1,25(OH)2D3对人肾小球系膜细胞增殖及mTOR/p70s6K表达的影响
2015-11-24唐玉玲马莉赵丹贾林杨锐杨晓萍
唐玉玲,马莉,赵丹,贾林,杨锐,杨晓萍△
细胞与分子生物学
1,25(OH)2D3对人肾小球系膜细胞增殖及mTOR/p70s6K表达的影响
唐玉玲1,马莉1,赵丹2,贾林1,杨锐1,杨晓萍2△
目的探讨1,25-二羟基维生素D3[1,25(OH)2D3]对人肾小球系膜细胞增殖的影响及其在肾小球系膜细胞调控中对mTOR/p70s6K信号通路的作用。方法体外培养人肾小球系膜细胞,取传代培养至3~7代细胞分为4组:正常对照组(加含5%胎牛血清DMEM培养基),VD组[加1,25(OH)2D310-8mol/L],R组(加雷帕霉素5 mg/L),R+ VD组[加雷帕霉素5 mg/L及1,25(OH)2D310-8mol/L],干预48 h。采用细胞增殖与活性检测试剂盒CCK-8检测细胞增殖情况,流式细胞术检测细胞周期时相分布,免疫荧光法检测细胞中mTOR、p70s6K的表达情况。结果正常对照组、VD组、R组、R+VD组:(1)吸光度值(A450)依次降低,组间多重比较差异均有统计学意义(均P<0.05);抑制率(IR)依次升高。(2)G1期细胞比例依次增加,S期、G2/M期依次减少,增殖指数(PI)依次降低,除R组与VD组比较差异无统计学意义外,其余组间多重比较差异均有统计学意义。(3)系膜细胞中mTOR、p70s6K蛋白表达强度均依次降低,除R组与VD组比较差异无统计学意义外,其余组间多重比较差异均有统计学意义。结论1,25(OH)2D3可显著抑制人系膜细胞增殖,且其可能通过抑制mTOR/p70s6K信号通路调控肾小球系膜细胞增殖。
肾小球系膜细胞;细胞增殖;细胞周期;核糖体蛋白质S6激酶类,70-kDa;1,25-二羟基维生素D3;mTOR/p70s6K信号通路
系膜细胞异常增殖是导致各种肾小球肾炎向终末期肾病(ESRD)发展的中心环节,有效抑制系膜细胞增殖是防治肾小球肾炎的重要策略。1,25-二羟基维生素D3[1,25(OH)2D3]具有多种新型生物学效应,包括促使肿瘤细胞生长停滞、诱导细胞凋亡和分化。哺乳动物雷帕霉素靶蛋白(mammalian target of rapamycin,mTOR)/p70s6K激酶(p70s6K)信号通路在正常及癌细胞的生长、增殖、分化、凋亡、代谢中扮演重要角色[1]。研究证实,mTOR/p70s6K信号通路的异常与多种恶性肿瘤有关,mTOR在骨肉瘤、肾细胞癌、结肠癌等多种肿瘤细胞中呈高表达[2-4]。但目前mTOR/p70s6K信号通路在人系膜细胞中的作用尚少见报道。本研究通过CCK-8法及流式细胞术观察活性维生素D3对人肾小球系膜细胞增殖的影响,并通过免疫荧光法观察人肾小球系膜细胞中mTOR、p70s6K蛋白的表达情况,探讨活性维生素D3在肾小球系膜细胞调控中对mTOR/p70s6K信号通路的作用。
1 材料与方法
1.1 实验材料
1.1.1 细胞株人肾小球系膜细胞株(湘雅医学院中心实验室提供),本课题组前期通过形态学观察及免疫荧光实验鉴定为人系膜细胞。
1.1.2 主要试剂1,25(OH)2D3、LY294002、碘化丙啶(美国Sigma公司),雷帕霉素(上海生工公司),胰蛋白酶(美国Gibco公司),CCK-8试剂盒、兔抗人mTOR单克隆抗体(美国cell signal公司),兔抗人p70s6K单克隆抗体(海艾博抗贸易有限公司),山羊抗兔IgG二抗(北京中杉金桥生物技术有限公司)。
1.1.3 仪器与设备细胞培养箱(美国Thermo Forma公司),普通光学显微镜、倒置相差显微镜(日本Olympus公司),ELX-800酶标仪(美国Biokit公司),流式细胞仪(德国Partec公司),激光共聚焦显微镜(德国ZEISS公司)。
1.2 实验方法
1.2.1 细胞培养用含10%胎牛血清的DMEM完全培养基复苏肾小球系膜细胞,置于5%CO2、37℃饱和湿度的培养箱中培养,当细胞生长至70%~80%融合时,用0.25%胰蛋白酶消化,传代继续培养,取3~7代用于实验。
1.2.2 实验分组及细胞干预收集生长良好的对数期肾小球系膜细胞,计数后接种于培养瓶中,待细胞完全贴壁后弃完全培养基,加入L-DMEM同步化24 h,设4组:(1)正常对照组,L-DMEM培养基(5%FBS)。(2)VD组,1,25(OH)2D3(10-8mol/L)。(3)R组,雷帕霉素(5 mg/L)。(4)R+VD组,雷帕霉素(终浓度为5 mg/L)+1,25(OH)2D3(终浓度为10-8mol/L)。
1.2.3 CCK-8法检测系膜细胞增殖情况参照CCK-8试剂盒说明书操作。取对数期人肾小球系膜细胞,调整浓度为1×104个/mL,200 μL/孔接种于96孔板内;按照实验分组干预48 h,干预结束后PBS冲洗1次,加入100 μL L-DMEM及10 μL CCK-8溶液,培养4 h后振荡15 min,酶标仪检测各孔在450 nm波长处的吸光度值(A450)。每组设4个复孔,设置空白调零孔(只加L-DMEM及CCK-8溶液)。实验重复3次,并计算抑制率(inhibition rate,IR)=[A(对照组)-A(实验组)]/A(对照组)× 100%。
1.2.4 流式细胞术检测系膜细胞细胞周期分布情况取对数期人肾小球系膜细胞,调整浓度为2×105个/mL,接种于培养瓶内;按照实验分组干预48 h,干预结束后收集细胞,4℃预冷的70%乙醇重悬,4℃固定过夜;上机前离心1次,PBS洗涤1次,1×Buffer液重悬细胞,加入RNA酶(终浓度为10 g/L),37℃孵育30 min,加入PI染液(终浓度为50 mg/L),4℃避光反应30 min,移入流氏管中,再加入PBS至2 mL,上机检测。实验重复3次,计算细胞增殖指数(proliferation index,PI)=(S期+ G2/M期细胞比例)(/G1期+S期+G2/M期细胞比例)×100%。
1.2.5 免疫荧光检测及结果判定取对数期人肾小球系膜细胞,调整浓度为8×103个/孔,接种于6孔板内的盖玻片上,加入10%DMEM培养基培养24 h后按实验分组干预48 h,干预结束后PBS浸洗3次,每孔加入4%多聚甲醛500 μL固定20 min;PBS浸洗3次,加10%正常山羊血清100 μL,室温封闭30 min,加一抗,4℃孵育过夜(一般大于18 h);PBS浸洗3次,避光滴加FITC标记兔抗山羊IgG(1∶100),室温反应1.5 h,5%甘油封片,最后采用激光共聚焦显微镜观察并采图,AIM软件测定荧光强度。
1.3 统计学方法应用SPSS 19.0统计软件处理数据,计量资料以均数±标准差表示,多组间比较采用方差分析,组间多重比较用LSD法,P<0.05为差异有统计学意义。
2 结果
2.1 CCK-8法检测各组干预48 h后对人肾小球系膜细胞增殖的影响正常对照组、VD组、R组、R+ VD组A450依次降低,组间多重比较差异均有统计学意义(均P<0.05);正常对照组、VD组、R组、R+VD组IR依次升高。见表1。
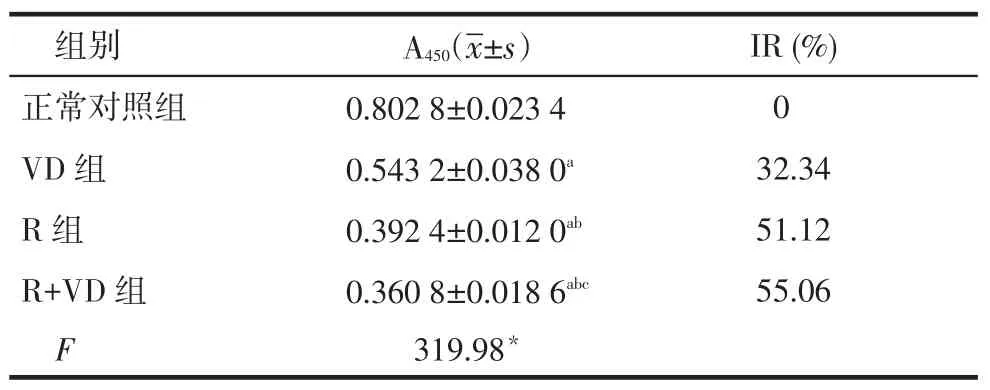
Tab.1The effects of 48-hour drug treatment on mesangial cell proliferation,which was measured by CCK-8 colorimetric assay表1 CCK-8法检测各组干预48 h后对人系膜细胞增殖的影响(n=10)
2.2 流式细胞术检测各组干预48 h后对人肾小球
系膜细胞细胞周期的影响正常对照组、VD组、R组、R+VD组G1期细胞比例依次增加,S期、G2/M期依次减少,PI依次降低,除R组与VD组比较差异无统计学意义外,其余组间多重比较差异均有统计学意义。见表2、图1。
Tab.2The effects of 48-hour drug treatment on mesangial cell cell cycles,which was measured by flow cytometry表2 流式细胞术检测各组干预48 h后对人肾小球系膜细胞细胞周期(n=10,%)
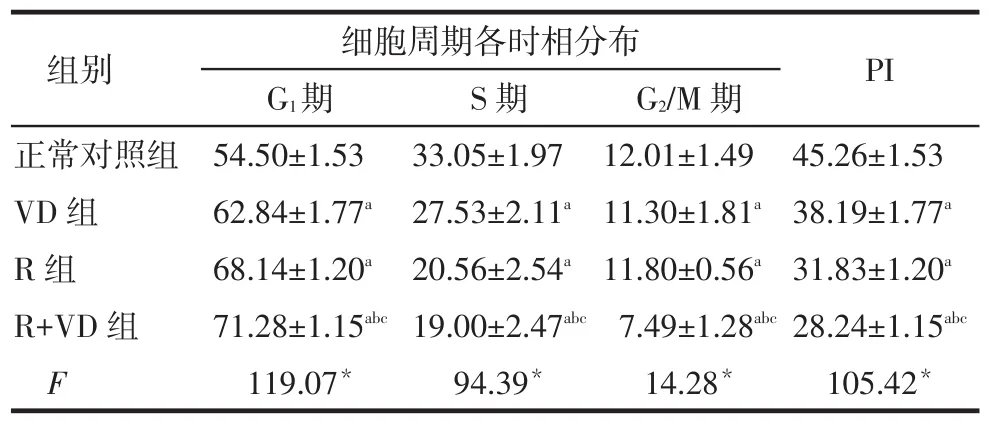
Tab.2The effects of 48-hour drug treatment on mesangial cell cell cycles,which was measured by flow cytometry表2 流式细胞术检测各组干预48 h后对人肾小球系膜细胞细胞周期(n=10,%)
细胞周期各时相分布G1期54.50±1.53 62.84±1.77a 68.14±1.20a 71.28±1.15abc 119.07*组别正常对照组VD组R组R+VD组F S期33.05±1.97 27.53±2.11a 20.56±2.54a 19.00±2.47abc 94.39*G2/M期12.01±1.49 11.30±1.81a 11.80±0.56a 7.49±1.28abc 14.28*PI 45.26±1.53 38.19±1.77a 31.83±1.20a 28.24±1.15abc 105.42*
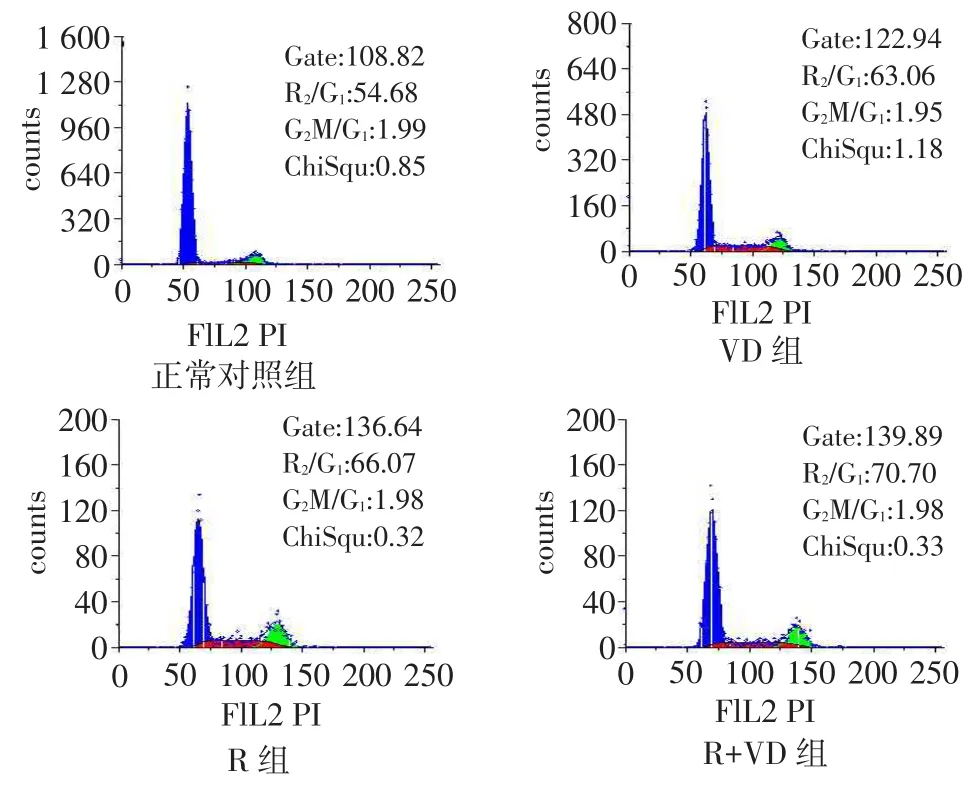
Fig.1The effects of 48-hour drug treatment on mesangial cell cell cycles,which was measured by flow cytometry图1 流式细胞术检测各组干预48 h后对人肾小球系膜细胞细胞周期的影响
2.3 免疫荧光检测各组干预48 h后肾小球系膜细胞中mTOR、p70s6K的表达情况正常对照组、VD组、R组、R+VD组系膜细胞中mTOR、p70s6K蛋白表达强度均依次降低,除R组与VD组比较差异无统计学意义外,其余组间多重比较差异均有统计学意义。见表3,图2、3。
Tab.3The expression of mTOR,p70s6K were detected by immunofluorescence in each group of mesangial cells表3 各组系膜细胞中mTOR、p70s6K免疫荧光强度比较(n=6)
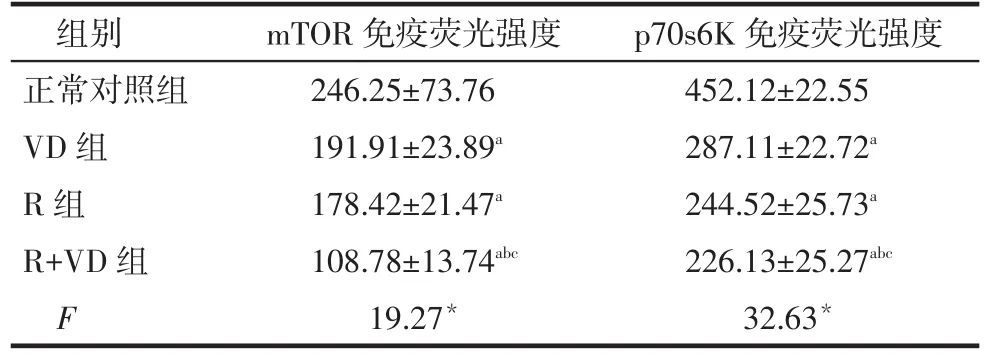
Tab.3The expression of mTOR,p70s6K were detected by immunofluorescence in each group of mesangial cells表3 各组系膜细胞中mTOR、p70s6K免疫荧光强度比较(n=6)
mTOR免疫荧光强度246.25±73.76 191.91±23.89a 178.42±21.47a 108.78±13.74abc 19.27*组别正常对照组VD组R组R+VD组F p70s6K免疫荧光强度452.12±22.55 287.11±22.72a 244.52±25.73a 226.13±25.27abc 32.63*
3 讨论
1,25(OH)2D3除了有调节钙磷代谢和免疫系统的功能外,还能调控多种组织来源细胞的生长和分化,主要表现为抑制多种肿瘤细胞、免疫细胞及肾脏系膜细胞增殖、分化相关的基因表达,最终影响细胞增殖、凋亡与分化[5]。有研究显示1,25(OH)2D3可抑制体外培养的小鼠系膜细胞的增殖[6]。本课题组前期研究也证实,1,25(OH)2D3可通过阻滞细胞周期而显著抑制正常人系膜细胞的增殖[7]。本研究结果显示:与正常对照组比较,VD组人系膜细胞的生长被显著抑制,并将细胞周期阻滞于G1期,进一步证实1,25(OH)2D3能显著抑制人系膜细胞的进程,阻滞系膜细胞增生,与前期研究结果一致。
细胞分裂增殖的实质是DNA复制,而细胞DNA复制及蛋白合成与mTOR信号通路的活化密切相关。mTOR属于PI3K蛋白激酶类家族,是PI3K/Akt信号通路下游的一个效应蛋白,其底物调控与细胞生长、存活和增殖相关蛋白的合成有关[8]。mTOR通过激活其下游靶蛋白p70s6K提高下游蛋白翻译效率,促进蛋白质合成及细胞生长、增殖[9]。阻断该信号通路的活化,mTOR靶向抑制剂雷帕霉素可通过抑制mTOR及其下游底物p70S6K、4E-结合蛋白1(4E-BP1)的活性,促进柯萨奇病毒(CVB3)所致HeLa细胞的病变效应及细胞凋亡,从而影响细胞增殖[10]。本研究结果显示,与正常对照组比较,R组系膜细胞增殖显著被抑制,并将细胞周期阻滞于G1期,提示mTOR/p70s6K信号通路在系膜细胞的增殖过程中可能起着重要的调控作用。
有研究显示,mTOR/p70s6K信号通路在细胞的生存、生长与增殖中起中心调控作用,其活化可以抑制多种刺激诱发的细胞凋亡,促进细胞周期进展,从而促进细胞的生存和增殖,同时参与血管形成,并参与肿瘤的侵袭和转移[11]。Li等[12]研究证实,丹参酮IIA(T2A)通过mTOR/p70s6K/4E-BP1信号通路可抑制乳腺癌细胞中缺氧诱导因子(HIF)-1α蛋白的合成及血管内皮生长因子(VEGF)的表达,进而抑制该肿瘤血管的生成与生长。Yang等[13]发现,依维莫司(mTORC1)可协同活性维生素D3下调mTOR底物p70s6K和起始因子4E-BP1的表达,诱导急性早幼粒细胞U937的增殖与分化,增加活性维生素D3介导的p21(waf1)转录活性及维生素D受体(VDR)乙酰化水平。α-硫辛酸可通过mTOR/p70s6K/4E-
BP1信号通路剂量依赖性地调控高糖诱导的肾小球系膜细胞增生及细胞外基质的形成[14]。本研究结果显示,R+VD组较另3组人肾小球系膜细胞的增殖抑制更为明显,提示1,25(OH)2D3可协同雷帕霉素加强对系膜细胞的抑制作用,1,25(OH)2D3可能通过阻滞mTOR/p70s6K信号通路抑制系膜细胞增殖。另外,免疫荧光结果显示,正常对照组中mTOR、p70s6K蛋白有表达,提示mTOR/p70s6K信号通路确实存在于正常人系膜细胞中;与正常对照组比较,VD和R组mTOR、p70s6K蛋白表达均显著降低,进一步证实1,25(OH)2D3能通过阻滞mTOR/p70s6K信号通路抑制系膜细胞增殖。与VD组比较,R+VD组mTOR、p70s6K蛋白表达显著降低,提示1,25(OH)2D3可协同雷帕霉素下调mTOR、p70s6K的表达,从而抑制系膜细胞增殖,与Yang等[13]研究结果一致。
综上所述,1,25(OH)2D3可显著抑制人肾小球系膜细胞增殖,将系膜细胞周期阻滞于G1期,其机制可能是通过抑制mTOR/p70s6K信号通路来实现的,但仍有待进一步研究证实。
(图2、3见插页)
[1]Parrales A,López E,Lee-Rivera I,et al.ERK1/2-dependent activa⁃tion of mTOR/mTORC1/p70S6K regulates thrombin-induced RPE cell proliferation[J].Cell Signal,2013,25(4):829-838.doi:10.1016/ j.cellsig.2012.12.023.
[2]Zhou Q,Deng Z,Zhu Y,et al.mTOR/p70s6K signal transduction pathway contributes to osteosarcoma progression and patients′prog⁃nosis[J].Med Oncol,2010,27(4):1239-1245.doi:10.1007/ s12032-009-9365-y.
[3]Maj-Hes A,Medioni J,Scotte F,et al.Rechallenge with mTOR in⁃hibitors in metastatic renal cel1 carcinoma patients who progressed on previous m TOR inhibitor therapy[J].Oncology,2013,85(1):8-13.doi:10.1159/000350005.
[4]Park JH,Kim JJ,Bae YS.Involvement of PI3K-AKT-mT0R path⁃way in protein kinase CKⅡinhibition-mediated senescence in hu⁃man colon cancer cells[J].Biochem Biophys Res Commun,2013, 433(4):420-425.doi:10.1016/j.bbrc.2013.02.108.
[5]IngralIam BA,BragdonB,NoheA.Molee ular basis of the potential of vitamin D to prevent cancer[J].Carr Med Res Opin,2008,24(1):139-149.doi:10.1185/030079908X253519.
[6]Hariharan S,Hong SY,Hsu A,et al.Effect of 1,25-dihydroxyvita⁃min D3on mesangial cell proliferation[J].J Lab Clin Med,1991, 117:423-429.
[7]Yin X,Zhang H,Chen JP,et al.Effects of 1,25(OH)2D3on prolifera⁃tion and expression of PCNA of human glomerular mesangial cells[J].Tianjin Med J,2015,43(1):17-19.[尹璇,张昊,陈建平,等.1, 25-二羟基维生素D3对人肾小球系膜细胞增殖及PCNA表达的影响[J].天津医药,2015,43(1):17-19].doi:10.3969/j.issn.0253-9896.2015.01.005.
[8]Weber JD,Gutmann DH.Deconvoluting mTOR biology[J].Cell Cy⁃cle,2012,11(2):236-248.doi:10.4161/cc.11.2.19022.
[9]Pópulo H,Lopes JM,Soares P.The mTOR signalling pathway in hu⁃man cancer[J].Int J Mol Sci,2012,13(2):1886-1918.doi:10.3390/ ijms13021886.
[10]Chen Z,Yang L,Liu Y,et al.LY294002 and Rapamycin promote coxsackievirus-induced cytopathic effect and apoptosis via inhibi⁃tion of PI3K/AKT/mTOR signaling pathway[J].Mol Cell Biochem, 2014,385(1-2):169-177.doi:10.1007/s11010-013-1825-1.
[11]Zhang X,Zanello LP.Vitamin D receptor-dependent 1 alpha,25(OH)2 vitamin D3-induced anti-apoptotic PI3K/Akt signaling in os⁃teoblasts[J].J Bone Miner Res,2008,23(8):1238-1248.doi: 10.1359/jbmr.080326.
[12]Li G,Shan C,Liu L,et al.Tanshinone IIA inhibits HIF-1α and VEGF expression in breast cancer cells via mTOR/p70S6K/RPS6/ 4E-BP1 signaling pathway[J].PLoS One,2015,10(2):e0117440. doi:10.1371/journal.pone.0117440.
[13]Yang J,Ikezoe T,Nishioka C,et al.Inhibition of mTORC1 by RAD001(everolimus)potentiates the effects of 1,25-dihydroxyvita⁃min D(3)to induce growth arrest and differentiation of AML cells in vitro and in vivo[J].Exp Hematol,2010,38(8):666-676.doi: 10.1016/j.exphem.2010.03.020.
[14]Lv C,Wu C,Zhou YH,et al.Alpha lipoic acid modulated high glu⁃cose-induced rat mesangial cell gysfunction via mTOR/p70s6K/4EBP1 pathway[J].Int J Endocrinol,2014,2014:658589.doi:10.1155/ 2014/658589.
(2015-04-17收稿 2015-05-11修回)
(本文编辑 陈丽洁)
Effects of 1,25(OH)2D3on proliferation and mTOR/p70s6K expressions of human glomerular mesangial cells
TANG Yuling1,MA Li1,ZHAO Dan2,JIA Lin1,YANG Rui1,YANG Xiaoping2△
1 Medical College of Shihezi University,Xinjiang 832000,China;2 Department of Nephrology,the First Affiliated Hospital of Medical College of Shihezi University△
ObjectiveTo investigate the effects of 1,25-dihydroxyvitamin D3[1,25(OH)2D3]on cell proliferation in hu⁃man glomerular mesangial cells and it′s effects on the regulation of mTOR/p70s6K signaling pathway in this cell line.MethodsThe cultured human mesangial cells at passage 3-7 were divided into four groups:control group,VD group(addition of 10-8mol/L of 1,25-dihydroxyvitamin D3),R group(addition of 5 mg/L of rapamycin)and R+VD group(addition of 5 mg/L ra⁃pamycin combined with 10-8mol/L of 1,25-dihydroxyvitamin D3).Drug incubation last 48 h.The effect of mesangial cell pro⁃liferation was measured by CCK-8 colorimetric assay.The cell cycles were measured by flow cytometry.The expression of mTOR and p70s6K were detected by immunofluorescence.Results(1)The absorbance of A450was higher in control group than that in VD group than that in R group than that in R+VD group.But the inhibition rate(IR)was lower in control group than that in VD group than that in R group than that in R+VD group.All comparisons were of statistic significance.(2)Cells in G1phase were higher while cells in G2/M and S phases as well as proliferation rate(PI)were lower in control group than those in VD group than those in R group than those in R+VD group.All comparisons were of statistic significance except in⁃dexes between group R and group VD.(3)mTOR and p30s6K expressions in mesangial cells were higher in control group than those in VD group than those in R group than those in R+VD group.All comparisons were of statistic significance ex⁃cept indexes between group R and group VD.Conclusion1,25-dihydroxyvitamin D3might inhibit mesangial cell prolifera⁃tion significantly through mTOR/p70s6K signaling pathways.
mesangial cells;cell proliferation;cell cycle;ribosomal protein S6 kinases,70-kDa;1,25(OH)2D3;mTOR/ p70s6K signaling pathway
R692
A
10.11958/j.issn.0253-9896.2015.10.001
国家自然科学基金资助项目(81160090)
1新疆石河子大学医学院(邮编832000);2新疆石河子大学第一附属医院肾病科
唐玉玲(1987),女,硕士在读,主要从事肾病系统疾病研究
△通讯作者E-mail:sbkyxp@163.com
