Rapid Propagation of Chirita ophiopogoides in Vitro
2015-11-08ChuanmingFUKanghuaXlANJinxiangHEFengluanTANGYunpingSHlNingzhenHUANG
Chuanming FU,Kanghua XlAN,Jinxiang HE,Fengluan TANG,Yunping SHl,Ningzhen HUANG
Guangxi Institute of Botany,Chinese Academy of Sciences,Guilin 541006,China
Rapid Propagation of Chirita ophiopogoides in Vitro
Chuanming FU,Kanghua XlAN,Jinxiang HE,Fengluan TANG,Yunping SHl,Ningzhen HUANG*
Guangxi Institute of Botany,Chinese Academy of Sciences,Guilin 541006,China
A method for in vitro culture and rapid propagation of Chirita ophiopogoides was developed using leaves as explants in this study.The results indicated that the medium MS+6-BA 0.1 mg/L+NAA 0.1 mg/L was suitable for bud induction and seedling regeneration from leaves in primary culture.The media MS+0.5 mg/L 6-BA+0.1 mg/L NAA+10%banana+5%potato and MS+0.5 mg/L 6-BA+0.5 mg/L NAA+2%banana were very suitable for callus multiplication and seedling hardening in subculture,and the proliferation coefficients were 7.9 and 5.6 per 60 d respectively.The optimal rooting medium was MS and the rooting rate was 100%on day 30 of culture.The rooted plantlets of C.ophiopogoides were transplanted in greenhouse with humus soil and 92.5%survived.Theoretically,using the rapid propagation system,about 20 176 seedlings can be reproduced from a sterile plantlet in a year.
Chirita ophiopogoides;Fleshy leaves;In vitro culture;Rapid propagation
C hirita ophiopogoides is a perennial herb of the genus Chirita in the family Gesneriaceae,endemic to China,and only found in Fusuiand Longzhou in southern Guangxi.It is an endangered species,and has been included in China Species Red List[1].It has fleshy leaves,lotus bud-shaped plant,numerous pink flowers,and thus is usu ally grown as an ornamental plant[2]. C.ophiopogoides is one of the few Gesneriaceae plants with fleshy leaves,and mostly distributed in bare rocky crevices where otherplants cannot survive.It is an important gene source for the breeding of stress-resistant species,as it is very tolerant to high temperature and drought[3-4].In addition,its shoot is widely used as a curefor rheumatism[5-6].It has importantval ues in application and research field.However,the rapid propagation of C.ophiopogoides has not been reported yet.As environmental factors have negative influences on the capsules of C.ophiopogoides at late growth stage,it is difficult to obtain fertile seeds.Therefore,its leaf was used as the explant to develop a system for in vitro tissue culture and rapid propagation to provide material basis and technology for the protection and application of C.ophiopogoides.
Materials and Methods
Disinfection and inoculation of plant materials
C.ophiopogoides was planted in a greenhouse,and the new healthy leaves at the upper and middle parts of plants were collected,soaked in 0.2%detergent solution for 10 min,washed with running water.Subsequently,they were sterilized by soaking in 70%alcohol for 30-60 s,0.1% HgCl2for 4-6 min,washed with sterile water 5 times on a clean bench.Finally,the leaves were cut to segments of 1.0-2.0 cm,and inoculated into induction medium,with one explant per flask.
Medium preparation,explant inoculation and culture
Preparation of media The media were prepared by supplementing 6-BA,IBA,NAA,mashedbanana,potato juice,3.0%sucrose and 0.5%agar at different combinations and concentrations to MS or 1/2 MS,adjusting pH to 5.8.Then,they were packaged into flasks and sterilized at 125℃ for 25 min.The ingredients and concentrations of the media were designed according to different culture stages and described in detail in following context. Primary induction culture According to the rapid propagation of other species in the genus Chirita those has been published,and our early work on other Gesneriaceae species,MS+0.5 mg/L 6-BA+0.05 mg/L IBA and MS+ 0.1 mg/L 6-BA+0.1 mg/L NAA were prepared for primary induction culture[7].After inoculation,the explants were cultured at(28±3)℃and a 12-h photoperiod at a light intensity of 30-40 μmol/(m2·s).Observation was conducted once every two days to remove the contaminated explants timely. From day 120 to day 160,callus growth and regenerated buds were recorded and compared to determine which medium was better.
Subculture Because the sterile explants obtained from primary induction culture were not enough,the goal of early subculture was to produce more,and the media were prepared based on those used in primary culture,with some mollifications:MS+0.1 mg/L 6-BA+0.1 mg/L NAA,MS+0.5 mg/L 6-BA+0.05 mg/L IBA and MS+0.5 mg/L 6-BA+0.2 mg/L NAA.The small sterile buds obtained from primary induction culture were separated,and the longer seedlings were cut into segments with axillary bud each,before they were inoculated into the media in flasks,with 5-6 explants in each flask.Subculture in bulk was conducted after a certain number of sterile explants were obtained.The results revealed that the explants grew slowly in above media,and formed a large amount of tiny seedlings,which were too fragile for later hardening and rooting tests.To improve the subculture efficiency,6-BA,NAA,mashed banana and potato juice in the medium were optimized according to the L16(45) orthogonal array as shown in Table 2. Five to six explants were inoculated into each flask,and 10-12 flasks were prepared in each treatment.During the subculture experimental period,seedling height, ratio of robust seedlings s,rooting rate,and number of regenerated buds etc.were measured and observed every 7 d.60 d later, the propagation coefficient(Propagation coefficient=The number of effective seedlings generated from one explant),ratio of robust seedlings,rooting rate and general growth of the seedlings in five flasks of each treatment were observed and recorded. SPSS software was adopted for statistical analysis to screen the optimal medium formula for subculture.Subculture should be performed no more than 20 generations.
Rooting The seedlings with three or four leaves and 2 cm high in subculture medium were cut off at base and transferred to rooting medium(MS and 1/2 MS without any additional ingredients),with 8-10 seedlings in each flask.On day 30 of culture,rooting rate,seedling height and ratio of robust seedlings were measured and compared to select the optimal rooting medium.
Culture conditions After explant inoculation,all the tissue cultures were carried out at(28±3)℃,and a 12-h photoperiod at a light intensity of 30-40 μmol/(m2·s).
Transplanting of rooted plantlets
The rooted seedlings were transferred out the incubator chamber and exposed to natural environment.Two days later,the seedlings were washed and transplanted with loose humus soil into greenhouse covered by shade net 70%,with regular watering.Seedling survival rate was calculated 30 d later.
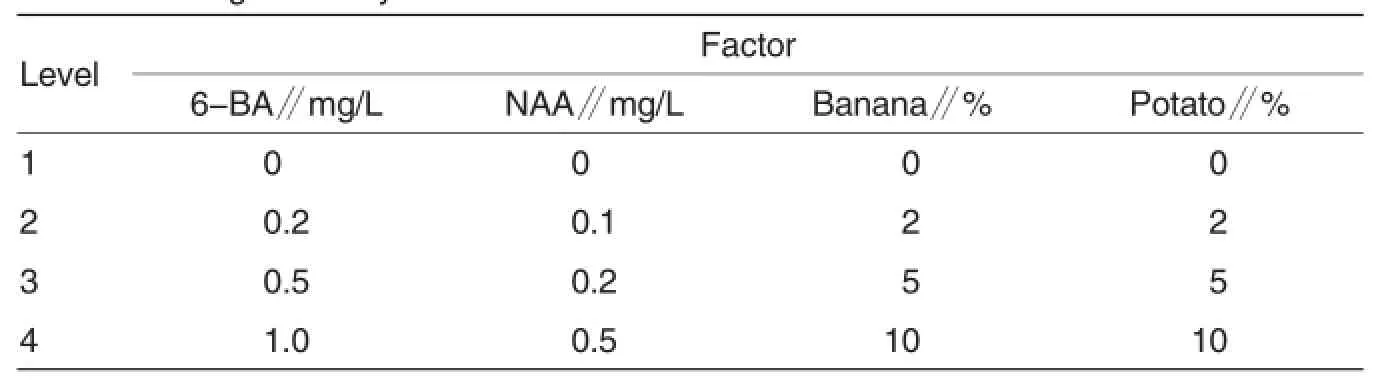
Table 1 Orthogonal array
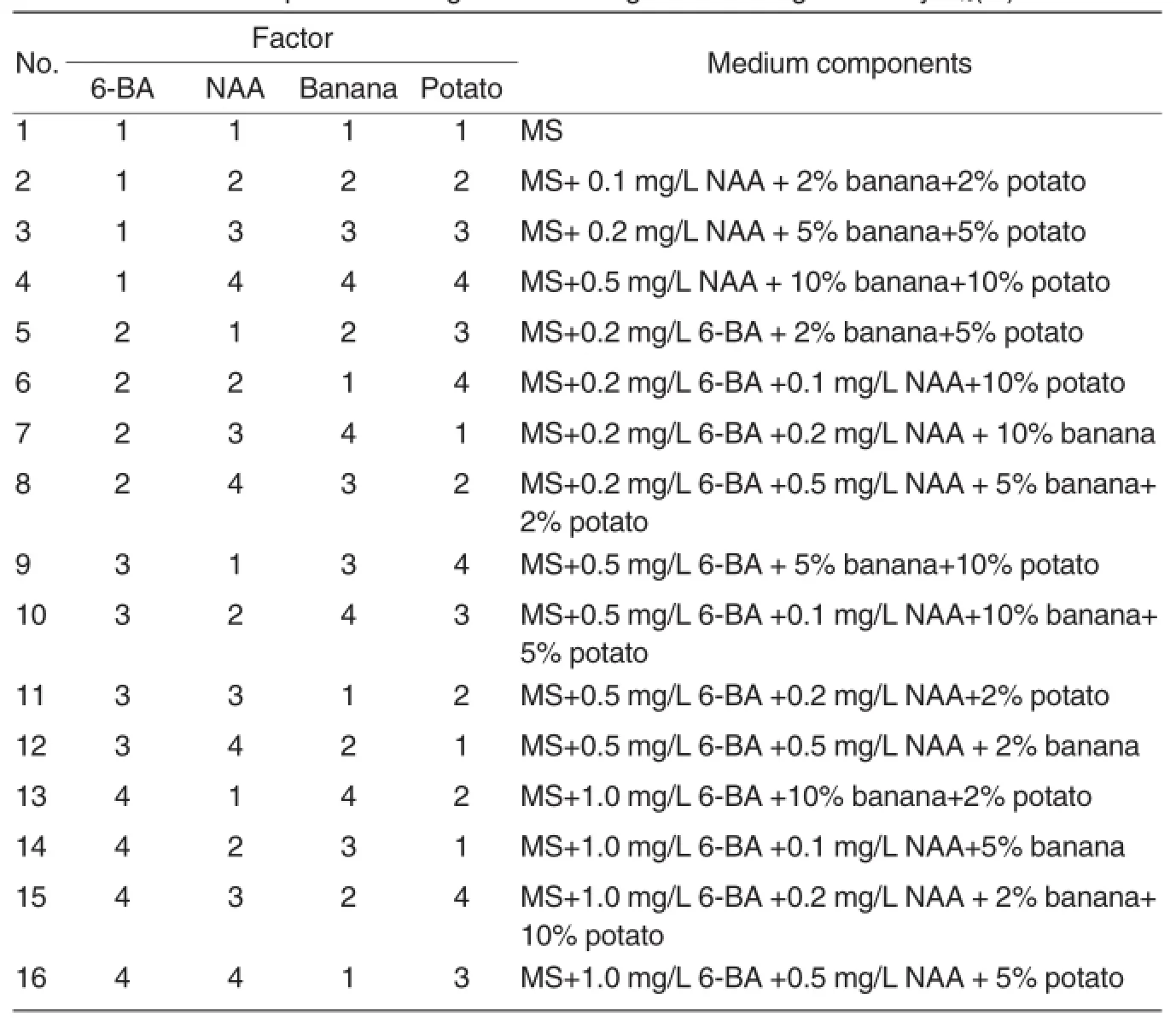
Table 2 Medium components designed according to the orthogonal array L16(45)
Results and Analysis
Primary induction and plant regeneration
The success rate of sterilizing the leaf explants of C.ophiopogoides with0.1%HgCl2for 4 min was only 6.5%,and it was improved to 22.2%by extending the time to 6 min.And the explants began to turning browning and died when the explants were sterilized more than 6 min.The results indicated that the leaf explants of C.ophiopogoides were sensitive to HgCl2.According to the success rate of sterilization,survival rate and the effect of Hg-Cl2on regenerated buds,sterilization with 0.1%HgCl2for 5 to 6 min was considered the most appropriate condition.
The sterilized leaf explants were inoculated into primary induction medium and cultured for 19 d.Then,the explants began to expand and thicken,and a small amount of callus appeared.On day 24 of culture,a small number of leaflets were differentiated.On day 33,leaflets were observed on all the explants.On day 48 of culture,more leaflets grew,and the leaf area increased.After that,the number and area of the leaflets continued to increase,and a small number of roots were observed.On both the media MS+0.5 mg/L 6-BA+0.05 mg/L IBA and MS+0.1 mg/L 6-BA+ 0.1 mg/L NAA,all the explants grew well within the first 50 d.The regenerated buds began to vitrify on day 90 around on the former medium,while the buds on the later medium continued to grow and part of the buds grew into robust seedlings after about 150 d of culture (Fig.1A).Therefore,MS+ 0.1 mg/L 6-BA+0.1 mg/L NAA was selected as the optimal medium for primary induction.
Subculture proliferation and seedling hardening
The calluses from primary induction were inoculated into three media MS+0.1 mg/L 6-BA+0.1 mg/L NAA,MS+0.5 mg/L 6-BA+0.05 mg/L IBA,MS+0.5 mg/L 6-BA+0.2 mg/L NAA for subculture.The results turned out that the calluses on all the three media grew slowly,and it took as long as 150 d for the calluses grew into seedlings. And numerous tiny seedlings obtained were too fragile and could not be used in later tests(Fig.1B).
The seedling growth was greatly improved by supplementing mashed banana and potato juice to the subculture medium.Therefore,the combinations and concentrations of 6-BA,NAA,mashed banana and potato juice in the medium were optimized via an orthogonal design.The proliferation coefficient,seedling height,ratio of robust seedlings and rooting rate were measured and compared to determine the best medium formula(Table 3 and Table 4).The results revealed that 6-BA,NAA,mashedbananaand potato had significant effects on the proliferation coefficient and the ratio of robust seedlings in subculture.
Since the propagation coefficient and shoot thickness directly affected the efficiency of subculture and the quality of generated seedlings,we conducted range analysis and multiple comparison on the two indices of all the treatments.
As shown in Table 5,the range analysis revealed that the among the four factors,banana had the most significant effects on proliferation coefficient,followed by 6-BA,NAA and potato(banana>6-BA>NAA>potato),while potato had the most significant effects on the ratio of robust seedlings,followed by 6-BA,NAA and banana(potato>6-BA>NAA=banana).

Table 3 Proliferation coefficient,seedling height and shoot thickness index in orthogonal design
The multiple comparison revealed that the treatments with 0.2-0.5 mg/L 6-BA,0.1-0.5 mg/L NAA,10%mashed banana and 5%-10%potato juice had higher proliferation coefficients than other treatments,while the treatments with 0.0-0.5 mg/L 6-BA,0.1-0.5 mg/L NAA,0%-10%mashed banana and 0%potato juice had stronger seedlings than other treatments.
Moreover,we also found that the addition of 6-BA,NAA,banana and potato were conducive to the formation of a large number of shoots,but a large number of invalid buds and weak shoots were induced by high concentration of 6-BA (1.0 mg/L)and potato juice (10%).0.2-0.5 mg/L NAA was beneficialto bud proliferation and seedling hardening,and banana also had significantly positive effects on bud proliferation and seedling growth(Fig.1C and D).
Proliferation efficiency(number of regenerated seedlings)and seedling quality (shoot thickness)are two important factors at subculture stage of rapid propagation of plant tissues.All above results suggested that MS+ 0.2-0.5 mg/L BA+0.1-0.5 mg/L NAA+ 10%banana+0%-5%potato was more suitable for the subculture of C.ophiopogoides leaf explants theoretically.
The further tests proved that MS+ 0.5 mg/L BA+0.1 mg/L NAA+10% banana+5%potato was most suitable for subculture proliferation,with the propagation coefficient of 7.9 per 60 d,similar-sized and relatively strong seedlings(Fig.1E).The medium MS+ 0.5 mg/L BA+0.5 mg/L NAA+2% banana was most suitable for seedling hardening,with the propagation coefficient of 5.6 per 60 d,well-grown and similar-sized seedlings(Fig.1F).
Rooting and transplanting
In MS medium less buds were regenerated,but the mother plants grew and rooted well.It only took 20 d to obtain developed root system.So,MS and 1/2 MS were considered suitable media for rooting the seedlings.The robust buds or seedlings over 2 cm high on the rooting media were cut and inoculated into the two media again. The result showed that the growth rate,thickness and rooting rate in MS were all better than in 1/2 MS(Fig.1G). Therefore,MS was selected as rooting medium in following tests,and the rooting rate could reach 100%after 30 d of culture.The rooted seedlings were transplanted to humus soil,and 92.5% of them survived 30 d later(Fig.1H).
Efficiency of the rapid propagation system
Based on above results,it took 60 d for subculture proliferation and seedling hardening,30 d for rooting,and 30 d for seedling survival after transplanting,so,within one year,four generations of subculture,one time of seedling hardening,one time of rooting culture and one time of transplanting could be completed.Discounting all the losses caused by contamination,vitrification and death,etc.,about 7.94×5.6×0.925=20 176 plants will be obtained through this system fromone sterile seedling ofC.ophiopogoides within one year in theory.
Table 4 The effects of 6-BA,NAA,banana and potato on proliferation coefficient and seedling height and seedling thickness
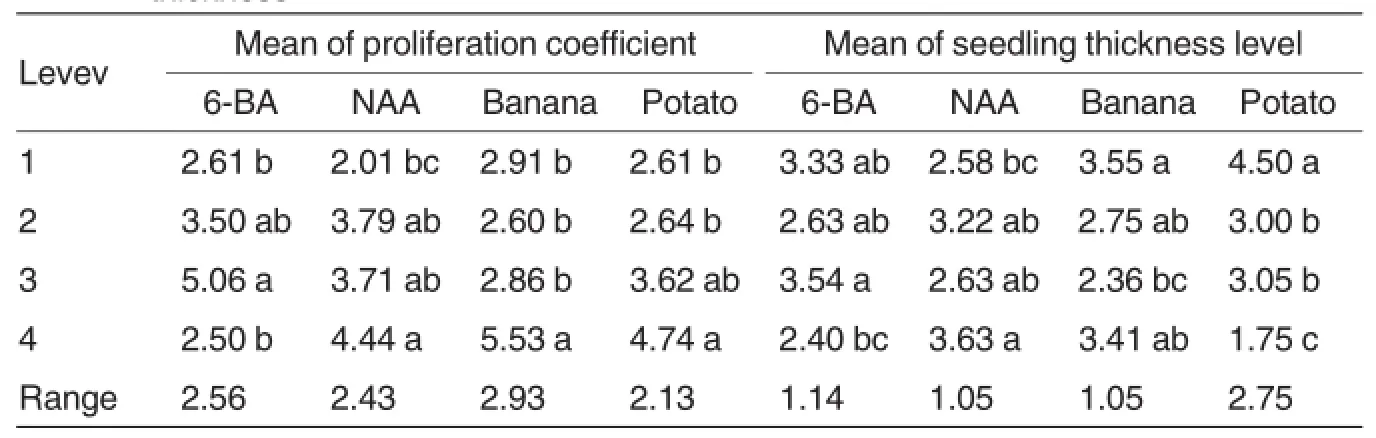
Table 5 Range analysis and multiple comparison on proliferation coefficient and seedling thickness
Discussion and Conclusion
Through the tissue culture of C.ophiopogoides we found that the callus and seedlings grew very slowly on MS medium,supplemented with only plant growth regulators 6-BA and NAA,and it took as long as 150 d for the callus developed into seedlings. And the seedlings were not strong enough.The orthogonal experiment revealed that in addition to the plant growth regulators at certain concentrations(6-BA at 0.5 mg/L,NAA at 0.1-0.5 mg/L),mashed banana had significant effects on seedling growth,as it not only improved the propagation coefficient in subculture,but also greatly accelerated the growth rate of seedlings,and thus shortened the subculture cycle from 150 d to only 60 d.Moreover,more robust seedlings were obtained after the addition of banana,which was completely different from the findings in C.spinulosa,and other plants in the same genus[7-9].The results of this study will provide some theoretical basis for the rapid propagation of C.ophiopogoides and other relative plants.
[1]WANG S(汪松),XIE Y(解焱).China species red list:Volume I(中国物种红色名录:第一卷)[M].Beijing:Higher Education Press(北京:高等教育出版社),2005.
[2]XING Q(邢全),SHI L(石雷),LIU LA(刘立安),et al.Fleshy ornamental plants in the family Gesneriaceae in China(中国苦苣苔科多肉观赏植物)[J].China Flower and Penjing(中国花卉盆景),2005,(1):2-4.
[3]WEI YG(韦毅刚).Plants in the family Gesneriaceae in China(华南苦苣苔科植物)[M].Nanning:Guangxi Science and Technology Press(南宁:广西科学技术出版社),2010.
[4]PU GZ(蒲高忠),PAN YM(潘玉梅),TANG SC(唐赛春),et al.Pollination biology and reproductive allocation of Chirita gueilinensis(Gesneriaceae)(桂林唇柱苣苔传粉生物学及生殖配置研究)[J].Bulletin of Botanical Research(植物研究),2009,29(2):169-175.
[5]Editorial Committee of the Flora of China(中国科学院中国植物志编委会).Flora of China:Volume 69(国植物志:第六十九卷)[M].Beijing:Science Press(北京:科学出版社),1990.
[6]LI ZY(李振宇),WANG YZ(王印政). Plants in the family Gesneriaceae in China(中国苦苣苔科植物)[M]. Zhengzhou:Henan Science and Technology Press(郑州:河南科学技术出版社),2004.
[7]TANG ZH(汤正辉),CHEN WL(陈维伦),SHI L(石雷),et al.In vitro micropropagation of Chirita spinulosa(刺齿唇柱苣苔的离体快速繁殖)[J].Plant Physiology Communications(植物生理学通讯),2004,40(2):211.
[8]YAO SE(姚绍嫦),LING ZZ(凌征柱),LI C(李翠),et al.Research on differentiation and plant regeneration from in vitro leaf tissues of Chirita medica D.Fang ex W.T.Wang(药用唇柱苣苔叶片分化及植株再生研究)[J].Seed(种子),2011,30(12):30-33.
[9]YU HX(余海霞),ZHANG ZJ(张占江),LU HZ(吕惠珍),et al.Study on tissue culture of Chirita swinglei(钟冠唇柱苣苔组织培养研究)[J].Hubei Agricultural Sciences(湖北农业科学),2012,12: 2606-2608.
Responsible editor:Qingqing YlN
Responsible proofreader:Xiaoyan WU
条叶唇柱苣苔离体快繁技术研究
付传明,冼康华,何金祥,唐凤鸾,石云平,黄宁珍*
(广西壮族自治区中国科学院广西植物研究所,广西桂林541006)
以叶片为外植体,对条叶唇柱苣苔(Chirita ophiopogoides)进行离体培养与快速繁殖研究。结果表明,培养基MS+0.1 mg/L 6-BA+0.1 mg/L NAA适用于初代培养中的芽诱导和植株再生;培养基MS+0.5 mg/L 6-BA+0.1 mg/L NAA+10%香蕉+5%马铃薯和MS+0.5 mg/L 6-BA+0.5 mg/L NAA+2%香蕉分别适用于继代增殖和壮苗培养,繁殖系数分别为7.9倍/60 d和5.6倍/60 d;培养基MS适用于生根培养,培养30 d后生根率达到100%;以腐质土为基质,将条叶唇柱苣苔生根试管苗移栽到大棚,成活率达92.5%。根据上述快繁技术,理论上每株试管苗每年可繁殖条叶唇柱苣苔种苗20 176株。
条叶唇柱苣苔;肉质叶;离体培养;快速繁殖
国家自然科学基金(31160055);广西科技攻关项目(桂科攻0992003B-31)。
付传明(1980-),男,湖北公安人,副研究员,从事植物生物技术与保育方面研究,
2015-09-09
Supported by National Natural Science Foundation of China (31160055);Key Science and Technology Research and Development Program of Guangxi(Gui Ke Gong 0992003B-31).
*Corresponding author.E-mail:hnzhen68@126.com
Received:September 9,2015 Accepted:November 15,2015
E-mail:470196422@qq.com。*通讯作者,E-mail:hnzhen68@126.com。
修回日期 2015-11-15
猜你喜欢
杂志排行
Agricultural Science & Technology的其它文章
- An lnnovative Strategy for Reciprocal Distant Hybridization between Spartina alterniflora and Rice
- Study on Absorptive Capacity to Formaldehyde and Physiological and Biochemical Changes of Scindapsus aureus Based on the Regulation of LaCl3
- Dynamic Variation in Sugar,Acid,and ASA Contents of‘Ganmi 6’Kiwifruit(Actinidia eriantha Benth)Fruits
- Construction and Development of GMS Agricultural lnformation Network Based on Stakeholder Analysis
- Effects of Different Decolorants on Retention Rate of Total Triterpenes in Fruit and Rattan Stems of Schisandra chinensis(Turcz.)Baill
- Determination of Heavy Metals inDendrobium candidiumWall.ex Lindl.by lCP-MS
