一维和二维镝(Ⅱ)配合物的慢磁弛豫
2015-12-01吴建锋张海霞张鹏赵朗唐金魁
吴建锋 张海霞 张鹏 赵朗 唐金魁
(中国科学院长春应用化学研究所,稀土资源与利用国家重点实验室,长春130022)
一维和二维镝(Ⅱ)配合物的慢磁弛豫
吴建锋张海霞张鹏赵朗唐金魁*
(中国科学院长春应用化学研究所,稀土资源与利用国家重点实验室,长春130022)
分别用稀土醋酸盐和稀土高氯酸盐与希弗碱配体和巯基烟酸配体反应得到了两例镝配合物[Dy2(OAc)6H2O]n(1)和{[DyL (H2O)4]ClO4·H2O}n(2)(L=2,2′-二硫代-二(3-吡啶甲酸)),并通过单晶X-射线衍射、元素分析、红外光谱和磁性测试对其进行了表征。结构研究和磁性测试表明:化合物1是羧基桥连的一维链结构,该化合物表现出慢磁弛豫性质,有效能垒为2 K;化合物2是通过原位生成的二硫键桥连的二维网状结构,表现出明显的铁磁相互作用和慢磁弛豫行为。
慢磁弛豫;铁磁相互作用;二硫键
0 Introduction
Magnetic materials play an important role in our modern civilization and have driven great advances in the field of information technology,especially in highdensity data storage[1-3].However,the conventional magneticmaterialsarestilllimitedbythe superparamagnetic effect,preventing further increase of the storage density[4].Since the discovery of single molecule magnet(SMM)[5-6],which exhibits magnetism at molecular level,the study of molecule based magnets has been the focus of chemistry,physics,and materials science[5].In the past two decades,a major breakthrough have been procured in the study of single molecule magnet(SMM)[7-14]and single ion magnet(SIM)[13,15-19]both based on 3d metals,4f metals[20-21],or both. Although single chain magnet(SCM)[22-25]is not dazzling enough to catch great attention among the research of molecule based magnets in contrast with SMM and SIM, itispromisingtodesignandsynthesizeone dimensional[26-29],even two and threedimensional magnets,which behave magnetic relaxation.Herein,weobtainedtwocarboxylato-bridgeddysprosium complexes,[Dy2(OAc)6H2O]n(1)and{[DyL(H2O)4]ClO4· H2O}n(2),with one and two dimensional structures. Magnetic study reveals that complex 1 shows slow magnetic relaxation behavior and complex 2 exhibits intra-chainferromagneticinteractionandslow magnetic relaxation.
1 Experimental
1.1Materials and general procedures
All chemicals and solvents were of analytical grade(Aladdin,Sigma-Aldrich)and used without any further purification.Elemental analysis for C,H,N and S wascarried out on a Perkin-Elmer 2400 analyzer.IR spectra(Fig.1)were recorded with a Perkin-Elmer Fourier transform infrared spectrophotometer using the reflectance technique(4 000~300 cm-1)with samples prepared as KBr disks.
Caution!Perchlorate salts of metal complexes are potentially explosive.They should be handled with care and prepared only in small quantities.
1.2X-ray crystal structure determination
Single-crystal X-ray data of the complexes were collected on a Bruker ApexⅡCCD diffractometer equippedwithgraphite-monochromatizedMo-Kα radiation(λ=0.071 073 nm)at 293(2)K.The structures were solved by direct methods and refined by fullmatrix least-squares methods on F2using SHELXTL-97[30].All non-hydrogen atoms were determined from the difference Fourier maps and refined anisotropically. The hydrogen atoms on the water molecules were determined from the difference Fourier maps.Other hydrogen atoms were introduced in calculated positions and refined with fixed geometry with respect to their carrier atoms.Crystallographic data are listed in Tables 1.
CCDC 1400117,1;1400116,2.
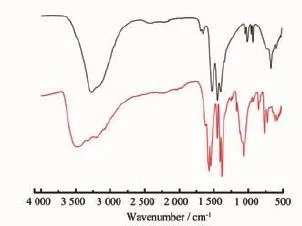
Fig.1 IR spectra of complexes 1(black)and 2(red)
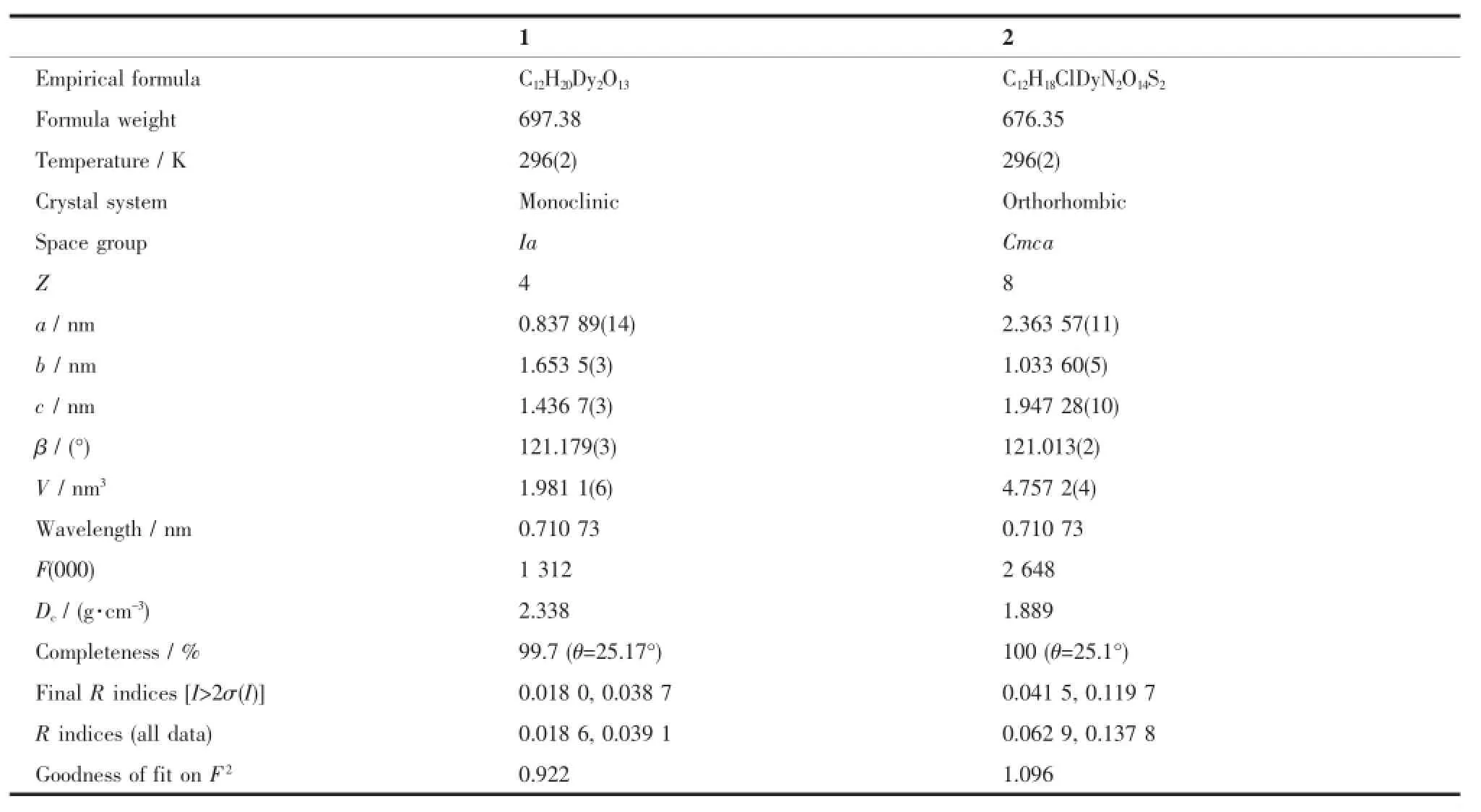
Table 1Crystallographic data for complexes 1 and 2
1.3Magnetic measurements
Magneticsusceptibilitywasrecordedona Quantum Design MPMS-XL7 SQUID magnetometer equipped with a 7 T magnet.The variable-temperature magnetization was measured in the temperature range of 1.9~300 K with an external magnetic field of 1 000 Oe. The dynamics of the magnetization were investigated from the ac susceptibility measurements with or without staticfieldsanda3.0Oeacoscillatingfield. Diamagnetic corrections were made with the Pascals constants[31]for all the constituent atoms as well as the contributions of the sample holder.

Scheme 1Structural representations of ligand L1(left)and H2L(right)
1.4Synthesis of complexes 1 and 2
[Dy2(OAc)6H2O]n(1).Dy(OAc)3·6H2O(0.1 mmol) was dissolved in 10 mL CH3CN/5 mL CH2Cl2,followed by the addition of L1(0.15 mmol).Then triethylamine (0.2 mmol,28 μL)was added,and the mixture was stirred for 3 h.Colorless block-shaped single crystals, suitable for X-ray diffraction analysis,were isolated (70%yield based on Dy(OAc)3·6H2O)after 7 days. Elemental analysis Calcd.for[Dy2(OAc)6H2O]n(C12H20Dy2O13,MW=697.38):C,20.67%;H,2.89%.Found:C, 20.60%;H,2.91%.
{[DyL(H2O)4]ClO4·H2O}n(2).Dy(ClO4)3·6H2O(0.2 mmol)was dissolved in 8 mL CH3OH/8 mL EtOH, followed by the addition of 2-thionicotinic acid(0.1 mmol).Then pyridine(0.1 mmol,16 μL)was added, and the mixture was stirred for 5 h.Yellow blockshaped single crystals,suitable for X-ray diffraction analysis,were isolated(45%yield based on Dy(ClO4)3· 6H2O)after 4 days.Elemental analysis Calcd.for{[DyL (H2O)4]ClO4·H2O}n(C12H18ClDyN2O14S2,MW=676.35):C, 21.31%;H,2.68%;N,4.14%;S,9.48%.Found:C, 21.32%;H,2.70%;N,4.14%;S,9.39%.

Fig.2 1D chain structure(left)and coordination model(right)of 1 with azure,dark and red spheres representing Dy, C and O,respectively
2 Results and discussion
2.1Structure description of complex 1
The reactions of Dy(OAc)3·6H2O with L1in the presence of triethylamine,afforded complex 1.Details forthestructuresolutionandrefinementare summarized in Table 1,and selected bond distances and angles are listed in Table 2.Single-crystal X-ray (Fig.2)studies reveal that complexe 1 crystallizes in monoclinic space group Ia with Z=4.The structure can be described as one dimensional dysprosium chain linked by OAc-without ligand L1,suggesting arelative poor coordination ability of ligand L1(Scheme 1).The asymmetric unit is composed of two DyIIIions, six OAc-anions and one coordinated water molecule, in which the two DyIIIions(Dy1 and Dy2)are linked by three OAc-anions in two bridging modes:two of them are μ2-η1:η2,the rest is μ2-η1:η1(Fig.2).The distance between DyIIIions is 0.396 nm due to the μ2-η1:η2bridge.In such a distance,strong intramolecular magnetic coupling is not necessarily precluded.A closer look at the coordination sphere of DyIIIions reveals that all the coordinating atoms are O atoms fromOAc-anionsandwatermolecule.The introduction of water makes a great difference between the coordination spheres of DyIIIions.Dy1 shows distorted mono-capped square-antiprismatic geometry as analyzed by the program SHAPE 2.1(Table 3), with a water molecule as the cap atom,while,Dy2 showstriangulardodecahedralgeometryintheabsence of coordinated water.Just because of the capped O atoms from water,the structure shows a zigzag shape chain with Dy1 in the corner.Whats more, the corners of each chain are close to each other with the shortest inter-chain distance of 0.639 5 nm,which does not necessarilytoprecludeanyinter-chain magnetic interactions.
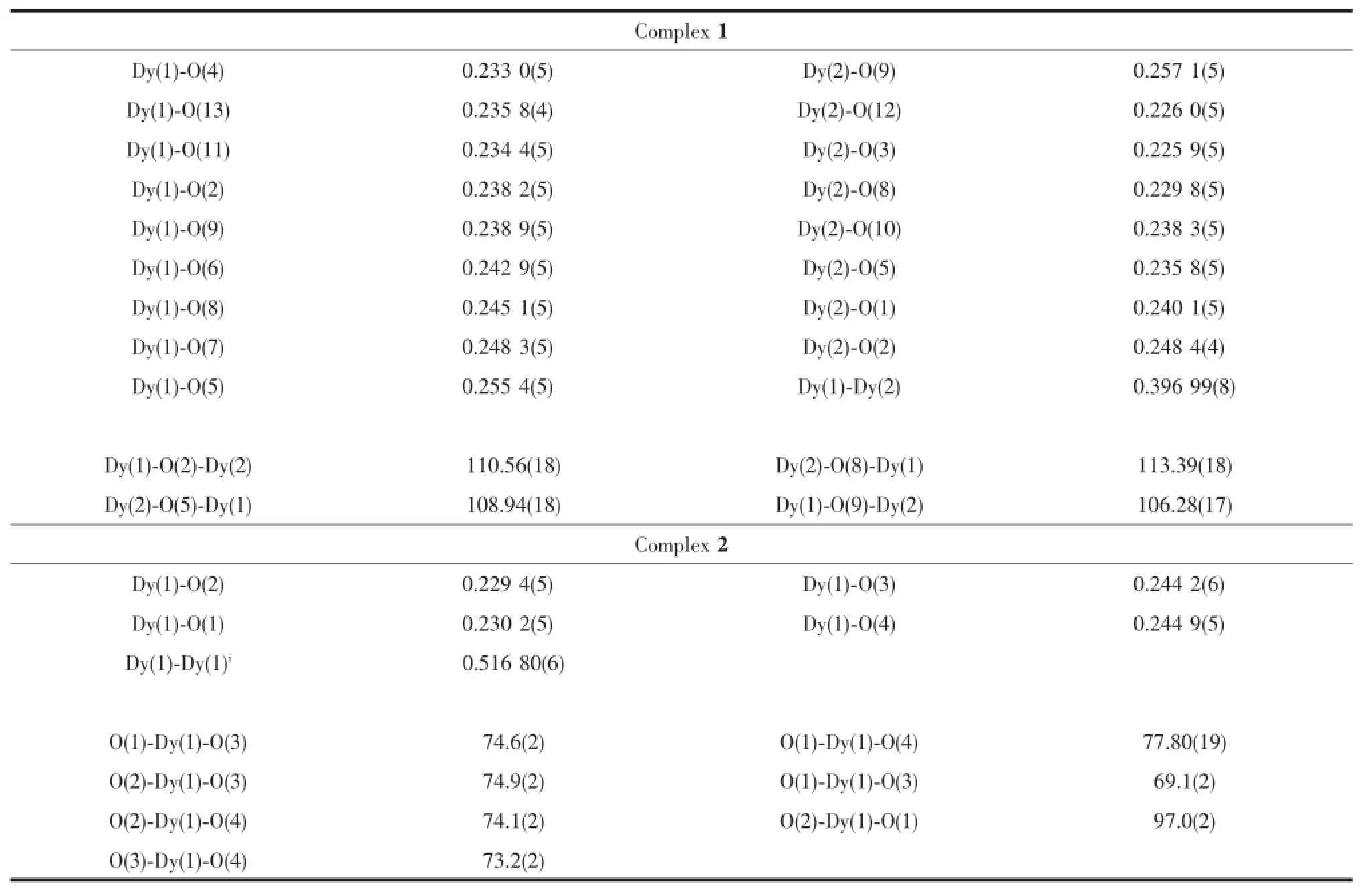
Table 2Selected bond distances(nm),angles(°)for complexes 1 and 2
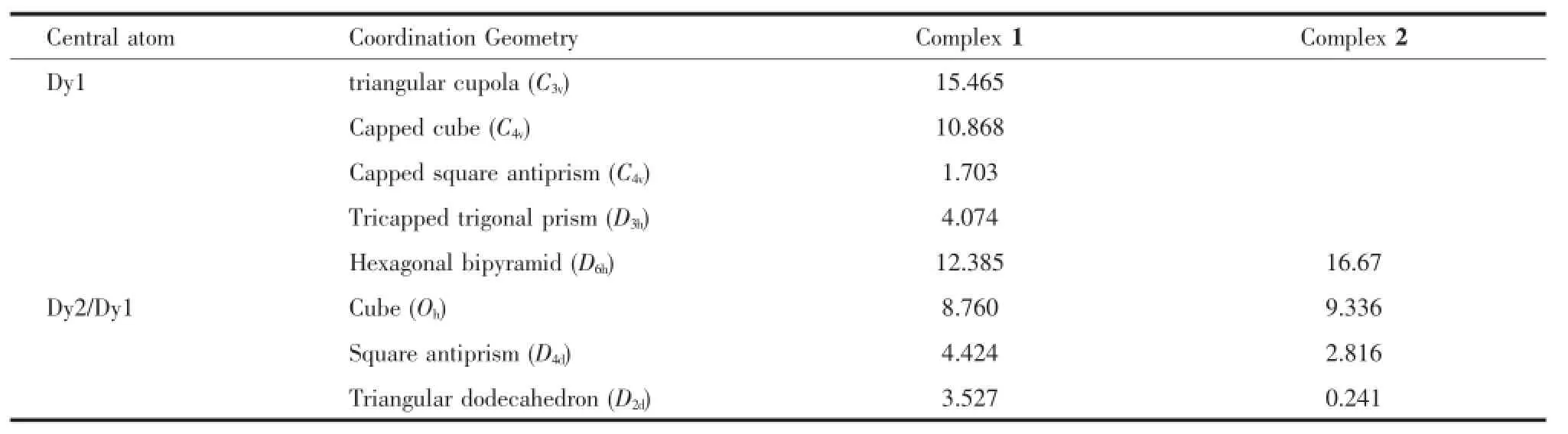
Table 3Continuous Shape Measure(CShM)values calculated by SHAPE 2.1 for 1 and 2
2.2Structure description of complex 2
The reactions of Dy(ClO4)3·6H2O and H2L with pyridine as the base afford complex 2.In contrast with complex 1,the structure of 2(Fig.3),crystallized in orthorhombic space group Cmca with Z=8,is more complex and interesting.The asymmetric unit is composed of one DyIIIion,one ligand L,one ClO4-and five water molecules,in which ligand L is derived from in-situ reaction of 2-thionicotinic acid.The structure can be described as two dimensional dysprosium network,in which one dimensional carboxyl bridged dysprosium chains are connected by ligand L.All DyIIIions,in the same coordinating geometry,i.e.,four O atoms from ligands L and four coordinated water molecules construct triangular dodecahedral geometry, are linked by carboxyls from ligand L with common synsynbridgingmode,formingonedimensional dysprosium chains.The dysprosium chains are linked by disulfide bonds,which are formed from in-situ reaction.The intra-and inter-chain distances are 0.517 nm and 0.974 nm,respectively.
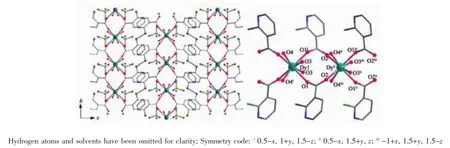
Fig.3 2D network structure(left)and coordination model(right)of 2 with azure,dark,blue,green,cyan and red spheres representing Dy,C,N,Cl,S and O,respectively
2.3Magnetic properties
The static magnetic susceptibilities of complexes 1 and 2(Fig.4)were collected in the temperature range of 2~300 K under an applied magnetic field of 1000 Oe,and the magnetization(M)data were collected in the field range of 0~7 T below 5 K.The room temperature values of χMT(28.26 cm3·K·mol-1and 13.91 cm3·K·mol-1,for 1 and 2,respectively)are close to the expected value of 14.17 cm3·K·mol-1for each DyIIIion,which indicates that the free-ion approximation applies.Upon cooling,however,the χMT values show different tendency,which is dependent on the magnetic interaction between DyIIIcenters.For complex 1,the χMT decreases slowly down to 10 K, reaching,after a sudden drop,the value of 17.23 cm3· K·mol-1at 2.0 K.The slow decrease and sudden drop are indicative of Stark level splitting at the lanthanide ions in weak crystal field and inter-chain or intrachainantiferromagneticmagneticinteractions, respectively[32].The field-dependent magnetization for 1(Fig.5)rises abruptly at low fields and reaches 11.73μBat 7 T without saturation,suggesting the presenceofmagneticanisotropy[33].In the case of complex 2,the χMT decrease slowly down to 11 K and reaches a minimal value of 12.22 cm3·K·mol-1,then increases suddenly and reaches the maximal value of 14.17 cm3·K·mol-1at 2.0 K.The inconstant χMT valuessuggesttwopossibilities:thepresenceof ferromagnetic coupling between DyIIIions within the chain because the interchain magnetic interaction is relatively weak due to the long interchain Dy…Dy distance,or the occupation of Stark sublevels with higher|Jz|values at low temperature[23].The fielddependent magnetization for 2(Fig.5)rises abruptly atlowfieldsandreaches6.26μBat7Twithout saturation,suggestingthepresenceofmagnetic anisotropy or the considerable crystal-field effects[33].

Fig.4 Temperature dependence of χMT products at 1 kOe,between 2 and 300 K for 1(left)and 2(right).Inset:plots of M-H/T for 1 and 2
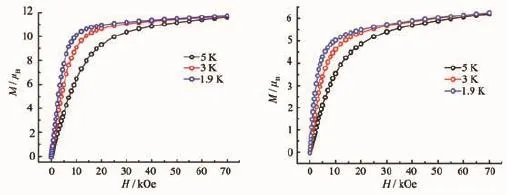
Fig.5 Field dependent magnetization for complexes 1(left)and 2(right)at 1.9,3.0,and 5.0 K
In order to investigate the SMM behavior of 1 and 2,alternating-current(ac)magnetic susceptibility measurements were also performed.Out-of-phase(χ″) susceptibilities signal aredetected for complexes 1 and 2(Fig.6)under zero dc field,indicating slow magnetic relaxation behavior.Interestingly,the χ″of complex 1 (Fig.7)under a 1 000 Oe dc field shows a small shoulder under 5 K.Upon cooling,the χ″increases again at lower temperature region,indicating quantum tunnelingprocess,whichhappensbetweenthe degeneratedgroundstates[34-36].Thetemperaturedependent ac susceptibility suggests the single-ion origin of such a slow relaxation behavior instead of single chain magnetism under 1 000 dc fields since the antiferromagnetic interaction detected in the static magnetic susceptibilities study is too weak to induce ordered magnetic moment arrangement.Although no maxima in χ″are observed here,the energy barrier and τ0can be evaluated through the equation bellow[37-38]:

Fig.6 Temperature-dependent out-of-phase(χ″) susceptibilitiesfor 1and2at1000Hz,under zero dc field

The best fitting of ln(χ″/χ′)vs.1/T plots(Fig.9) gives an estimate of the activation energy and the characteristic time of~2 K and 1×10-7s,respectively.
Compared with 1,χ″for 2 below 5 K increase slower under zero dc field(Fig.6),indicating that the magnetic coupling between DyIIIions is not strong enough to suppress the quantum tunneling effect. While,a new small shoulder appears at 7 K when 400Oedc field is applied(Fig.8),indicatingslow relaxationofcomplex2.Whenloweringthe temperature,χ″increasesrapidly,implyingthat quantum tunneling mechanism plays the dominant role in the low temperature region.
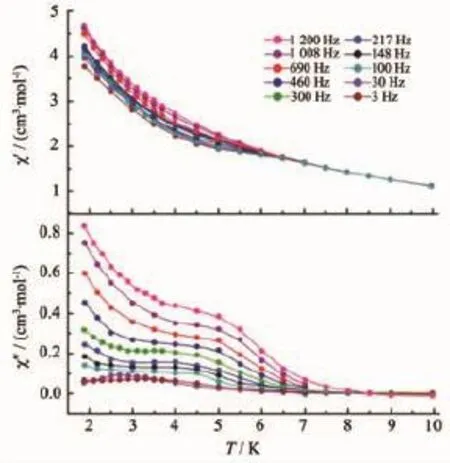
Fig.7 Temperature-dependent ac susceptibility for 1 under 1 000 Oe dc field

Fig.8 Temperature-dependent out-of-phase(χ″) susceptibilities for 2 at 1 000 Hz,under zero and 400 Oe dc field
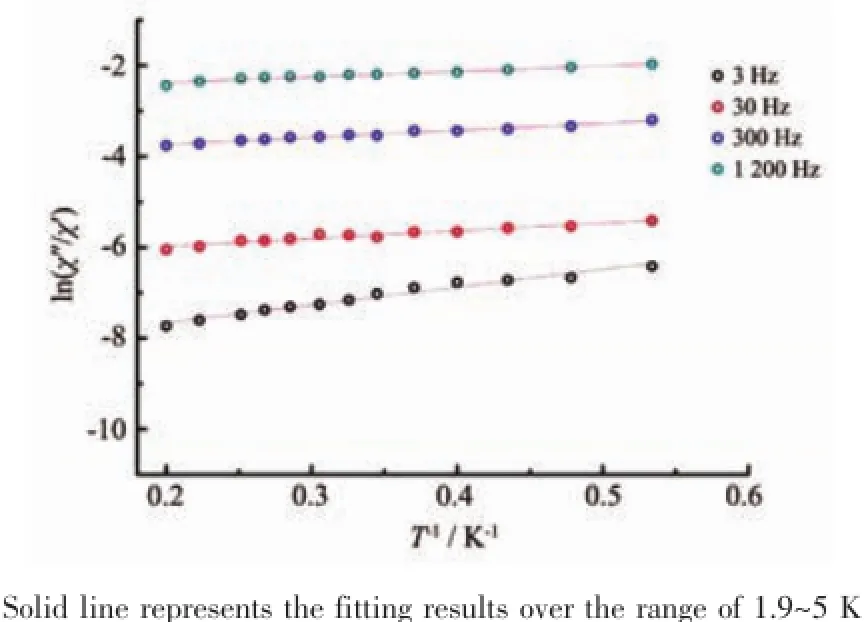
Fig.9 Plot of natural ln(χ″/χ′)vs 1/T for 1
3 Conclusions
In summary,1D and 2D dysprosium complexes, 1 and 2,have been isolated.Single crystal X-ray studies reveal that complex 1 is one dimensional zigzag chain linked by OAc-while complex 2 is two dimensional network constructed by disulfide bond bridged one dimensional dysprosium chains.Magnetic study shows that complex 1 exhibits slow magnetic relaxation behavior with energy barrier of 2 K,while complex 2 shows ferromagnetic magnetic interaction and slow magnetic relaxation.
References:
[1]Zhang P,Guo Y N,Tang J.Coord.Chem.Rev.,2013,257: 1728-1763
[2]Gatteschi D,Sessoli R,Villain J.Molecular Nanomagnets. Oxford:Oxford University Press,2006.
[3]Zhang P,Zhang L,Tang J.Dalton Trans.,2015,44:3923-3929
[4]Stipe B C,Strand T C,Poon C C,et al.Nat.Photonics, 2010,4:484-488
[5]Zhang P,Tang J.Lanthanide Single Molecule Magnets. Heidelberg:Springer,2015.
[6]LIN Shuang-Yan(林双燕),GUO Yun-Nan(郭云南),TANG Jin-Kui(唐金魁),et al.Chinese J.Appl.Chem.(应用化学), 2010,27(12):1365-1371
[7]Blagg R J,Muryn C A,McInnes E J,et al.Angew.Chem. Int.Ed.,2011,50:6530-6533
[8]Blagg R J,Ungur L,Tuna F,et al.Nat.Chem.,2013,5:673-678
[9]Liu J L,Wu J Y,Chen Y C,et al.Angew.Chem.Int.Ed., 2014,53:12966-12970
[10]Lin S Y,Wernsdorfer W,Ungur L,et al.Angew.Chem. Int.Ed.,2012,51:12767-12771
[11]Tang J,Hewitt I,Madhu N T,et al.Angew.Chem.Int.Ed., 2006,45:1729-1733
[12]Guo Y N,Xu G F,Wernsdorfer W,et al.J.Am.Chem. Soc.,2011,133:11948-11951
[13]Zhang P,Zhang L,Wang C,et al.J.Am.Chem.Soc.,2014, 136:4484-4487
[14]Guo Y N,Ungur L,Granroth G E,et al.Sci.Rep.,2014,4: 5471
[15]Rinehart J D,Fang M,Evans W J,et al.J.Am.Chem.Soc., 2011,133:14236-14239
[16]Joseph M Z,Dianne J X,Mihail Atanasov,et al.Nat.Chem.,2013,5:577-581
[17]Rinehart J D,Fang M,Evans W J,et al.Nat.Chem., 2011,3:538-542
[18]Ishikawa N,Sugita M,Ishikawa T,et al.J.Am.Chem.Soc., 2003,125:8694-8695
[19]Ganivet C R,Ballesteros B,de la Torre G,et al.Chem. Eur.J.,2013,19:1457-1465
[20]Woodruff D N,Winpenny R E P,Layfield R A.Chem.Rev., 2013,113:5110-5148
[21]Dechambenoit P,Long J R.Chem.Soc.Rev.,2011,40: 3249-3265
[22]Bogani L,Sangregorio C,Sessoli R,Gatteschi D.Angew. Chem.Int.Ed.,2005,44:5817-5821
[23]Jia L,Chen Q,Meng Y S,et al.Chem.Commun.,2014,50: 6052-6055
[24]Pejo C,Guedes G P,Novak M A,et al.Chem.Eur.J., 2015,21:8696-8700
[25]Shao D,Zhang S L,Zhao X H,et al.Chem.Commun.,2015, 51:4360-4363
[26]Caneschi A,Gatteschi D,Lalioti N,et al.Angew.Chem.Int. Ed.,2001,40:1760-1763
[27]Norio I,Yoshitomo O,Susumu C,et al.J.Am.Chem.Soc., 2008,130:24-25
[28]Feng X,Harris T D,Long J R.Chem.Sci.,2011,2:1688-1694
[29]Zhang Y Z,Zhao H H,Funck E,et al.Angew.Chem.Int. Ed.,2015,54:5583-5587
[30]Sheldrick G M.SHELXS-97,Program for Crystal Structure Solution,University of Göttingen:Germany,1997.
[31]Boudreaux E A,Mulay L N.Theory and Applications of Molecular Paramagnetism.New York:John Wiley&Sons, 1976.
[32]Kahn O.Molecular Magnetism.New York:VCH,1993.
[33]Xu G F,Wang Q L,Gamez P,et al.Chem.Commun.,2010, 46:1506-1508
[34]Leuenberger M N,Loss D.Nature,2001,410:789-793
[35]Plokhov D I,Popov A I,Zvezdin A K.Phys.Rev.B,2011, 84:224436
[36]Wernsdorfer W,Sessoli R.Science,1999,284:133-135
[37]Bartolomé J,Filoti G,Kuncser V,et al.Phys.Rev.B,2009, 80:014430
[38]Lin S Y,Xu G F,Zhao L,et al.Dalton Trans.,2011,40: 8213-8217
Magnetic Relaxation in 1D and 2D Dysprosium(Ⅱ)Coordination Polymers
WU Jian-FengZHANG Hai-XiaZHANG PengZHAO LangTANG Jin-Kui*
(State Key Laboratory of Rare Earth Resource Utilization,Changchun Institute of Applied Chemistry,Chinese Academy of Sciences,Changchun 130022,China)
Two dysprosium complexes,[Dy2(OAc)6H2O]n(1)and{[DyL(H2O)4]ClO4·H2O}n(2)(L=2,2′-dithiobis(3-pyridinecarboxylic acid)),were synthesized and structurally and magnetically characterized.Complex 1 is a 1D dysprosium chain,in which the dysprosium ions are bridged by OAc-anions,exhibiting slow magnetic relaxation behavior with an energy barrier of 2 K.Complex 2 is a 2D dysprosium network,in which the carboxylato-bridged dysprosium chains are linked by disulfide bonds,showing ferromagnetic interaction and slow magnetic relaxation. CCDC:1400117,1;1400116,2.
slow magnetic relaxation;ferromagnetic interaction;disulphide bond
O614.342
A
1001-4861(2015)09-1847-08
10.11862/CJIC.2015.236
2015-05-29。收修改稿日期:2015-07-13。
国家自然科学基金(No.21371166)资助项目。
*通讯联系人。E-mail:tang@ciac.ac.cn;会员登记号:S06N9843M1004。
