QCM Sensors Based on PEI Films for CO2Detection
2015-11-18GuangZhongXieTingKangYongZhouTaoXieHuiLingTaiandYaDongJiang
Guang-Zhong Xie, Ting Kang, Yong Zhou, Tao Xie, Hui-Ling Tai, and Ya-Dong Jiang
QCM Sensors Based on PEI Films for CO2Detection
Guang-Zhong Xie, Ting Kang, Yong Zhou, Tao Xie, Hui-Ling Tai, and Ya-Dong Jiang
—In this paper, quartz crystal microbalance(QCM) gas sensors coated with polyehtyleneimine (PEI)was utilized for carbon dioxide (CO2) detection. The sensing mechanism is based on the availability of reversible acid-base reactions between CO2molecules and PEI at room temperature. The experimental results revealed that the PEI/starch sensor exhibited much higher sensitivity than that of pure PEI, and showed approximate linearity over a concentration region ranging from 500 ppm to 8000 ppm. The influence of humidity had also been investigated. Furthermore, the response and recovery time deceased as the operation temperatures increased. Finally, sensitivity loss after conservation for several days and reversibility of the sensors had been discussed.
Index Terms—Humidity, polyehtyleneimine, sensor,starch, temperature.
1. Introduction
As a green house gas contributing to global warming,carbon dioxide (CO2) is relatively inert, so it is an important and difficult task to monitor CO2with convenient and reliable sensors. The qualitative detection and thereafter the control of CO2concentration are very important in many areas, such as air conditioning,agriculture, biological technology, and medical services[1]. To meet these increasing requirements, a large number of continuous efforts have been made to develop high performance CO2sensors. Various principles, including infrared absorption[2],[3], field-effect transistors[4],resistance[5]-[7], and piezoelectric[8]have so far been adopted for developing CO2sensors. Quartz crystal microbalance (QCM) CO2sensors have advantages of large sensitivity and digital frequency output. When the sensor exposes to ambient environment, the adsorption of CO2molecules on CO2sensing layer will make the sensor produce a frequency shift.
In recent years, various sensitive materials have been investigated for CO2sensors, such as carbonates (BaCO3[9],Na2CO3[10], SrCO3[11]), metal oxides (CuO-BaTiO3,La2O3)[12],[13], Versamide 900, as well as CO2sensitive materials containing amino groups, such as monomeric amines, polymeric amines[14]-[19], and modified silicates,also named as siloxanes[20],[21]. Therefore, the polyehtyleneimine (PEI) is often chosen as the preferred sensitive material to detect CO2. In this paper, PEI and PEI/starch films were selected for QCM CO2detection based on the hard soft acids bases (HSAB) theory. Some methods were utilized to improve the response, and the respective sensitive properties were studied in detail as well.
2. Experimental
2.1 Device Preparation
An 8 MHz AT-cut quart crystal with Ag electrode(purchased from Jingbao Company, Chengdu) was used as the substrate. Before depositing the sensitive film, the devices were cleaned with acetone, ethanol, and de-ionized water in sequence. Each step lasted 15 minutes by sonication to remove impurities and pollutants from the devices surface, and then the treated devices were dried in a vacuum drying oven for 2 hours at 60°C.
2.2 Materials and Films Fabrication
PEI (mass fraction was 50% in water) was purchased from Aldrich. Soluble starch (C12H22O11) was purchased from J&K Scientific. The other reagents were analytically pure, and de-ionized (DI) water was used for preparation of samples and solutions. QCM CO2gas sensors were fabricated as follows.
Monolayer film: PEI solution was diluted 70 times with DI water, then spin-coated on the active area of QCM electrode. Finally, the devices were put into the vacuum drying oven under 60°C for 48 hours.
Composite film: A small amount of starch (A: 2 mg, B: 6 mg, C: 10 mg) was added into 35 ml PEI solution to mix mechanically until uniform solution was obtained. Then thecomposite film was formed by spin coating the composite material on the active area of the QCM electrode. Finally,the devices were put into the vacuum drying oven under 60°C for 48 hours.
The sensors’ frequencies were measured in dry state after the coating process to confirm the formation of the needed layer. A constant frequency shift of around 4 kHz was kept in all these devices following the same procedure.
2.3 Test Facility
研究表明:饲喂牛奶、牛奶替代品提高0.1千克日增重,可使产奶量提高65千克;饲喂谷物提高0.1千克日增重,可使产奶量提高266千克,所以应重视谷物采食量,谷物摄入量对牛奶产量的影响大于牛奶摄入量。尽早断奶,瘤胃才能更好的发育,如图2。
The sensitive properties of the prepared QCM gas sensors were measured real-timely with a flow gas system,as shown in Fig. 1. The air was used for dilution of CO2gas. The temperature in the sensor cell was stabilized at 27°C. Gas concentration was controlled by a mass flow controller. The frequency of the QCM sensor was recorded with a measurement system consisting of a film thickness oscillator, frequency counter, and computer. Scanning electron microscopy was used to analyze the morphology of the sensitive films. The Fourier transform infrared (FTIR)spectrum of PEI in the range of 667 cm-1to 4000 cm-1was recorded to analysis the contained chemical group.
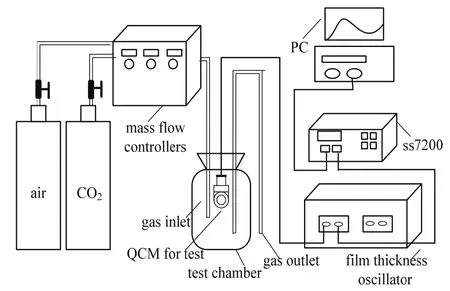
Fig. 1. Experimental facility.
3. Result and Discussion
Basically, the frequency of the QCM sensor changes upon adsorption or absorption of the corresponding gas. The frequency shift Δf results in an increase in the oscillating mass Δm, described as
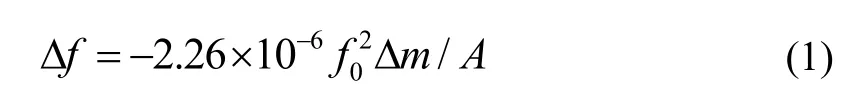
where A is the area of the electrode surface and f0is the fundamental frequency of QCM.
3.1 Characterization
The scanning electron microscope ( SEM ) image of a pure PEI thin film is shown in Fig. 2 (a). It shows that there are a large number of wrinkles on the surface. And the upper left corner of Fig. 2 (a) gives a magnification of a wrinkles. It can be found that the surface is raised, which seems to contribute to the short response time and more gas adsorption sites of the sensor. Fig. 2 (b) shows traces of starch particles dispersed unevenly in the PEI polymer. And the upper left corner of the Fig. 2 (b) gives a magnification of a starch particles, which shows that the starch does not dissolve in the PEI and just attaches on the polymer membrane.
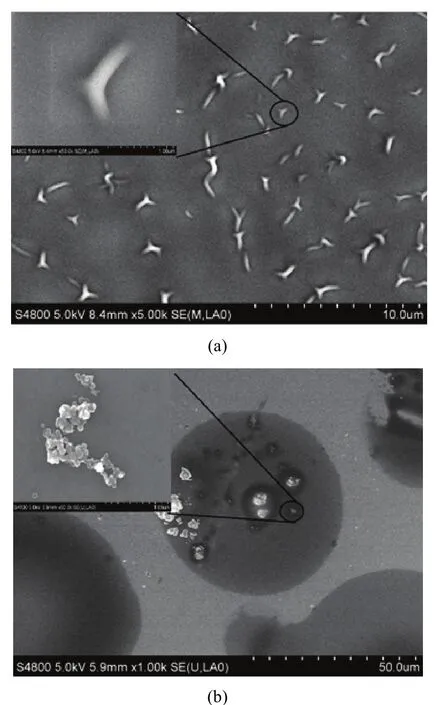
Fig. 2. SEM images: (a) PEI film and (b) PEI/Starch composite film.
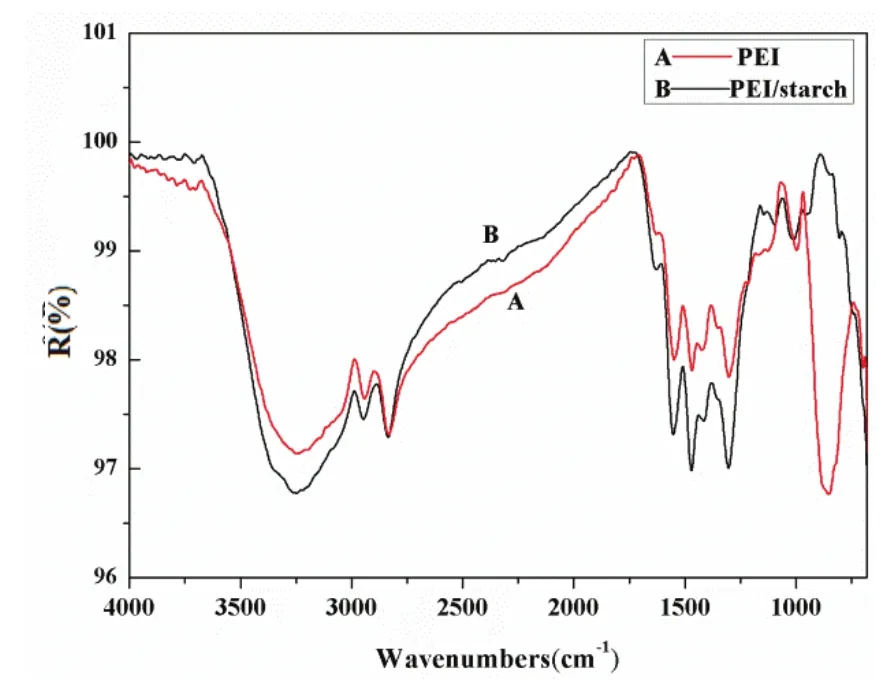
Fig. 3. FTIR spectrum of PEI and PEI/Starch.
The FTIR spectrum of PEI is shown as curve A in Fig. 3, and the main characteristic peaks of PEI were assigned as follows: The broad peak centered at 3400 cm-1was an envelope of νO-Hfor the adsorbed water, and νN-Hfor theammino groups.The 2941 cmm-1and 28333 cm-1bandswere asssigned to νC-HHfor the -CHH2groups, andthe bands at1548 cmm-1and 851 ccm-1were attrributed to thee in plane bennding viibration and oout of plane bending vibratiion of N-H foor the unnits of PEI, rrespectively, bboth of whichh were assocciated wwith amino skeeleton[22],[23]. CConsidering thhe above anallysis,thhe PEI containned -NH and-CH2groups.Curve B in FFig. 3 shhows that thepeak 3100 cmm-1to 3400 cmm-1is much wwider thhan curve A,because -NHH associated wwith -OH exiist in sttarch. Besidess, the peak at851 cm-1groows weaker, wwhich mmay also be thee result of theaddition of sttarch.
3..2 Responseto CO2
A. Improvemennt of Responsee
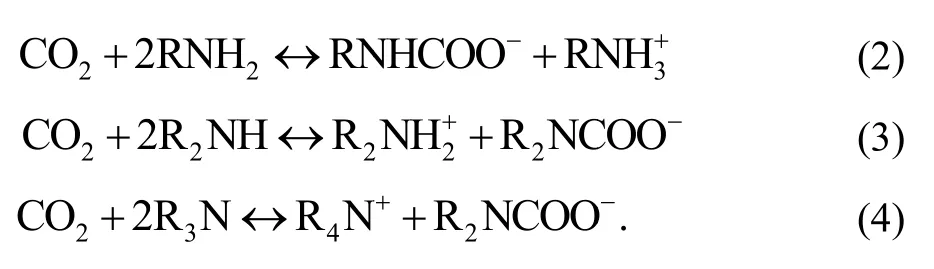
The sensibiility (S) of thee sensors to CCO2gas is giveen by ΔΔfΔC , wherre Δf represeents the frequuency shift off the seensor exposedd to CO2gas aand ΔC is the cconcentrationshift off CO2gas. Tyypical responses of QCM seensors coatedwith PEI and PEI/staarch for CO2ggas are shownn in Fig. 4 andd Fig. 5, respectively..
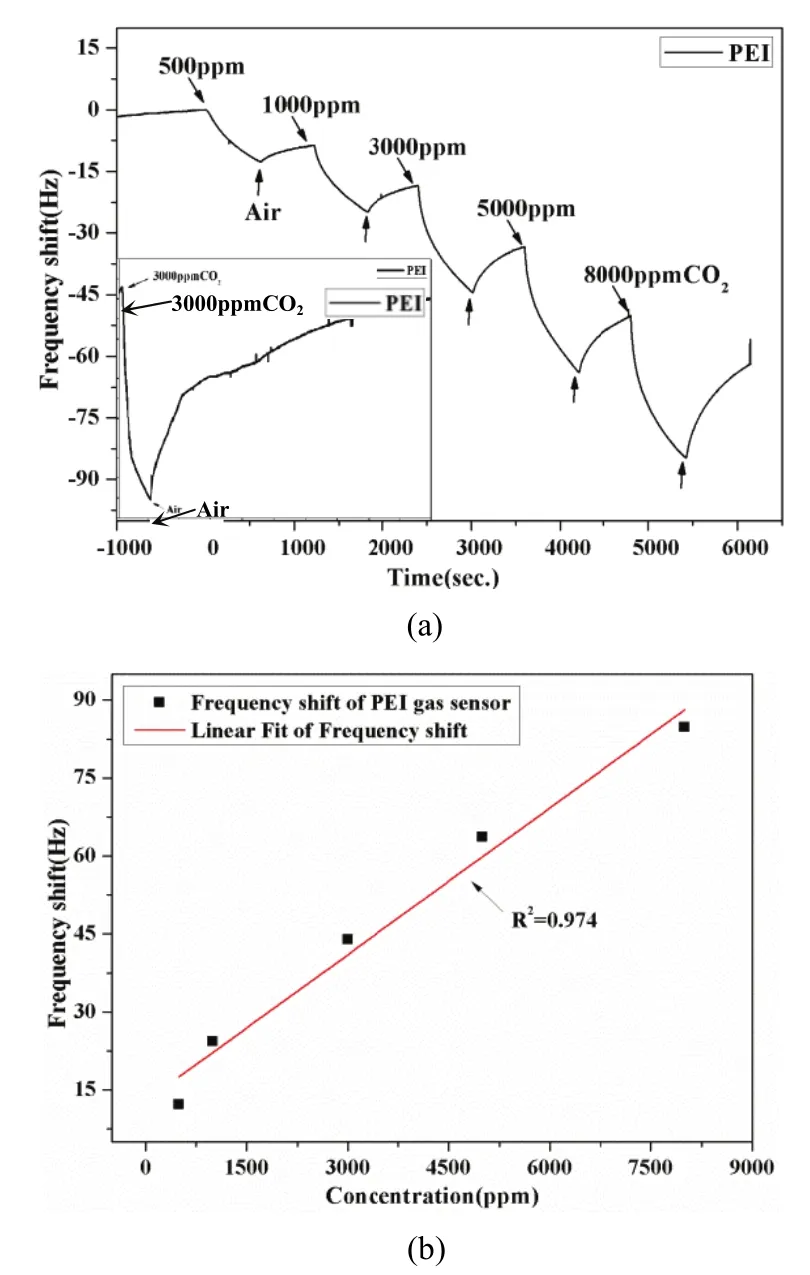
Fiig. 4. Responnse of PEI gaas sensor to CCO2: (a) reall-time frrequency shift aand (b) linear fitt of the frequenncy shift.
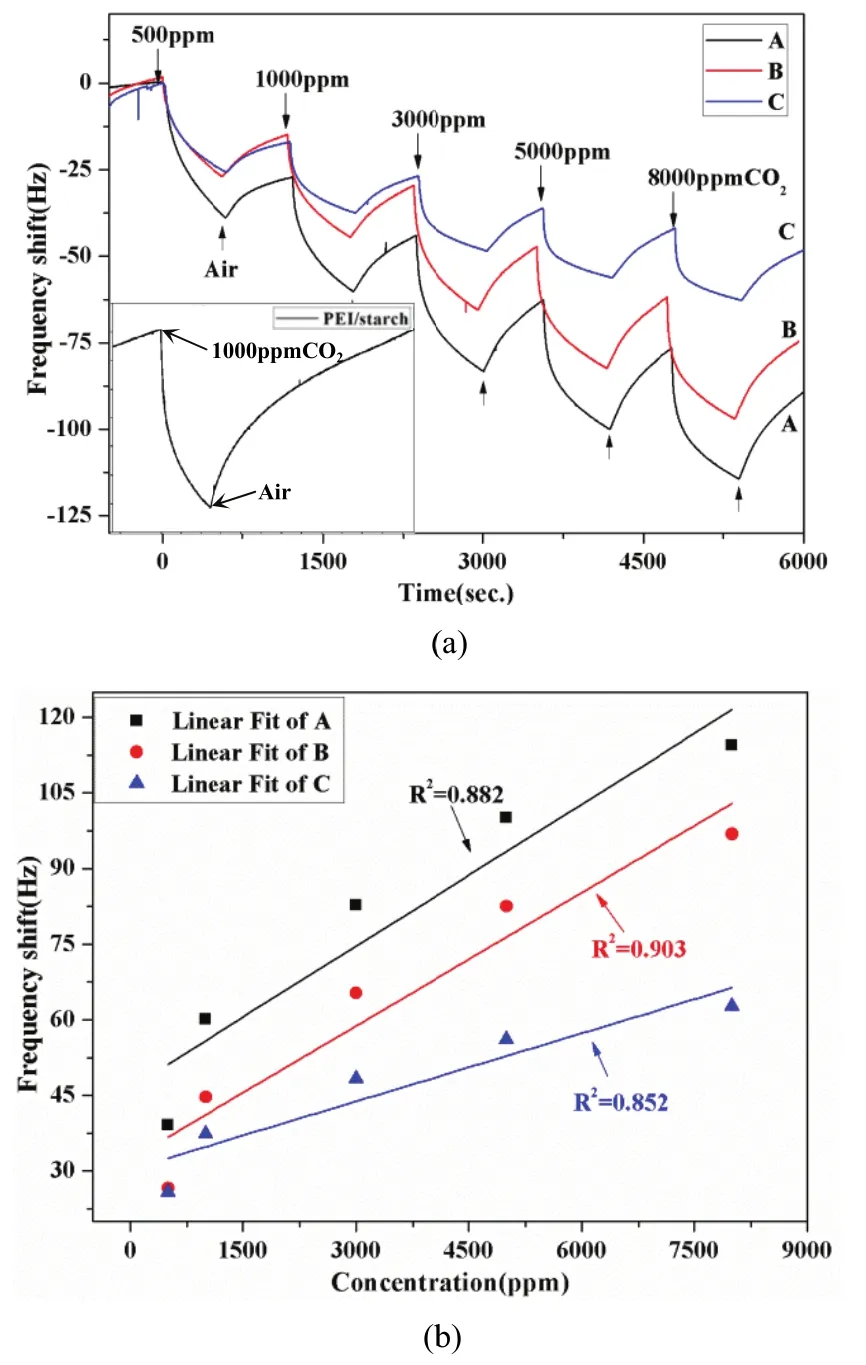
Fig.5. Response oof PEI/starch ggas sensor to CCO2: (a) real-timme frequuency shift and(b) linear fit off the frequencyshift.
FFig. 4 (a) andd Fig. 5 (a) sshow the real--time frequenncy shifftts Δf of the ssensors duringg exposures too different COO2conccentrations bbetween 500ppm and 8000 ppm ata ambbient temperatture (27°C). FFrom the figuures, PEI senssor andPEI/starch seensor both dessorption incommpletely dueto theshort test timme. Thereforre an experimment had been perfformed for aa single conncentration ((5000 ppm)to inveestigate the recoverability oof the sensor aas a supplemeent showwn in the botttle-left corners of Fig. 4 (a)) and Fig. 5 (aa),whicch show thatthe sensor haad a good revversibility. Froom Fig.4 (a) and Figg. 5 (a), the mooderate quanttity of the starrch dopeed PEI sensorr shows a betteer response thhan the pure one,andboth of themm can restorre to its origginal frequenccy,althoough a longtime (about30 minutes)will be takeen. Therrefore, the innfluence of diifferent amouunt of starch oon theproperties off the sensorhas been innvestigated. AAs illusstrated in Fig.5 (a), the sensing responseof the sensorr A(2 mmg starch) iis larger thaan the otherss, and reducces graddually with thee increasing aamount of starrch. In additioon,modderate starch-ddoped compoosite film sennsors has largger senssing responsethan the monoolayer one. Thhe reason is thhat morre adsorptionsites were emmerged due tothe PEI, which ensuured more pphysical inteeraction betwween the COO2moleecules and thhe sensitive ffilms. Meanwwhile the starrch attraacts more waater to the fiilm due to thhe hygroscoppic natuure which ennhanced CO22reaction wiith PEI aminnogroups. However, the addition of excessive dopant prevented the direct contact between CO2gas and adsorption sites in PEI polymer which weakened the response of CO2gas. In addition, the linearly coefficients of the PEI/starch sensor (A) and PEI sensors were 0.882 and 0.974, respectively, as shown in Fig. 4 (b) and Fig. 5 (b). B. Response to H2O Vapor
Moderate moisture will not resist the adsorption of CO2,but can promote it. Because the presence of H2O molecules will react with the reaction product of (2) and (3):
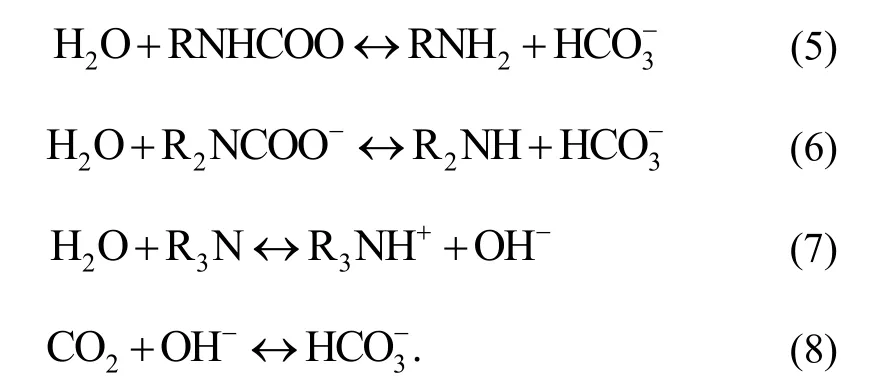
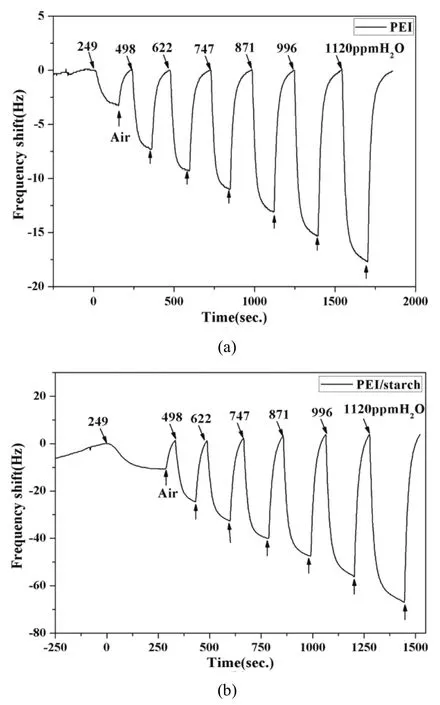
Fig. 6. Influence of H2O molecules to different sensors: (a) PEI sensor and (b) PEI/starch sensor.
Because of the acid-base interaction of CO2with amino groups and the hydrophilicity of starch, the CO2response might be affected by the presence of starch. The following test was used for investigating the relevance of humidity and CO2absorption. As shown in Fig. 6, both of the PEI sensor and PEI/starch sensor exhibit a good responsivity to H2O vapor. According to Fig. 7, the PEI/starch sensor displays greater response to H2O vapor than PEI sensor. It reveals clearly that the starch has good hygroscopicity. Besides that, the frequency shift of the two sensor exhibits a good linearity versus the H2O vapor concentration over the range investigated.

Fig. 7. Linear fit of frequent shifts of PEI sensor and PEI/starch sensor to H2O molecules.
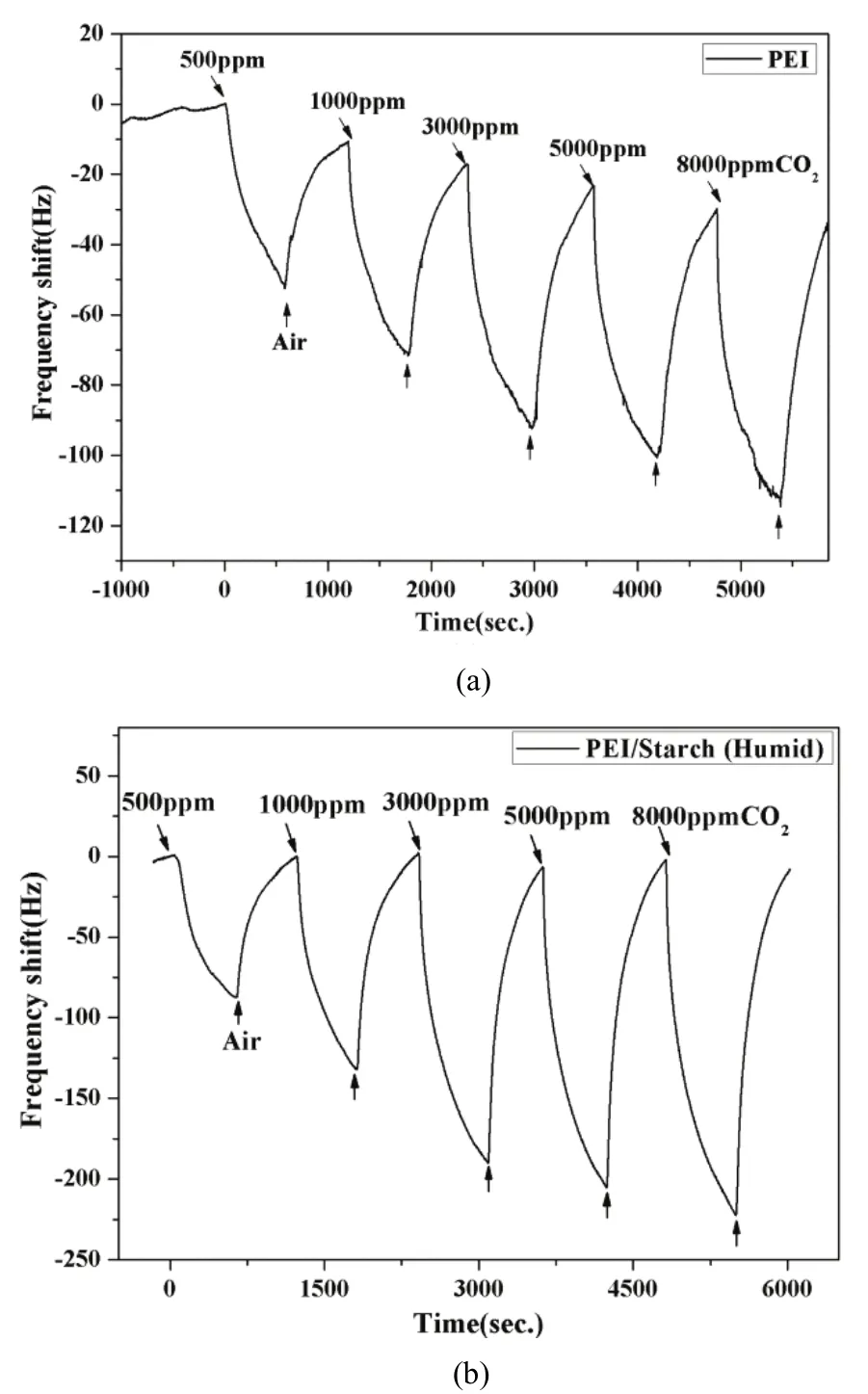
Fig. 8. Real-time frequency shift of sensors in humid environment:(a) PEI sensor and (b) PEI/starch sensor.
In order to investigate the relevance of humidity and CO2absorption, the following test was carried out. Firstly,10 ml clean water was added into the bottom of the test chamber for simulating the humid condition. Secondly,5000 ppm CO2was injected into the test chamber for 30 minutes to saturate the clean water and ensure that it would not absorb CO2anymore in the later test. Finally, QCM gas sensors were connected and clean air was injected as carrier gas to test the performance of the sensors.
As shown in Fig. 8, on exposure to different CO2gas concentrations, the frequency shifts of PEI sensor and PEI/starch sensor in the humid condition are much larger than that in the dry condition. It shows that the appropriate amount of moisture can promote CO2adsorption. Due to the fact that the existence of H2O molecules greatly affects the response property, it can be inferred that the H2O molecules participation results in higher sensitivity.
C. Effect of Temperature
As physical absorption and chemical reactions are affected by temperature, the responses of PEI-coated and PEI/starch sensors exposed to various CO2concentration in different working temperature such as 27°C, 40°C, and 60°C are studied, as shown in Fig. 9.
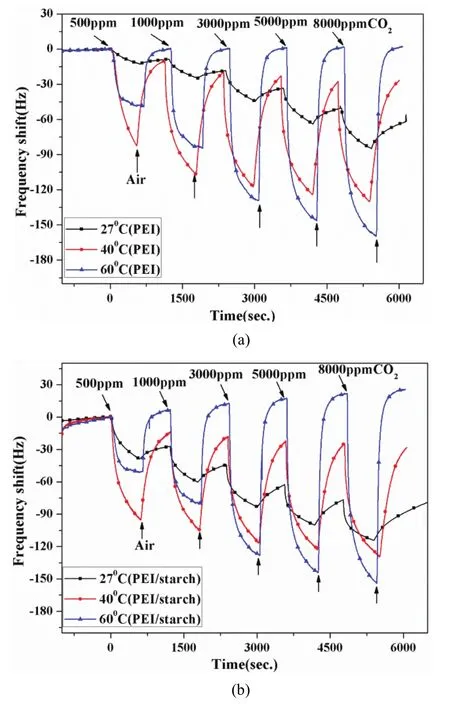
Fig. 9. Influence of temperature (27°C, 40°C, and 60°C) to sensors: (a) PEI sensor and (b) PEI/starch sensor.
Fig. 9 (a) and Fig. 9 (b) illustrates the effect of the temperature on the responses of two sensors. When the concentration was higher than 3000 ppm, the sensing response increased with the increase of temperature. But 40°C was optimal when the sensors exposed to CO2with the concentration lower than 3000 ppm. And the sensors at a higher temperature exhibited better recoverability, shorter response time, and recovery time, which was owing to the difference in thermally expanded volumes leading to a difference in the sensor frequency response. And it also shows that the responses of two sensors to CO2at 40°C and 60°C were almost similar.
D. Study of Stability
The PEI and PEI/starch sensors were exposed to the air of ambient environment without package for several days. Their sensing responses to CO2at room temperature several days later are exhibited in the Fig. 10.
Fig. 10 (a) and Fig. 10 (b) show that the responses of the PEI and PEI/starch sensors shrink after a long time exposed to air, but they still exhibit a distinct response to CO2. In addition, the two sensors exhibit good stability,providing a better way to detect CO2.
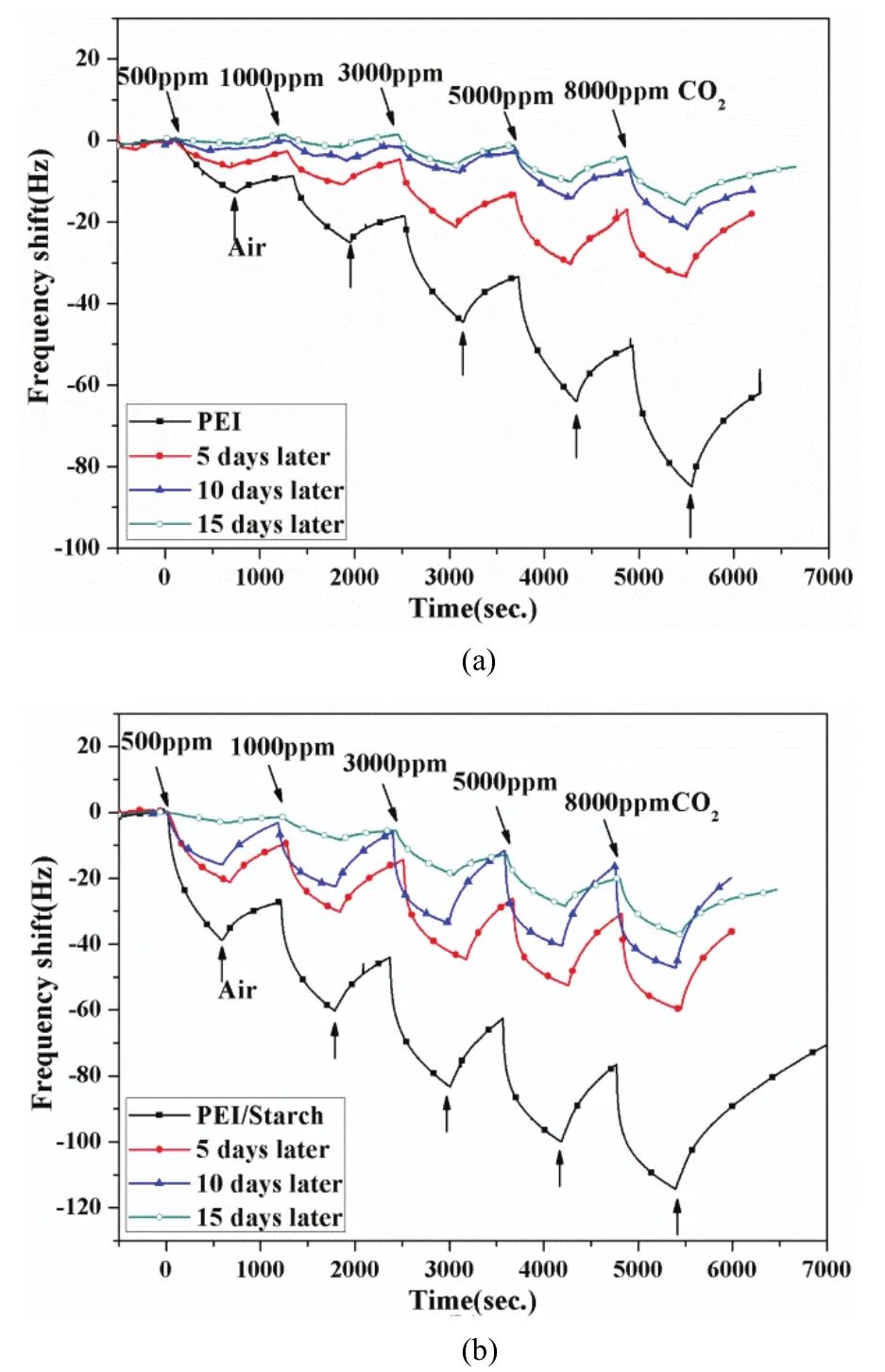
Fig. 10. Stability of sensors: (a) PEI sensor and (b) PEI/starch sensor.
4. Conclusions
The experimental results have confirmed that CO2could be successfully detected in a concentration of 500 ppm to 8000 ppm at room temperature using PEI-basedQCM gas sensors. By adding the starch to the PEI polymer film, the sensing response to CO2was enhanced, and it seemed suitable for CO2detection at room temperature. The starch worked as a dopant can improve the adsorption of H2O molecules in the PEI layer, which resulted in an increase of sensor sensitivity and response speed. However,the interference could be removed by increasing the working temperature. Especially when the sensors worked at a high temperature atmosphere, PEI and PEI/starch coated sensors showed a shorter response and recovery time. Further, the sensors showed a modest long-term stability on exposure to the air atmosphere. These properties would be promising for future development of room-temperature CO2sensors.
[1] P.-M. Eisenberger, R.-W. Cohen, G. Chichilnisky, et al.,“Global warming and carbon-negative technology: Prospects for a lower-cost route to a lower-risk atmosphere,”Energy and Enviroment., vol. 20, no. 6, pp. 973-984, 2009.
[2] S. Neethirajan, D.-S. Jayas, and S. Sadistap, “Carbon dioxide (CO2) sensors for the agri-food industry—A review,”Food Bioprocess Technology, vol. 2, no. 2, pp. 115-121,2009.
[3] A. Marshal, A. Cornet, and J.-R. Morante, “Study of the CO2and humidity interface in La doped tin oxide CO2gas sensor,” Sensors and Actuators B, vol. 94, pp. 324-329,2003.
[4] H. Koezuka and A. Tsumura, “Field effect transistor utilizing conducting polymers,” Synthetic Metals, vol. 28, no. 1-2, pp. 753-760, 1989.
[5] N.-V. Hieu, “Highly reproducible synthesis of very large-scale tin oxide nanowires used for screen-printed gas sensor,” Sensors and Actuators B, vol. 144, no. 2, pp. 425-431, 2010.
[6] I.-A. Al-Homoudi, J.-S. Thakur, R. Naik, and G.-W. Auner,“Anatase TiO2films based CO gas sensor: Film thickness,substrate and temperature effects,” Applied Surface Science,vol. 253, no. 21, pp. 8607-8614, 2007.
[7] N.-D. Hoa, N.-V. Quy, M.-A. Tuan, and N.-V. Hieu, “Facile synthesis of p-type semiconducting cupric oxide nanowires and their gas-sensing properties,” Physica E, vol. 42, no. 2,pp. 146-149, 2009.
[8] R. Zhou, S. Vaihinger, K.-E. Geckeler, and W. Gpeiö,“Reliable CO2sensors with silicon-based polymers on quartz microbalance transducers,” Sensors Actuators B: Chemical, vol. 19, no. 1-3, pp. 415-420, 1994.
[9] B. Ostrick, M. Fleischer, and H. Meixner, “The influence of interfaces and interlayers on the gas sensitivity in work function type sensors,” Sensors Actuators B: Chemical, vol. 95, no. 1-3, pp. 271-274, 2003.
[10] N. Miura, M. Lio, G. Lu, et al. “Solid-state amperometric NO2sensor using a sodium ion conductor,” Sensor and Actuators B: Chemical, vol. 35, no. 1, pp. 124-129, 1996.
[11] T. Goto, G. He, T. Narushima, and Y. Iguchi, “Application of Srß-alumina solid electrolyte to a CO2gas sensor,” Solid State Ionics., vol. 156, pp. 329-336, 2003.
[12] A. Haeusler and J.-U. Meyer, “A novel thick film conductive type CO2sensor,” Sensor and Actuators B: Chemical, vol. 34, no. 1-3, pp. 388-395, 1996.
[13] M.-Y. Kim, Y.-N. Choi, J.-M. Bae, et al., “Carbon dioxide sensitivity of La-doped thick film tin oxide gas sensor,”Ceramics International, vol. 38, no. 1, pp. 657-660, 2012.
[14] S. Stegmeier, M. Fleischer, A. Tawil, P. Hauptmann, K. Egly,and K. Rose, “Sensing mechanism of room temperature CO2sensors based on primary amino groups,” Sensor and Actuators B: Chemical, vol. 154, no. 2, pp. 270-276, 2011.
[15] M.-S. Nieuwenhuizen and A.-J. Nederlof, “A SAW gas sensor for carbon dioxide and water. Preliminary experiments,” Sensor and Actuators B: Chemical, vol. 2, no. 2, pp. 97-101, 1990.
[16] K. Korsah, C.-L. Ma, and B. Dress, “Harmonic frequency analysis of SAW resonator chemical sensors: application to the detection of carbon dioxide and humidity,” Sensor and Actuators B: Chemical, vol. 50, no. 2, pp. 110-116, 1998.
[17] R. Zhou, D. Schmeiser, and W. Gopel, “Mass sensitive detection of carbon dioxide by amino group-functionalized polymers,” Sensor and Actuators B: Chemical, vol. 33, no. 1-3, pp. 188-193, 1996.
[18] C. Caliedo, P. Verardi, E. Verona, et al., “Advances in SAW-based gas sensors,” Smart Material Structures, vol. 6,no. 6, pp. 689-699, 1997.
[19] A. Star, T.-R. Han, V. Joshi, J.-C.P. Gabriel, and G. Gruner,“Nanoelectronic carbon dioxide sensors,” Advanced Materials, vol. 16, no. 22, pp. 2049-2052, 2004.
[20] S. Stegmeier, M. Fleischer, A. Tawil, et al., “Stepwise improvement of (hetero-) polysiloxane sensing layers for CO2detection operated at room temperature by modification of the polymeric network,” Sensor and Actuators B: Chemical, vol. 148, no. 2, pp. 450-458, 2010.
[21] S. Stegmeier, M. Fleischer, A. Tawil, et al., “Sensing of CO2at room temperature using work function readout of (hetero-)polysiloxanes sensing layers,” Sensor and Actuators B: Chemical, vol. 154, no. 2, pp. 206-212, 2011.
[22] A. Barau, V. Budarin, A. Caragheorgheopol, et al., “A simple and efficient route to active and dispersed silica supported palladium nanoparticles,” Catalysis Letters, vol. 124, no. 3-4, pp. 204-214, 2008.
[23] G. Martra, L. Bertinetti, C. Gerbaldi, et al., “Pd/SiO2as heterogeneous catalyst for the heck reaction evidence for a sensitivity to the surface structure of supported particles,”Catalysis Letters, vol. 132, no. 2, pp. 50-57, 2009.

Guang-Zhoong Xie wasborn in Sicchuan Province, CChina in 1968. HHe received hiss B.S. and M.S. ddegrees in phyysics from Sicchuan University,Chengdu in1991 and1996,respectivelyy, and receivedd his Ph.D. ddegree from Univeersity of Elecctronic Sciencee and Technologyy of China (UEESTC) in 20077. He is a professsor with Schoool of Optoelecttronic Innformation, (UUESTC). Hisresearch intee rests are senn sitive mmaterials and sennsors.

Ting Kangg was born inn Shaanxi Provvince,China in 19989. She receivved her B.S. ddegree from Jiangsu University inn 2011. Currentlly she is pursuingg the M.S.degree in optical engineeringg with UESTTC. Her ressearch interests incclude the CO2ggas sensor and QQCM gas sensingmechanism.

Yong Zhouu was born in Henan Provvince,China in 19988. He receivved the B.S. ddegree from the Chongqing Univversity of Posts and Telecommuunications (CQUUPT), Chongqiing in 2009. Hereceived hiss M.S. degreee in microelectroonics from UEESTC and nowhe is pursuing tthe Ph.D. ddegree in optical engineeringg with UESTTC. His research innterests includee the preparattion of gas seensors, gas seensing mmaterials, and ffabrication ofmicro-electro-mmechanical systems(MMEMS) gas sennsors array.

Tao Xie wasborn in Heilonngjiang Provincce,China in 19866. He receivedd the B.S. degrree from the JilinUniversity, Changchun in 20110. He receivedd his M.SS. degreein microelectronics from UESTTC and now heis pursuing thee Ph.D. deggree in opticcal engineering wiith UESTC. Hisresearch interests include the preeparation of OTTFT gas sensorrs, OTFFT gas sensingg mechanism and fabricatioon of OTFT ggas sensoors array.

Hui-Ling Taiwas born in NNingxia Proxincce,China in 19799. She receivedd her B.S. degrree and Ph.D. deggrees from UESSTC, Chengduin 2003 and 20008, respectively. She isan associate proofessor withthe Schoolof Optoelectronicc Information,, UESTC. HHer scientific interrests are condducting polymeers and their compoosites for gas seensor applicatioon.

Ya-Dong Jiang was born in SSichuan Provincce,China in 1964. He received hhis B.S. degreein 1986 from UEESTC. Then hegot his M.S. aand Ph.D. degreesin 1989 and 20001 from UESTTC,respectively. HHe is a professorr and the Deanof School of Optooelectronic Infoormation, UESTTC. His majorresearch innterests incluude optoelectronicmaterial anddevices, sensitiive material sand ssensors.
Manuscript received September 1, 2014; revised November 4, 2014. This work was supported by the National Natural Science Foundation of China under Grant No. 61176006 and No. 61006036, and the Specialized Research Fund for the Doctoral Program of Higher Education under Grant No. 20120185110012.
G.-Z. Xie is with the School of Optoelectronic Information, University of Electronic Science and Technology of China, Chengdu 610054, China(Corresponding author e-mail: gzxie@uestc.edu.cn)
T. Kang, Y. Zhou, T. Xie, H.-L. Tai, and Y.-D. Jiang are with the School of Optoelectronic Information, University of Electronic Science and Technology of China, Chengdu 610054, China (e-mail: xiaobu0401@126.com; zhyfly68@126.com; Xietaohlj@163.com;taitai1980@uestc.edu.cn; jiangyd@uestc.edu.cn ).
Digital Object Identifier: 10.3969/j.issn.1674-862X.2015.02.017
猜你喜欢
杂志排行
Journal of Electronic Science and Technology的其它文章
- Energy-Based Collaborative Spectrum Sensing for Cognitive UWB Impulse Radio
- Monitoring of PON System Using Compound Surveillance Technique
- Design and Realization of an NFC-Driven Smart Home System to Support Intruder Detection and Social Network Integration
- Modeling of a Planar Nine-Way Metamaterial Power Divider/Combiner
- Robust Stability of a Class of Fractional Order Hopfield Neural Networks
- Novel Sequence Number Based Secure Authentication Scheme for Wireless LANs
