 10.1002/adfm.200500778
(3)Borisov, S. M.; Wolfbeis, O. S. Anal. Chem. 2006, 78, 5094. doi: 10.1021/Ac060311d
(4)Kose, M. E.; Carroll, B. F.; Schanze, K. S. Langmuir 2005, 21, 9121. doi: 10.1021/La050997p
(5)Lupo, F.; Fragala, M. E.; Gupta, T.; Mamo, A.; Aureliano, A.; Bettinelli, M.; Speghini, A.; Gulino, A. J. Phys. Chem. C 2010, 114, 13459. doi: 10.1021/Jp1028917
(6)Schroder, C. R.; Polerecky, L.; Klimant, I. Anal. Chem. 2007, 79, 60. doi: 10.1021/Ac0606047
(7)Borisov, S. M.; Krause, C.; Arain, S.; Wolfbeis, O. S. Adv. Mater. 2006, 18, 1511. doi: 10.1002/adma.200600120
(8)Lo, K. K. W.; Li, S. P. Y.; Zhang, K. Y. New J. Chem. 2011, 35, 265. doi: 10.1039/C0nj00478b
(9)Zhang, S. J.; Hosaka, M.; Yoshihara, T.; Negishi, K.; Iida, Y.; Tobita, S.; Takeuchi, T. Cancer Res. 2010, 70, 4490. doi: 10.1158/0008-5472.Can-09-3948
(10)Williams, J. A. Chem. Soc. Rev. 2009, 38, 1783. doi: 10.1039/b804434c
(11)Dixon, I. M.; Collin, J. P.; Sauvage, J. P.; Flamigni, L.; Encinas, S.; Barigelletti, F. Chem. Soc. Rev. 2000, 29, 385. doi: 10.1039/B000704h
(12)Ulbricht, C.; Beyer, B.; Friebe, C.; Winter, A.; Schubert, U. S. Adv. Mater. 2009, 21, 4418. doi: 10.1002/adma.200803537
(13)Whittle, V. L.; Williams, J. A. Dalton Trans. 2009, 3929. doi: 10.1039/b821161b
(14)You, Y.; Park, S. Y. Dalton Trans. 2009, 1267. doi: 10.1039/b812281d
(15)Fernandez-Moreira, V.; Thorp-Greenwood, F. L.; Coogan, M. P. Chem Commun (Camb). 2010, 46, 186. doi: 10.1039/b917757d
(16)Zhao, Q.; Li, F.; Huang, C. Chem. Soc. Rev. 2010, 39, 3007. doi: 10.1039/b915340c
(17)Lo, K. K. W.; Louie, M. W.; Zhang, K. Y. Coordin. Chem. Rev. 2010, 254, 2603. doi: 10.1016/j.ccr.2010.01.014
(18)Lo, K. K. W. Photophysics of Organometallics 2010, 29, 115. doi: 10.1007/3418_2009_3
(19)Chi, Y.; Chou, P. T. Chem. Soc. Rev. 2010, 39, 638. doi: 10.1039/b916237b
(20)Yue, Y.; Xu, H. X.; Hao, Y. Y.; Xie, X. D.; Qu, L. T.; Wang, H.; Xu, B. S. Acta Phys. -Chim. Sin. 2012, 28, 1593. [岳 岩, 许慧侠, 郝玉英, 解晓东, 屈丽桃, 王 华, 许并社. 物理化学学报, 2012, 28, 1593.] doi: 10.3866/PKU.WHXB201204181
(21)Wei, C. D.; Ge, G. P.; Li, C. Y.; Lei, K. W.; Liang, H. Z.; Yu, G.; Liu, Z. W. Acta Phys. -Chim. Sin. 2015, 31, 17. [韦传东, 葛国平,李春艳, 雷克微, 梁洪泽, 禹 钢, 刘志伟. 物理化学学报, 2015, 31, 17.] doi: 10.3866/PKU.WHXB201411212
(22)Wang, L. X.; Mei, Q. B.; Yan, F.; Tian, B.; Weng, J. N.; Zhang, B.; Huang, W. Acta Phys. -Chim. Sin. 2012, 28, 1556. [王玲霞,梅群波, 颜 芳, 田 波, 翁洁娜, 张 彬, 黄 维. 物理化学学报, 2012, 28, 1556.] doi: 10.3866/PKU.WHXB201205043
(23)Ren, J. K.; Xu, H. X.; Qu, L. T.; Hao, Y. Y.; Wang, H.; Xu, B. S. Acta Phys. -Chim. Sin. 2013, 29, 1115. [任静琨, 许慧侠, 屈丽桃, 郝玉英, 王 华, 许并社. 物理化学学报, 2013, 29, 1115.] doi: 10.3866/PKU.WHXB201302253
(24)DeRosa, M. C.; Mosher, P. J.; Yap, G. P. A.; Focsaneanu, K. S.; Crutchley, R. J.; Evans, C. E. B. Inorg. Chem. 2003, 42, 4864. doi: 10.1021/Ic026230r
(25)Huynh, L.; Wang, Z. U.; Yang, J.; Stoeva, V.; Lough, A.; Manners, I.; Winnik, M. A. Chem. Mater. 2005, 17, 4765. doi: 10.1021/cm047794r
(26)Ohsawa, Y.; Sprouse, S.; King, K. A.; Dearmond, M. K.; Hanck, K. W.; Watts, R. J. J. Phys. Chem. 1987, 91, 1047. doi: 10.1021/J100289a009
(27)Lowry, M. S.; Hudson, W. R.; Pascal, R. A.; Bernhard, S. J. Am. Chem. Soc. 2004, 126, 14129. doi: 10.1021/Ja047156+
(28)Medina-Castillo, A. L.; Fernandez-Sanchez, J. F.; Klein, C.; Nazeeruddin, M. K.; Segura-Carretero, A.; Fernandez-Gutierrez, A.; Grätzel, M.; Spichiger-Keller, U. E. Analyst 2007, 132, 929. doi: 10.1039/b702628e
(29)Habibagahi, A.; Mebarki, Y.; Sultan, Y.; Yap, G. P.; Crutchley, R. J. ACS Appl. Mater. Interfaces 2009, 1, 1785. doi: 10.1021/am900306a
(30)Di Marco, G.; Lanza, M.; Mamo, A.; Stefio, I.; Di Pietro, C.; Romeo, G.; Campagna, S. Anal. Chem. 1998, 70, 5019. doi: 10.1021/ac980234p
(31)Xu, W. Y.; Ma, W. T.; Li, K. Y.; Hu, J. M.; Shen, L. R.; Li, H. Y.; Cao, L. X. Sensor Actuat. B-Chem. 2002, 86, 174. doi: 10.1016/s0925-4005(02)00165-x
(32)Lu, X.; Manners, I.; Winnik, M. A. Macromolecules 2001, 34,1917. doi: 10.1021/Ma001454j
(33)Hocker, G. B. Appl. Opt. 1979, 18, 1445. doi: 10.1364/Ao.18.001445
(34)Klimant, I.; Wolfbeis, O. S. Anal. Chem. 1995, 67, 3160. doi: 10.1021/Ac00114a010
(35)Wang, H. Y.; Xu, G. B.; Dong, S. J. Analyst 2001, 126, 1095. doi: 10.1039/b100376n
(36)Hubner, J. P.; Carroll, B. F.; Schanze, K. S.; Ji, H. F. Exp. Fluids 2000, 28, 21. doi: 10.1007/s003480050003
(37)Tang, Y.; Tehan, E. C.; Tao, Z. Y.; Bright, F. V. Anal. Chem. 2003, 75, 2407. doi: 10.1021/Ac030087h
(38)Bedlek-Anslow, J. M.; Hubner, J. P.; Carroll, B. F.; Schanze, K. S. Langmuir 2000, 16, 9137. doi: 10.1021/La0011679
(39)Lu, X.; Winnik, M. A. Chem. Mater. 2001, 13, 3449. doi: 10.1021/Cm011029k
(40)Li, H. R.; Lin, J.; Zhang, H. J.; Li, H. C.; Fu, L. S.; Meng, Q. G. Chem. Commun. 2001, 1212. doi: 10.1039/b102160p
(41)Xavier, M. P.; Garcia-Fresnadillo, D.; Moreno-Bondi, M. C.; Orellana, G. Anal. Chem. 1998, 70, 5184. doi: 10.1021/Ac980722x
(42)Wang, Z.; McWilliams, A. R.; Evans, C. E. B.; Lu, X.; Chung, S.; Winnik, M. A.; Manners, I. Adv. Funct. Mater. 2002, 12, 415. doi: 10.1002/1616-3028(20020618)12:6/7<415::Aid-Adfm415>3.0.Co;2-Y
(43)Franville, A. C.; Mahiou, R.; Zambon, D.; Cousseins, J. C. Solid State Sci. 2001, 3, 211. doi: 10.1016/S1293-2558(00)01114-6
(44)Sprouse, S.; King, K. A.; Spellane, P. J.; Watts, R. J. J. Am. Chem. Soc. 1984, 106, 6647. doi: 10.1021/Ja00334a031
(45)dos Santos, C. M. G.; Gunnlaugsson, T. Supramol. Chem. 2009, 21, 173. doi: 10.1080/10610270802588285
(46)Reetz, M. T.; Rentzsch, M.; Pletsch, A.; Taglieber, A.; Hollmann, F.; Mondiere, R. J. G.; Dickmann, N.; Hocker, B.; Cerrone, S.; Haeger, M. C.; Sterner, R. ChemBioChem 2008, 9, 552. doi: 10.1002/cbic.200700413
(47)Lee, S. J.; Bae, D. R.; Han, W. S.; Lee, S. S.; Jung, J. H. Eur. J. Inorg. Chem. 2008, 2008, 1559. doi: 10.1002/ejic.200701073
(48)Lo, K. K. W.; Ng, D. C. M.; Chung, C. K. Organometallics 2001, 20, 4999. doi: 10.1021/Om010652b
(49)Li, H. R.; Lin, J.; Zhang, H. J.; Fu, L. S.; Meng, Q. G.; Wang, S. B. Chem. Mater. 2002, 14, 3651. doi: 10.1021/Cm0116830
(50)Okutsu, T.; Ishihara, A.; Kounose, N.; Suzuki, H. H. T.; Ichimura, T.; Hiratsuka, H. J. Photochem. Photobiol. A 2007, 186, 229. doi: 10.1016/j.jphotochem.2006.08.019
(51)Sun, Y.; Liang, X. H.; Zhao, Y. Y.; Fan, J. Spectrochim. Acta A 2013, 102, 194. doi: 10.1016/j.saa.2012.10.013
(52)Rodríguez-Romero, J.; Aparicio-Ixta, L.; Rodríguez, M.; Ramos-Ortíz, G.; Maldonado, J. L.; Jiménez-Sánchez, A.; Farfán, N.; Santillan, R. Dyes Pigments 2013, 98, 31. doi: 10.1016/j.dyepig. 2012.12.029
(53)Malins, C.; Glever, H. G.; MacCraith, B. D.; Fanni, S.; Vos, J. G. Anal. Commun. 1999, 36, 3. doi: 10.1039/a808731h
(54)Tang, L.; Qi, Z. J.; Hong, M. X.; Li, N.; Wei, S.; Yang, F.; Ji, X.; Hu, A. J. Acta Chim. Sin. 2012, 70, 1081. [唐兰兰, 祁争健, 洪满心, 李 楠, 沈 伟, 杨 帆, 吉 昕, 胡爱江. 化学学报, 2012, 70, 1081.] doi: 10.6023/A1112121
(55)Wang, J. X.; Xia, H. Y.; Liu, W. Q.; Zhao, F.; Wang, Y. B. Inorg. Chim. Acta 2013, 394, 92. doi: 10.1016/j.ica.2012.07.032
(56)Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; et al. Gaussian 03, Revision B.05; Gaussian Inc.; Pittsburgh, PA, 2003.
Photoelectric Properties of Novel Amide-Functionalized Ir(III) Complexes
SHEN Wei TIAN Wen-Wen QI Zheng-Jian*SUN Yue-Ming*
(College of Chemistry and Chemical Engineering, Southeast University, Nanjing 211189, P. R. China)
A series of luminescent cyclometalated Ir(III) complexes functionalized with amide derivatives were prepared and compared with [Ir(ppy)2phen-NH2]Cl. The complexes were [Ir(ppy)2phen-Br]Cl, [Ir(ppy)2phen-COOH]Cl, and [Ir(ppy)2phen-Si]Cl, where ppy is 2-phenylpyridine, phen-NH2is 5-amino-[1,10]-phenanthroline, phen-Br is 2-bromo-2-methyl-N-(1,10-phenanthrolin-5-yl)propanamide, phen-COOH is 4-[(1,10-phenanthrolin-5-yl)amino]-4-oxobut-2-enoic acid, and phen-Si is 5-[N,N-bis-3-(triethoxysilyl) propyl]ureyl-1,10-phenanthroline. They were characterized using nuclear magnetic resonance (NMR) spectroscopy, mass spectrometry (MS), ultraviolet-visible (UV-Vis) absorption spectroscopy, photoluminescence (PL) spectroscopy, and cyclic voltammetry (CV). The three novel complexes have intense absorptions in the blue-purple region. The complexes show bright yellow to orange PL emissions under UV irradiation, and the quantum yields (Φ) of these complexes are higher than 12%. The excited-statelifetimes of the novel complexes are 9.18–12.00 μs, much longer than that of [Ir(ppy)2phen-NH2]Cl (5.78 μs). With both the highest quantum yield (32%) and longest lifetime (12.00 μs), [Ir(ppy)2phen-Br]Cl also shows the best oxygen-sensing properties and the largest I0/I factor, 10.91 (I0: the PL intensity of the complex in the absence of O2, I: the PL intensity of the complex under pure oxygen). These results suggest that [Ir(ppy)2phen-Br]Cl may be a promising candidate for use in oxygen sensors based on covalent grafting. Time-dependent density functional theory (TD-DFT) calculations were used to supplement the photoelectric property studies. Theoretical calculations indicate that all the mononuclear complexes have approximately octahedral structures with Ir(III) as the coordination center. The computational results agree well with the experimental data.
Photoelectric property; Ir(III) complex; Phenanthroline; Oxygen quenching; Luminescence sensor
O644
10.3866/PKU.WHXB201510084
Received: August 24, 2015; Revised: October 8, 2015; Published on Web: October 8, 2015.
*Corresponding authors. QI Zheng-Jian, Email: qizhengjian@seu.edu.cn; Tel: +86-13605186011. SUN Yue-Ming, Email: sun@seu.edu.cn;
Tel: +86-13770338286.
The project was supported by the National Key Basic Research Program of China (973) (2013CB932902), National Natural Science Foundation of China (21173042), Science and Technology Support Program (Industry) Project of Jiangsu Province, China (BE2013118), Transformation of
Scientific and Technological Achievements in Jiangsu Province Special Funds, China (BA2014123), and National Key Scientific Instrument and
Equipment Development Project, China (2014YQ060773).
国家重点基础研究发展规划项目(973) (2013CB932902), 国家自然科学基金(21173042), 江苏省科技支撑计划(工业)项目(BE2013118), 江苏省科技成果转化专项资金(BA2014123)及国家重大科学仪器设备开发专项(2014YQ060773)资助
©Editorial office of Acta Physico-Chimica Sinica
