CeO2对CuCl/活性炭吸附剂C2H4/C2H6吸附分离性能促进作用
2015-12-07邢建东敬方梨孙红丽罗仕忠
邢建东 敬方梨 储 伟,* 孙红丽 喻 磊 张 欢 罗仕忠,*
(1四川大学化学工程学院, 成都 610065; 2四川大学新能源与低碳技术研究院, 成都 610207)
CeO2对CuCl/活性炭吸附剂C2H4/C2H6吸附分离性能促进作用
邢建东1,2敬方梨1,2储 伟1,2,*孙红丽1喻 磊1张 欢1罗仕忠1,*
(1四川大学化学工程学院, 成都 610065;2四川大学新能源与低碳技术研究院, 成都 610207)
通过等体积浸渍方法制备了添加CeO2助剂的用于C2H4/C2H6吸附分离的CuCl/活性炭(AC)吸附剂, 使用氮气吸附-脱附曲线、X射线衍射(XRD)、X射线光电子能谱(XPS)、扫描电子显微镜(SEM)、能量分散X射线光谱(EDX)等分析方法对吸附剂进行了表征. 结果表明, 吸附剂表面Cu(II)在氮气气氛焙烧过程中被部分还原成Cu(I). 重点研究了Ce元素的添加对于吸附剂的C2H4/C2H6吸附分离性能的影响, 等温吸附曲线结果表明添加了CeO2的吸附剂通过降低乙烷的吸附容量从而显著提高了吸附分离性能. XRD及XPS结果表明, 和未添加助剂样品相比, 其表面晶体团簇较小, 分散性更好, Cu(II)还原程度更高. 添加CeO2的吸附剂样品5Ce50Cu (CeO2和CuCl2的质量分数(w)分别为5%和50%)获得了最好的吸附分离效果, 相对于未添加CeO2的样品50Cu,其在660 kPa下的吸附选择性由4.2提升到8.7.
C2H4; 变压吸附; 吸附选择性; CeO2助剂; Cu(I)活性位; 表面表征
1 Introduction
Ethylene (C2H4) is widely used in synthesizing the fiber and rubber as an important chemical raw material. It is usually produced by cracking the long-chain hydrocarbons such as diesel fuel and naphtha;1,2nevertheless ethane (C2H6) is also unexpectedly co-produced together with the desired C2H4.3The cryogenic distillation separation technology of C2H4/C2H6mixture must be operated at low temperature of –29 °C and high pressure of 1.9 MPa because of their similar molecule size and volatility,1,4which is considered as the most energy intensive unit operations in petrochemical industry.5,6On the contrary, adsorptive separation of C2H4and C2H6provides an alternative and competitive technique whatever for energy consumption or economic matter.
The metal-organic frameworks (MOFs), molecular sieve, polymer, and activated carbon (AC) are potential materials as adsorbents7due to their large specific surface area, pore volume, and the amount of adsorption sites. It has been reported that MOF-74 exhibited high separation factor towards C2H4/C2H6and well adsorption capacity about C2H4.8,9The MMOF-74 (M = Zn, Ni, Co, Mn, Mg, Fe) such as Fe-MOF-74 can further promote the performance, leading to the separation factors between 13 and 18 calculated by ideal ads orbed solution theory, and the C2H4capacity was 6.02 mmolg–1at about 100 kPa.1In despite of that, the drawbacks of MOFs are also obvious like high cost, hydrothermal instability,10,11and facile poisoning by adsorbed moisture.12,13Furthermore, MOF-74 becomes less attractive for industrial application because both C2H6and C2H4can reach adsorption equilibrium at low pressure (20 kPa) over it. Anson et al.14,15studied the cation exchanged forms of the titanosilicate molecular sieves (ETS) by various dopants like Na+, K+, Li+, Ba2+, and La3+. The results showed that the modified ETS-10 have strong adsorption of C2H6and C2H4, with the saturated adsorption capacities being 1–2 mmolg–1. Meanwhile, the adsorption equilibrium points appeared at low pressures and the adsorption isotherms were nearly rectangular, which implied that the materials have low adsorption swing capacity. Activated carbon (AC) is one of the most favorable porous materials and it has many industrial applications such as gas adsorption, energy storage, catalysts or catalyst supports.16As adsorbent, the large amounts of micropore volume, specific adsorption capacity, and a highly heterogeneous surface are the key properties which are rather stable compared to those MOFs and zeolites.17Moreover, it could be easily and cheaply manufactured in large quantities, representing the low cost.18Several investigations have focused on the C2H4adsorption performance on the activated carbon adsorbent. The activated carbon exhibited large adsorption capacity for both C2H4and C2H6with a value up to 6 mmolg–1, while the adsorption selectivity is unsatisfactory.19,20Fortunately, it was recently reported that the Cu(I) and Ag(I) can improve the C2H4adsorption selectivity by π-complexation,21–23Ag(I) coordinated phenanthroline-based polymer24or selective reduction of Cu(II) to Cu(I) using vapor-induced reduction (VIR) with HCHO or CH3OH,25,26exhibiting good performance in adsorptive separation of olefins/paraffins. The Cu(I) acts as active site and plays a significant role in the separation of olefins/paraffins. Consequently the π-complexation has become an effective method for selective adsorption of C2H4.27Up to now, numerous studies and reports have documented that the Ce can promote the stability and dispersion of the active component nanoparticles.28–30Hence, it can be speculated that the CeO2should have the promoting effect on the dispersion of CuCl, thus leading to the improvement of adsorptive separation of C2H4/C2H6for the modified adsorbent.
In the current work, we thus prepared the Cu(I) supported on activated carbon with the addition of Ce as promoter in order to improve the separation performances of C2H4/C2H6mixture. The Ce element was introduced into the materials just because it may play an important role in improving the stability and enhancing the dispersion of Cu(I) active sites.
2 Experimental
2.1 Adsorbent preparation
All chemicals were commercially obtained and used as received without further purification. In a typical preparation, 1g activated carbon (Chengdu Changzheng Chemical Co., Ltd., China, CP) was firstly dried in oven at 110 °C for 12 h to remove the water. 0.634 g CuCl22H2O (Chengdu Kelong Chemical Co., Ltd., China, AR) and 0.126 g Ce(NO3)36H2O (Chengdu Kelong Chemical Co., Ltd., China, AR) were dissolved with 5 mL deionized water to get a clear solution. The metal elements were loaded on activated carbon by incipient wetness impregnation method. The resulting solids were calcined at 350 °C for 4 h in nitrogen atmosphere (50 mLmin–1) after drying at 110 °C for 12 h. The final sample was denoted as 5Ce50Cu (the CeO2and CuCl2mass fractions (w) were 5% and 50%, respectively). The dried samples were named with a prefix “as-made”. Analogously, the samples 50Cu, 2Ce50Cu, 5Ce50Cu, 8Ce50Cu, 10Ce50Cu, 5Ce were prepared by using the same protocol and to further investigate the effect of Ce on the adsorptive separation performance for C2H4/C2H6.
2.2 Characterizations
The specific surface areas and pore size distributions determined by the N2adsorption/desorption isotherms at 77 K were measured on NOVA1000e (Quantachrome Company of America). The specific surface areas and pore size distributions were calculated by the Brunauer-Emmett-Teller (BET) method and density functional theory (DFT) method,18,31,32respectively.
The X-ray diffraction (XRD) analysis of the samples was recorded by Philips X'pert PRO (Philips Company of Netherlands) with Cu KαX-ray source and an internal standard of silicon power. The 2θ ranges from 10° to 80° at 40 kV and 35 mA with 0.03° per step.
The chemical states and quantitative analysis of Cu species in the samples were revealed by X-ray photoelectron spectro-scopy (XPS), and were performed using an XSAM800 spectrometer (Kratos Company of British) with an Al Kα(hv = 1486.6 eV) X-ray source.
The morphology of the samples was investigated by scanning electron microscopy (SEM) and the surface distribution of element was performed using energy dispersive X-ray spectroscopy (EDX) from Hitachi S-4800, Japan.
2.3 C2H4and C2H6adsorption
The C2H4(purity of 99.99%) and C2H6(purity of 99.99%) adsorption experiments were performed at 75 °C within the pressure up to 700 kPa and measured by the static volumetric method as it is flexible and accurate.33Helium (purity of 99.999%) was usually used for the void volume calibration in the adsorption setup because it cannot be adsorbed at pressures below 10 MPa.161 g sample (60–80 mesh) was pretreated at 110 °C for 8 h in a vacuum oven prior to data collection and then loaded in the adsorption cell. The adsorption cell and the reference cell were kept at the required temperatures using an isothermal oven, and the detail experimental steps were described previously.12,18,34Each test was carried out twice to ensure the veracity of the experimental data.
2.4 Data analysis and fit
The adsorption selectivity (s) was defined as the following Eq.(1)

The q(C2H4) and q(C2H6) are the adsorption capacities of C2H4and C2H6, respectively.
The Langmuir model is a classical adsorption model and suitable for physical adsorption and chemical adsorption. It is the most widely used isothermal adsorption equation in practical application,12,21which can be described as Eq.(2)

where q is the adsorption quantity, p is the equilibrium pressure, qmand b are Langmuir parameters.35
3 Results and discussion
3.1 Adsorption performance
The adsorption isotherms of C2H4and C2H6were carried out on the six adsorbents at 75 °C and the results were graphically presented in Fig.1. In these figures, solid points represented the experimental data and the solid lines represented the results fitted by the Langmuir model. The parameters were listed in Table 1. All the C2H4adsorption data exhibited the characteristic of type I isotherms according to the IUPAC,36which suggested that C2H4adsorbed on the adsorbent surface was strongly dependent on chemical adsorption.

Fig.1 (a) C2H4adsorption isotherms on different adsorbents, (b) C2H6adsorption isotherms on different adsorbents, (c) C2H4/C2H6adsorption selectivity calculated by the Langmuir model fitting data
The C2H4and C2H6adsorption isotherms showed that 50Cu adsorbent has higher adsorption capacity for both C2H4and C2H6, but the adsorbents with CeO2promoter have higher C2H4/C2H6adsorption selectivity. Fig.1(a, b) showed that the quantity of adsorbed C2H4and C2H6declined with the increasing content of CeO2. Precisely, the adsorbed C2H6dropped down significantly by 63% from 7.81 to 2.87 mLg–1at 660 kPa when the content of CeO2rose from 0% for 50Cu to 5% (w) for 5Ce50Cu. The decrement of that slowed down as CeO2content further increased for the mixed samples. However, for the adsorbed C2H4, the difference of the C2H4adsorption abilities for the adsorbents was smaller than that of C2H6. As far as the selectivity of C2H4was concerned, the 50Cu showed a selectivity value of 4.2 at the pressure of 660 kPa from the Fig.1(c), while it increased more than 2 times to 8.7 for 5Ce50Cu at 660 kPa. Such a change was obviously helpful to improve the selectivity of C2H4. The adsorption isotherms on 5Ce showed that it did not adsorb C2H4or C2H6selectively, although it showed better adsorption capacity. These results suggested that the introduction of CeO2into CuCl/AC could improve the adsorption selectivity efficiently.
3.2 Adsorbent characterization
As showed in Fig.2(a), the N2adsorption/desorption isotherms of the adsorbents: as-made 50Cu, 50Cu, as-made 5Ce50Cu, and 5Ce50Cu. From the shapes of the obtained isotherms, it can be concluded that all the samples presented a micro-mesoporous character. According to the IUPAC classification, all the isotherms exhibited obvious type H4 hysteresis loops, reflecting the presence of the uniformly sized slit mesopores on all the samples.36–38The adsorption isotherm for AC increased more rapidly at low relative pressures than those of other samples, suggesting that the AC possessed higher amount of micropores, an d that a lot of microp ores were blocked after adding CuCl22H2O and Ce(NO3)36H2O onto the AC. In Fig.2(b), pore size distribution diagram calculated by nonlocal density functional theory37,39,40also demonstrated the consistent results that the pure AC had more micropore structures, while other samples had more mesporous structures. All data above clearly evidenced that some micropore structures were plugged in the prepared samples and some bigger pore structures shrank as mesporous structures.
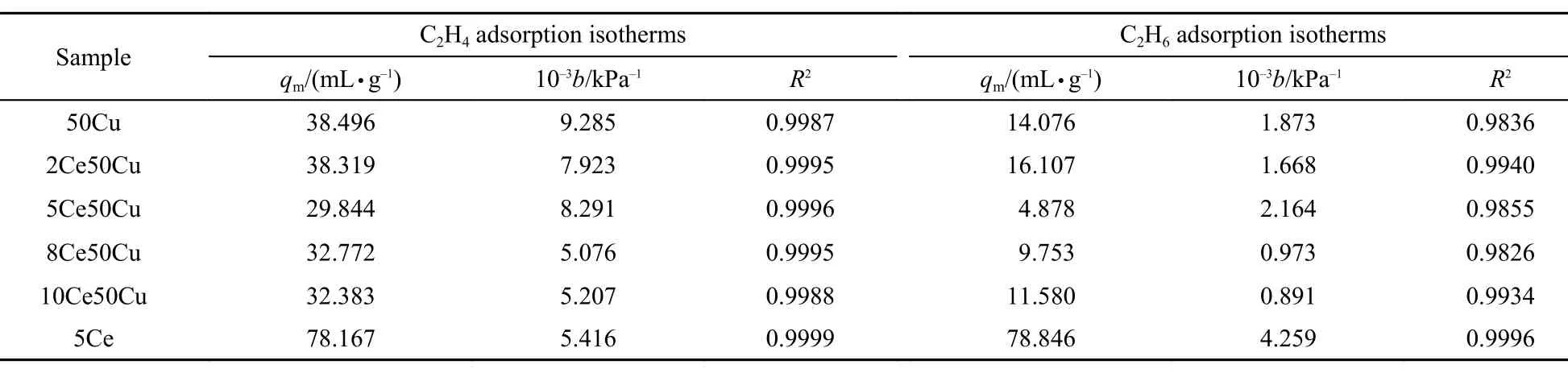
Table1 Langmuir parameters of equilibrium isotherms obtained from the Langmuir model
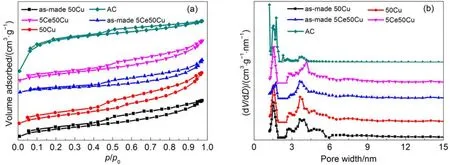
Fig.2 (a) N2adsorption/desorption isotherms (77 K) of the samples and (b) the pore size distributions obtained from the DFT equation
The pore parameters of the samples were presented in Table 2. After impregnating CuCl22H2O onto the AC, some micropores were blocked and the specific surface area and pore volume of the adsorbents declined seriously. After calcination at 350 °C for 4 h under nitrogen atmosphere, the specific surface area and pore volume of the adsorbents increased little. Similarly, the samples with the Ce addition have smaller specific surface area and pore volume, indicating that the CeO2particles from the thermal decomposition of Ce(NO3)36H2O could further plug the porous channel.
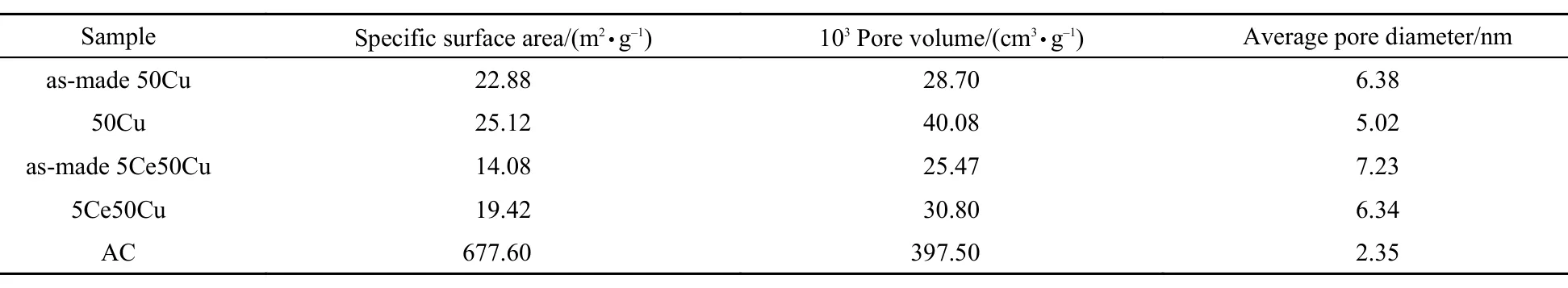
Table2 Textural properties obtained by N2adsorption analyses of the samples
The XRD results for the 50Cu and 5Ce50Cu samples before and after calcination were depicted in Fig.3. The precursors asmade 50Cu and as-made 5Ce50Cu showed three apparent diffraction peaks at 16.2°, 32.3°, and 39.6°, which can be attributed to CuCl2. After treatment at 350 °C for 4 h in nitrogen atmosphere, three major diffraction peaks at 28.7°, 47.6°, and 56.5° were observed for 50Cu and 5Ce50Cu samples, which can be ascribed to CuCl, indicating that the CuCl22H2O could be dispersed on the surface of AC and partially reduced to CuCl.41At the same time, the diffraction peaks of CuCl2of the 50Cu and 5Ce50Cu samples weakened. Compared with the 50Cu adsorbent, the intensities of CuCl and CuCl2diffractionpeaks of the sample 5Ce50Cu were obviously weaker, meaning that the CuCl and CuCl2crystal particles were smaller, thus leading to the higher dispersion. The CuCl crystallite sizes of 50Cu and 5Ce50Cu samples calculated by Scherrer's equation from the (111) and (220) reflections (corresponding to two major diffraction peaks) were presented in Table 3. The CuCl crystallite sizes of 5Ce50Cu sample were 25.6 and 28.6 nm for (111) and (220) reflections, which decreased by 23.4% and 17.8%, respectively, when they compared with those of 50Cu sample (33.4 and 34.8 nm). Furthermore, the CuCl crystallite could disperse in the mesoporous and macroporous or on the surface of the AC. The smaller CuCl crystallite could provide more bare surface of crystallite, thus more adsorption active sites. It is clear from these results that the CeO2can promote the dispersion of CuCl on AC surface.28,42

Fig.3 XRD patterns of the samples

Table3 CuCl crystallite size of 50Cu and 5Ce50Cu samples
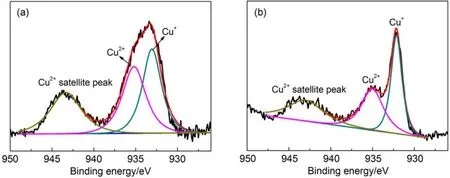
Fig.4 Cu 2p3/2XPS spectra of (a) 50Cu and (b) 5Ce50Cu samples

Fig.5 SEM images of (a) 50Cu and (b) 5Ce50Cu
The chemical states of surface copper for samples 50Cu and 5Ce50Cu were measured by XPS technique, the spectra were depicted in Fig.4. An overlapped peak between ca 928 eV and ca 939 eV could be observed for 50Cu sample, which actually consisted of two peaks by deconvolution treatment, indicating two types of chemical states of copper. The peak at 934.6 eV was the characteristic of Cu2+species, which could be further confirmed by the satellite peak at 943.8 eV, while the peak at 932.8 eV was assigned to Cu+.25,43It was split into two differential peaks as small amount of Ce was introduced for 5Ce50Cu, showing a decreased peak intensity of Cu2+. Correspondingly, the shape of the satellite peak at 943.8 eV became wider. On the contrary, the peak assigned to Cu+(932.8 eV) became much intenser, the surface content increased from 52.5% for Ce-free sample 50Cu to 63.9% for Ce-contained sample 5Ce50Cu ac-cording to the quantitative calculation. In other words, the introduction of Ce can promote the selective reduction of Cu2+to Cu+, leading to a Cu+-rich surface. By linking with the fact that Cu+is the active phase dedicating to selective adsorption of C2H4, it can explain why the best separation performance was obtained on sample 5Ce50Cu.
Fig.5 presented SEM images of the 50Cu and 5Ce50Cu samples. Apparently, the crystal particles on the 5Ce50Cu surface was smaller than that of the 50Cu sample, indicating that CeO2did promote the dispersion of the crystal particles on the adsorbent surface.28,42,44,45EDX surface scanning images of Cu and Ce elements for the 5Ce50Cu adsorbent were shown in Fig.6(a) and Fig.6(b), respectively, and the cross distribution of Cu/Ce elements on the sample surface was displayed in Fig.6(c). With the addition of CeO2, the Cu component was homogeneously distributed over the whole AC surfaces; more importantly, CeO2was observed to be practically deposited on the bare AC surface and well separated from the Cu ensembles. Thus, such a unique spatial positioning of Ce and Cu over the AC surface can dramatically decrease the accessibility of the AC surface adsorption sites for C2H6. The Ce presence in the CuCl-AC adsorbent system can actually exert a peculiar siteblocking effect and reduce the C2H6adsorption volume upon the separation performance for C2H4/C2H6.
4 Conclusions
Effect of CeO2on the adsorptive separation performance for C2H4/C2H6was investigated by the CeO2promoted CuCl/AC adsorbents. The results showed that CeO2could promote the dispersion and reduction of CuCl2on the AC surface, with the CuCl grain size decreased by 23.4% and the Cu(I) content increased 11.4%, thus leading to an obvious improvement of adsorptive separation selectivity for C2H4/C2H6. The CeO2acts as promoter in the adsorbent, it can promote the reduction of Cu2+to Cu+which is responsible for selective adsorbing C2H4. On the other hand, the Ce element and reduced Cu+can occupy the bare surface of the activated carbon, lowering the capacity of adsorbing C2H6. In addition, the separation performance got its optimum value when the CeO2addition amount was 5% (w), and the separation selectivity increased from 4.2 to 8.7 at 660 kPa. The performance in C2H4/C2H6separation was improved here, the simplicity of the adsorbent preparation technique made it be promising even in industrial scale. At the same time, the adsorbent system could be further extended by introducing other rare earth elements.

Acknowledgment: The authors would like to thank FENG Yan-Yan, LIU Xing, and WANG Jia-Jie (Sichuan University) for the helpful discussion.
(1)Bloch, E. D.; Queen, W. L.; Krishna, R.; Zadrozny, J. M.; Brown, C. M.; Long, J. R. Science 2012, 335 (6076), 1606. doi: 10.1126/science.1217544
(2)Shi, M.; Lin, C. C. H.; Kuznicki, T. M.; Hashisho, Z.; Kuznicki, S. M. Chem. Eng. Sci. 2010, 65 (11), 3494. doi: 10.1016/j.ces. 2010.02.048
(3)Narin, G.; Martins, V. F. D.; Campo, M.; Ribeiro, A. M.; Ferreira, A.; Santos, J. C.; Schumann, K.; Rodrigues, A. E. Sep. Purif. Technol. 2014, 133, 452. doi: 10.1016/j.seppur.2014.06.060
(4)Duan, X.; Zhang, Q.; Cai, J.; Yang, Y.; Cui, Y.; He, Y.; Wu, C.; Krishna, R.; Chen, B.; Qian, G. J. Mater. Chem. A 2014, 2, 2628. doi: 10.1039/c3ta14454b
(5)Gucuyener, C.; van den Bergh, J.; Gascon, J.; Kapteijn, F. J. Am. Chem. Soc. 2010, 132 (50), 17704. doi: 10.1021/ja1089765
(6)Li, J.; Fu, H. R.; Zhang, J.; Zheng, L. S.; Tao, J. Inorg. Chem. 2015, 54 (7), 3093. doi: 10.1021/acs.inorgchem.5b00316
(7)Bao, Z. B.; Alnemrat, S.; Yu, L.; Vasiliev, I.; Ren, Q. L.; Lu, X. Y.; Deng, S. G. Langmuir 2011, 27 (22), 13554. doi: 10.1021/la2030473
(8)Geier, S. J.; Mason, J. A.; Bloch, E. D.; Queen, W. L.; Hudson, M. R.; Brown, C. M.; Long, J. R. Chem. Sci. 2013, 4 (5), 2054. doi: 10.1039/c3sc00032j
(9)Wu, X. F.; Bao, Z. B.; Yuan, B.; Wang, J.; Sun, Y. Q.; Luo, H. M.; Deng, S. G. Microporous Mesoporous Mat. 2013, 180, 114. doi: 10.1016/j.micromeso.2013.06.023
(10)Ma, D. Y.; Li, Y. W.; Li, Z. Chem. Commun. 2011, 47 (26), 7377. doi: 10.1039/c1cc11752a
(11)Kusgens, P.; Rose, M.; Senkovska, I.; Frode, H.; Henschel, A.; Siegle, S.; Kaskel, S. Microporous Mesoporous Mat. 2009, 120, 325. doi: 10.1016/j.micromeso.2008.11.020
(12)Luo, J. J.; Liu, Y. F.; Sun, W. J.; Jiang, C. F.; Xie, H. P.; Chu, W. Fuel 2014, 123, 241. doi: 10.1016/j.fuel.2014.01.053
(13)Policicchio, A.; Maccallini, E.; Agostino, R. G.; Ciuchi, F.; Aloise, A.; Giordano, G. Fuel 2013, 104, 813. doi: 10.1016/j.fuel.2012.07.035
(14)Anson, A.; Wang, Y.; Lin, C. C. H.; Kuznicki, T. M.; Kuznicki, S. M. Chem. Eng. Sci. 2008, 63 (16), 4171. doi: 10.1016/j.ces. 2008.05.038
(15)Anson, A.; Lin, C. C. H.; Kuznicki, T. M.; Kuznicki, S. M. Chem. Eng. Sci. 2010, 65 (2), 807. doi: 10.1016/j.ces. 2009.09.033
(16)Feng, Y. Y.; Yang, W.; Chu, W. Chin. Phys. B 2014, 23 (10), 8.
(17)Sun, W. J.; Feng, Y. Y.; Jiang, C. F.; Chu, W. Fuel 2015, 155, 7. doi: 10.1016/j.fuel.2015.03.083
(18)Luo, J. J.; Liu, Y. F.; Jiang, C. F.; Chu, W.; Wen, J.; Xie, H. P. J. Chem. Eng. Data 2011, 56 (12), 4919. doi: 10.1021/je200834p
(19)Costa, E.; Calleja, G.; Marron, C.; Jimenez, A.; Pau, J. J. Chem. Eng. Data 1989, 34 (2), 156. doi: 10.1021/je00056a003
(20)Choi, B. U.; Choi, D. K.; Lee, Y. W.; Lee, B. K.; Kim, S. H. J. Chem. Eng. Data 2003, 48 (3), 603. doi: 10.1021/je020161d
(21)Huang, H. Y.; Padin, J.; Yang, R. T. Ind. Eng. Chem. Res. 1999, 38 (7), 2720. doi: 10.1021/ie990035b
(22)Jiang, W. J.; Sun, L. B.; Yin, Y.; Song, X. L.; Liu, X. Q. Sep. Sci. Technol. 2013, 48, 968. doi: 10.1080/01496395.2012.712600
(23)Li, B. Y.; Zhang, Y. M.; Krishna, R.; Yao, K. X.; Han, Y.; Wu, Z. L.; Ma, D. X.; Shi, Z.; Pham, T.; Space, B.; Liu, J.; Thallapally, P. K.; Liu, J.; Chrzanowski, M.; Ma, S. Q. J. Am. Chem. Soc. 2014, 136 (24), 8654. doi: 10.1021/ja502119z
(24)Yu, C.; Cowan, M. G.; Noble, R. D.; Zhang, W. Chem. Commun. 2014, 50 (43), 5745. doi: 10.1039/c4cc02143f
(25)Qin, J. X.; Wang, Z. M.; Liu, X. Q.; Li, Y. X.; Sun, L. B. J. Mater. Chem. A 2015, 3, 12247. doi: 10.1039/C5TA02569A
(26)Jiang, W. J.; Yin, Y.; Liu, X. Q.; Yin, X. Q.; Shi, Y. Q.; Sun, L. B. J. Am. Chem. Soc. 2013, 135 (22), 8137. doi: 10.1021/ja4030269
(27)Cowan, M. G.; McDanel, W. M.; Funke, H. H.; Kohno, Y.; Gin, D. L.; Noble, R. D. Angew. Chem. Int. Edit. 2015, 54 (19), 5740. doi: 10.1002/anie.201500251
(28)Wang, K.; Li, X. J.; Ji, S. F.; Shi, X. J.; Tang, J. J. Energy Fuels 2009, 23, 25. doi: 10.1021/ef800553b
(29)Ren, H. P.; Song, Y. H.; Wang, W.; Chen, J. G.; Cheng, J.; Jiang, J. Q.; Liu, Z. T.; Liu, Z. W.; Hao, Z. P.; Lu, J. Chem. Eng. J. 2015, 259, 581. doi: 10.1016/j.cej.2014.08.029
(30)Wang, N.; Shen, K.; Huang, L. H.; Yu, X. P.; Qian, W. Z.; Chu, W. ACS Catal. 2013, 3 (7), 1638. doi: 10.1021/cs4003113
(31)Liu, J. X.; Jiang, X. M.; Huang, X. Y.; Wu, S. H. Energy Fuels 2010, 24, 3072. doi: 10.1021/ef100142t
(32)Feng, Y. Y.; Jiang, C. F.; Liu, D. J.; Chu, W. J. Anal. Appl. Pyrolysis 2013, 104, 559. doi: 10.1016/j.jaap.2013.05.013
(33)Ahmed, M. J.; Theydan, S. K. J. Porous Mat. 2014, 21 (5), 747. doi: 10.1007/s10934-014-9821-8
(34)Hao, S. X..; Wen, J.; Yu, X. P.; Chu, W. Appl. Surf. Sci. 2013, 264, 433. doi: 10.1016/j.apsusc.2012.10.040
(35)Feng, Y. Y.; Yang, W.; Liu, D. J.; Chu, W. Chin. J. Chem. 2013, 31 (8), 1102. doi: 10.1002/cjoc.v31.8
(36)Kruk, M.; Jaroniec, M. Chem. Mat. 2001, 13 (10), 3169. doi: 10.1021/cm0101069
(37)Thommes, M. Chem. Ing. Tech. 2010, 82 (7), 1059. doi: 10.1002/cite.201000064
(38)Sing, K. S. W.; Williams, R. T. Adsorpt. Sci. Technol. 2004, 22 (10), 773. doi: 10.1260/0263617053499032
(39)Neimark, A. V.; Ravikovitch, P. I.; Vishnyakov, A. Phys. Rev. E 2000, 62 (2), 1493. doi: 10.1103/PhysRevE.62.R1493
(40)Ravikovitch, P. I.; Neimark, A. V. Colloid Surf. A-Physicochem. Eng. Asp. 2001, 187, 11.
(41)Ma, J. H.; Li, L.; Ren, J.; Li, R. F. Sep. Purif. Technol. 2010, 76, 89. doi: 10.1016/j.seppur.2010.09.022
(42)Zhang, X. R.; Shi, P. F. J. Mol. Catal. A-Chem. 2003, 194 (1), 99.
(43)Jing, F. L.; Zhang, Y. Y.; Luo, S. Z.; Chu, W.; Zhang, H.; Shi, X. Y. J. Chem. Sci. 2010, 122 (4), 621. doi: 10.1007/s12039-010-0097-5
(44)Han, T.; Huang, W.; Wang, X. D.; Tang, Y.; Liu, S. Q.; You, X. X. Acta Phys. -Chim. Sin. 2014, 30 (11), 2127. [韩 涛, 黄 伟,王晓东, 唐 钰, 刘双强, 游向轩. 物理化学学报, 2014, 30 (11), 2127.] doi: 10.3866/PKU.WHXB201409121
(45)Xie, X. X.; Fei, Z. Y.; Zou, C.; Li, Z. Z.; Chen, X.; Tang, J. H.; Cui, M. F.; Qiao, X. Acta Phys. -Chim. Sin. 2015, 31 (6), 1153. [谢兴星, 费兆阳, 邹 冲, 李郑州, 陈 献, 汤吉海, 崔咪芬,乔 旭. 物理化学学报, 2015, 31 (6), 1153.] doi: 10.3866/PKU. WHXB201504145
Improvement of Adsorptive Separation Performance for C2H4/C2H6Mixture by CeO2Promoted CuCl/Activated Carbon Adsorbents
XING Jian-Dong1,2JING Fang-Li1,2CHU Wei1,2,*SUN Hong-Li1YU Lei1ZHANG Huan1LUO Shi-Zhong1,*
(1School of Chemical Engineering, Sichuan University, Chengdu 610065, P. R. China;2Institute of New Energy and Low-Carbon Technology, Sichuan University, Chengdu 610207, P. R. China)
CeO2promoted CuCl/activated carbon (AC) adsorbents were prepared using an incipient wetness impregnation method, and characterized using N2adsorption/desorption isotherms, X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), scanning electron microscopy (SEM), and energy-dispersive X-ray spectroscopy (EDX). The Cu(II) on the AC surface was reduced to Cu(I) when calcination was performed in a nitrogen flow. The effects of Ce on the C2H4/C2H6adsorptive separation performance were investigated. The adsorption isotherms showed that the addition of CeO2improved the separation performance by decreasing the C2H6adsorption capacity compared with that of the nonpromoted sample. The XRD and XPS results indicated that the active crystal particles on the AC surface became smaller, leading to higher dispersion and a higher degree of Cu(II) reduction. The best adsorption selectivity was obtained using the 5Ce50Cu [CeO2and CuCl2mass fractions (w) 5% and 50%, respectively] sample, i.e., with CeO2in the adsorbent; the adsorption selectivity increased from 4.2 to 8.7 at 660 kPa compared with that of the 50Cu sample.
C2H4; Pressure swing adsorption; Adsorption selectivity; CeO2promoter; Cu(I) active site; Surface characterization
O647
10.3866/PKU.WHXB201510091
Received: July 10, 2015; Revised: October 8, 2015; Published on Web: October 9, 2015.
*Corresponding authors. CHU Wei, Email: chuwei1965scu@163.com. LUO Shi-Zhong, Email: luosz@scu.edu.cn; Tel: +86-28-85403836.
The project was supported by the National Natural Science Foundation of China (21476145).
国家自然科学基金(21476145)资助项目
©Editorial office of Acta Physico-Chimica Sinica
