Antioxidant and α-glucosidase inhibitor activities of natural compounds isolated from Quercus gilva Blume leaves
2015-10-31AnastasiaWheniIndrianingsihSanroTachibanaRiznaTrianaDewiKazutakaItohTheUnitedGraduateSchoolofAgriculturalSciencesEhimeUniversityTarumiMatsuyamaEhime7908566Japan
Anastasia Wheni Indrianingsih, Sanro Tachibana, Rizna Triana Dewi, Kazutaka ItohThe United Graduate School of Agricultural Sciences, Ehime University, 3-5-7 Tarumi, Matsuyama, Ehime 790-8566, Japan
2Research Unit for Development of Chemical Engineering Process, Indonesian Institute of Sciences, Gading, Playen, Gunungkidul, Yogyakarta 55581,Indonesia
3Department of Applied Biosciences, Faculty of Agriculture, Ehime University, 3-5-7 Tarumi Matsuyama, Ehime 790-8566, Japan
4Research Center for Chemistry, Indonesian Institute of Sciences, Kawasan Puspiptek Serpong, Tangerang Selatan 15314, Indonesia
Antioxidant and α-glucosidase inhibitor activities of natural compounds isolated from Quercus gilva Blume leaves
Anastasia Wheni Indrianingsih1,2, Sanro Tachibana3*, Rizna Triana Dewi4, Kazutaka Itoh31The United Graduate School of Agricultural Sciences, Ehime University, 3-5-7 Tarumi, Matsuyama, Ehime 790-8566, Japan
2Research Unit for Development of Chemical Engineering Process, Indonesian Institute of Sciences, Gading, Playen, Gunungkidul, Yogyakarta 55581,Indonesia
3Department of Applied Biosciences, Faculty of Agriculture, Ehime University, 3-5-7 Tarumi Matsuyama, Ehime 790-8566, Japan
4Research Center for Chemistry, Indonesian Institute of Sciences, Kawasan Puspiptek Serpong, Tangerang Selatan 15314, Indonesia
ARTICLE INFO
Article history:
in revised form 19 May 2015
Accepted 10 Jun 2015
Available online 5 Aug 2015
Quercus gilva Blume
Antioxidative activity
α-Glucosidase inhibitor
Lineweaver-Burk plot
Objective: To isolate and investigate antioxidant and α-glucosidase inhibitor compounds in the leaves of Quercus gilva Blume (Q. gilva).
Methods: Dry leaves of Q. gilva were extracted with methanol and the methanolic extract was further separated by silica gel column chromatography using several solvents with increasing polarity. The antioxidant activities of the isolated compounds were evaluated using various in vitro assays: 1,1-diphenyl-2-picrylhydrazyl radical scavenging activity, hydrogen peroxide radical scavenging activity, β-carotene bleaching assay, and reducing power assay. The α-glucosidase inhibitory assay was conducted against α-glucosidase from Saccharomyces cerevisiae.
Results: Three compounds were isolated and their structures were identified as catechin (1),epicatechin (2), and tiliroside (3) using an instrumental analysis. Compound 2 had higher antioxidant activity with inhibitory concentrations (IC50) of (22.55 ± 2.23) µmol/L than that of quercetin, which was used as the standard, with an IC50of (28.08 ± 2.39) µmol/L, followed by compound 1 with IC50of (40.86 ± 3.45) µmol/L. On the other hand, compound 3 had the lowest antioxidant activity with an IC50of (160.24 ± 8.15) µmol/L. However, compound 3 had the highest α-glucosidase inhibitory activity with an IC50of (28.36 ± 0.11) µmol/L, followed by compounds 1 and 2 with (168.60 ± 5.15) and (920.60 ± 10.10) µmol/L, respectively.
Conclusions: The results obtained for the antioxidant activities and α-glucosidase inhibitory activities in a methanolic extract from the leaves of Q. gilva confirmed the potential of this plant as a source of natural antioxidants and antidiabetic medicine.
Original article doi: 10.1016/j.apjtb.2015.07.004 ©2015 by the Asian Pacific Journal of Tropical Biomedicine. All rights reserved.
1. Introduction
The long-term over-production of free radicals may cause oxidative damage in the human body, eventually leading to chronic diseases such as cancer and neurodegenerative disease[1]. Although free radicals typically come from the surrounding environment,some physiological and biochemical processes in the human body also produce reactive oxygen species, such as the superoxide radical,hydroxyl radicals, and peroxyl radicals, as by-products[2]. Therefore,antioxidants are considered important because of their many health benefits. Plants such as vegetables, fruits, herbs, and spices contain awide variety of free radical scavenging molecules, such as phenolic compounds, nitrogen compounds, vitamins, and terpenoids, which have high antioxidant activities[3,4]. In view of these potential health benefits, intensive research has been conducted on natural antioxidants derived from plants.
On the other hand, free radicals may also cause diabetes mellitus(DM)[5]. DM is a serious, chronic metabolic disorder that is characterized by high blood glucose levels. One therapeutic approach for diabetes is to postpone the absorption of glucose by inhibiting carbohydrate-hydrolyzing enzymes, e.g., α-glucosidase in the digestive organs. A previous study examined established α-glucosidase inhibitors from plants and their effects on blood glucose levels after food uptake[6]. The inhibition of α-glucosidase was shown to delay the digestion of carbohydrates[7]. Thus,α-glucosidase inhibitors have potential as therapeutic agents for the treatment of type 2 DM and hyperglycemia[8]. Acarbose is the most widely used α-glucosidase inhibitor, but has gastrointestinal side effects[9]. Plants are potential sources of drugs and many of the currently available drugs have been derived from plants. Therefore,α-glucosidase inhibitors screened from plants have attracted increasing attention in recent years[10].
Quercus gilva Blume (Q. gilva) of the family Fagaceae is a tall evergreen tree distributed in the lowland mountain regions of Jeju Island in Korea[11]. Moreover, Q. gilva as an oak species in warm temperate regions also grows in Japan, in which it is mainly distributed in the southern part of the country[12]. The wood of this evergreen oak was selected to make various tools for agriculture and processing in Japan such as hoes, spades, mallets, and axe handles. Previous phytochemical studies on Q. gilva led to the identification of terpenes from the fruit of this plant[13]. A recent study identified antioxidative constituents in the branches of Q. gilva using free radical scavenging activities[14]; however, the other bioactivities of Q. gilva have not yet been examined in detail. To the best of our knowledge, this study is the first to isolate active compounds from the leaves of Q. gilva and evaluate antioxidant and α-glucosidase inhibitory activities.
The evaluation of antioxidant and α-glucosidase inhibitory activities may be used for preliminary observations on pharmacological activities because natural compounds from plants that are considered to be safe have therapeutic effects and fewer health side effects than synthetic medicines[15]. In the present study, the antioxidant and α-glucosidase inhibitor activities of isolated compounds from the leaves of Q. gilva were tested. An in vitro assay of α-glucosidase inhibitory activity was conducted using α-glucosidase enzyme from Saccharomyces cerevisiae(S. cerevisiae) yeast while an in vitro antioxidant activity assay was conducted using several methods including 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging, β-carotene bleaching assay, hydrogen peroxide radical scavenging assay, and reducing power assay. These assays may be used for preliminary observations on the evaluation of pharmacological activities. The results of these assays may then be used to verify the medicinal effects of these active compounds isolated from plants.
2. Materials and methods
2.1. General instrumentation and reagents
The UV-vis absorption spectra of the isolated compounds were recorded on a Hitachi U-1600 spectrophotometer (Hitachi, Japan)and λ max was expressed in nanometers. All melting points were determined on a Yanaco micro melting point apparatus (Yanaco Co., Ltd., Kyoto, Japan) and uncorrected. Optical rotation was determined using a Jasco P-2100 polarimeter. Electron ionization mass spectra (EI-MS) were recorded on a gas chromatograph-mass spectrometer (Shimadzu, Japan) and fast atomic bombardment mass spectrometer (Shimadzu, Japan). Nuclear magnetic resonance(NMR) spectra were recorded at 500 MHz for1H and 125 MHz for13C on a JEOL JNM-AL 500 spectrometer using tetramethylsilane as the internal standard [chemical shift values (δ) in parts per million(µg/mL) and coupling constant (J) in Hz]. The symbols s, d, dd, and ddd stand for singlet, doublet, double doublet, and double double doublet. Thin-layer chromatography (TLC) was run on silica gel 60 F254 pre-coated plates (Merck 5554) and spots were detected using UV light.
DPPH, β-carotene, α-glucosidase [(EC 3.2.1.20)] type I from S. cerevisiae, p-nitrophenyl α-D-glucopyranoside (p-NPG), potassium ferricyanide [K3Fe(CN)6], trichloroacetic acid, ferric chloride(FeCl3), and hydrogen peroxide were purchased from Wako Pure Chemicals, Ltd. (Osaka, Japan). Tween 40, gallic acid, and quercetin were purchased from Sigma-Aldrich Co. Ltd. (Tokyo,Japan). All solvents used in this study (methanol, ethanol, toluene,ethyl acetate, chloroform, hexane, and acetone) were purchased from Wako Pure Chemicals, Ltd. (Osaka, Japan).
2.2. Plant material
The leaves of Q. gilva were collected from a site in Ehime University, Matsuyama, Japan, in October 2013. Voucher specimens have been deposited in the Department of Plant Chemistry, Faculty of Agriculture, Ehime University, Japan. The leaves were naturally dried.
2.3. Extraction and isolation procedures
The dried leaves of Q. gilva were powdered and extracted twice with methanol (1:8 w/w) at room temperature for 3 days. The methanol filtrate was concentrated under reduced pressure. The methanolic extract was partitioned successively using solvents with increasing polarity from hexane, chloroform, ethyl acetate,and methanol to obtain hexane soluble, chloroform soluble, ethyl acetate soluble, and methanol soluble. All extracts were screened for antioxidant activity using the DPPH test and, as a result, methanol soluble showed stronger activity than the others. Active methanol soluble (70 g) was separated by column chromatography over silica gel (100 mesh). The column was eluted with solvents of increasing polarities and a stepwise gradient from hexane (100%), ethyl acetate(50%) in hexane, ethyl acetate (100%), and ethyl acetate-methanol mixture with increasing polarity to 100% methanol to obtain eight fractions (F1-F8). Fraction F5 (6.25 g), which exhibited the highest antioxidant activity among the fractions, was further separated by silica gel column chromatography with a gradient solvent using hexane, ethyl acetate, and methanol repeatedly to obtain four fractions (F51-F54). Compounds 1 (190 mg) and 2 (270 mg) were isolated as a white yellowish solid compound from fraction F52 by preparative reversed-phase TLC (RP-TLC) eluted with methanolwater (4:5) followed by the recrystallization of compound 1 from hot water and recrystallization of compound 2 from ethyl acetate. With further silica gel column chromatography of fraction F53,compound 3 was isolated as a yellow amorphous powder (280 mg)after recrystallization from methanol.
A solution of compound 3 (10 mg) in 5% ethanolic H2SO4was refluxed and resulted in kaempferol on cooling[16]. The residue was partitioned between ethyl acetate and water to obtain D-glucose and p-coumaric acid, respectively, as hydrolysis products. The results were confirmed by high performance liquid chromatography and TLC with an available standard.
2.4. DPPH free radical scavenging activity
The antioxidant activities of compounds 1-3 were determined by a DPPH radical scavenging assay as conducted according to Sahu et al.[17] with slight modifications. Samples were dissolved in methanol at various concentrations, treated with DPPH (1 mmol/ L in methanol), and left to stand for 30 min at room temperature in the dark. Absorbance was measured at 517 nm using a UV-vis spectrophotometer. The ability of the samples to scavenge the DPPH radical was calculated using the equation 1:
where A0is the absorbance of the control and A1is absorbance in the presence of the sample. The inhibitory concentration (IC50) of the samples was calculated using a regression analysis from the graph plotting scavenging activity against concentration. Assays were carried out in triplicate.
2.5. Hydrogen peroxide radical scavenging activity
The abilities of compounds 1-3 to scavenge hydrogen peroxide were determined according to the method of Khan et al.[18].A solution of hydrogen peroxide (40 mmol/L) was prepared in phosphate buffer saline (pH 7.4). Samples at various concentrations in 4 mL distilled water were added to hydrogen peroxide solution(0.6 mL). The solution was left to stand for 10 min and absorbance was measured at 230 nm. The ability of the samples to scavenge the hydrogen peroxide radical was calculated using equation 1. All experiments were carried out in triplicate and the results were expressed as the mean ± SD of three determinations.
2.6. Reducing power assay
The reducing power assay was performed according to a previously described method by Jayanthi and Lalitha[19] with minor modifications. A test sample solution (1 mL, 20 g/mL) was mixed with phosphate buffer (2.5 mL) and potassium ferricyanide (2.5 mL). The mixture was incubated at 50°C for 20 min. Trichloroacetic acid(2.5 mL) were added to the mixture, which was then centrifuged at 3 000 r/min for 10 min. The upper layer of the solution (2.5 mL)was mixed with distilled water (2.5 mL) and a freshly prepared ferric chloride solution (0.5 mL). Absorbance was measured at 700 nm. Antioxidant activity was calculated using equation 1. All experiments were carried out in triplicate and results were expressed as the mean ± SD of three determinations.
2.7. β-Carotene-linoleate model assay
The antioxidant activities of compounds 1-3 in the β-carotenelinoleate model system were assessed as reported by Ramazan et al.[20]. A solution of β-carotene was prepared by dissolving 2 mg of β-carotene in 10 mL of chloroform. Two milliliters of the solution was then transferred into a boiling flask containing 20 mg linoleic acid and 200 mg Tween 40. Chloroform was removed using a rotary evaporator and 50 mL of distilled water was slowly added. Aliquots of the emulsion (4.8 mL) were transferred into different test tubes containing 0.2 mL of samples in methanol. These tubes were incubated at 50 °C in a water bath. As soon as the emulsion was added to each tube, the zero time absorbance was measured at 470 nm using a spectrophotometer. Absorbance readings were then recorded at 20 min intervals. Antioxidant activity was calculated using equation 1. All experiments were carried out in triplicate and the results were expressed as the mean ± SD of three determinations.
2.8. α-Glucosidase inhibitory activity
The inhibitory activity of α-glucosidase was evaluated as reported by Moradi-Afrapoli et al.[21]. Samples were dissolved in dimethyl sulfoxide at various concentrations (10 µL) and then treated with p-NPG (250 µL, 3 mmol/L) in phosphate buffer solution (490 µL,100 mmol/L, pH 7). The solution was pre-incubated at 37 °C for 5 min. Two hundred and fifty microliters of α-glucosidase enzyme(0.065 IU/mL) was then added and the reaction continued for 15 min. The reaction was stopped by the addition of 1 mL of 0.2 mol/ L Na2CO3. The mixtures were measured at 400 nm using a UV-vis spectrophotometer. The percentage inhibition of α-glucosidase inhibitory activity was calculated using equation 1. All experiments were carried out in triplicate and results were expressed as the mean ± SD of three determinations.
2.9. Enzyme kinetics
All isolated compounds were evaluated for their kinetics in inhibiting α-glucosidase activity. The type of inhibition of the active compounds against α-glucosidase was determined using increasing concentrations of p-NPG as a substrate in the absence or presence of active compounds as inhibitors at different concentrations. The type of inhibition was determined using a Lineweaver-Burk plot analysis. 2.10. Statistical analysis
All assays were conducted in triplicate. Statistical analyses were performed with SPSS 16.0 for an analysis of variance (ANOVA)followed by Duncan's test. Differences at P < 0.05 were considered to be significant.
3. Results
3.1. Isolation and structure identification
The activity-guided isolation procedures for active compounds in the leaves of Q. gilva are shown in Figure 1.
The methanol soluble fraction in the methanolic extract of Q. gilva was fractionated using silica gel chromatography and followed by recrystallization to give compounds 1, 2, and 3. The1H NMR and13C NMR spectral data of all isolated compounds were compared with reported data, and their structures were identified as catechin (1),epicatechin (2), and tiliroside (3).
Compound 1: A white yellowish solid; melting point 174-175 °C. UV spectra (MeOH) λ max (log ε) 280 nm (3.21). [α]15D+ 16° (c: 0.1, MeOH).1H NMR (500 MHz, CD3OD): δ 2.50 (1H, dd, J = 16.1,8.1 Hz, H-4a), 2.84 (1H, dd, J = 16.1, 5.4 Hz, H-4b), 3.97 (1H, ddd,J = 7.8, 7.8 and 5.5 Hz, H-3), 4.56 (1H, d, J = 7.5 Hz, H-2), 5.84(1H, d, J = 2.2 Hz, H-8), 5.92 (1H, d, J = 2.3 Hz, H-6), 6.71 (1H, dd,J = 8.0, 1.9 Hz, H-6'), 6.76 (1H, d, J = 8.0 Hz, H-5'), 6.83 (1H, d, J = 1.9, H-2');13C-NMR (125 MHz, CD3OD): δ 29.3 (C-4), 69.6 (C-3),83.6 (C-2), 96.3 (C-8), 97.1 (C-6), 101.6 (C-10), 116.0 (C-2'), 116.9(C-5'), 120.8 (C-6'), 133.0 (C-1'), 147.0 (C-3'), 147.0 (C-4'), 157.7(C-9), 158.4 (C-7), 158.5 (C-5).
EI-MS [M]+: m/z 290 for C15H14O6
Compound 2: A white yellowish solid; melting point 240-242 °C. UV spectra (MeOH) λ max (log ε) 279.5 nm (3.43). [α]15D-31° (c: 0.1, MeOH).1H NMR (500 MHz, CD3OD): δ 2.73 (1H, dd, J = 16.8,2.7 Hz, H-4a), 2.85 (1H, dd, J = 16.7, 4.6 Hz, H-4b), 4.16 (1H, ddd,J = 1.5, 2.9 and 2.8 Hz, H-3), 4.80 (1H, d, J = 1.5 Hz, H-2), 5.91(1H, d, J = 2.2 Hz, H-6), 5.93 (1H, d, J = 2.4 Hz, H-8), 6.75 (1H, d,J = 8.2 Hz, H-5'), 6.79 (1H, dd, J = 8.3, 1.8 Hz, H-6'), 6.96 (1H, d,J = 1.8 Hz, H-2');13C-NMR (125 MHz, CD3OD): δ 30.0 (C-4), 68.2(C-3), 80.6 (C-2), 96.7 (C-8), 97.1 (C-6), 100.8 (C-10), 116.1 (C-2'),116.7 (C-5'), 120.2 (C-6'), 133.0 (C-1'), 146.5 (C-3'), 146.7 (C-4'),158.1 (C-9), 158.4 (C-7), 158.7 (C-5).
EI-MS [M]+: m/z 290 for C15H14O6
Compound 3: A yellow amorphous powder; melting point 265-267°C. UV λ max (MeOH) nm (log ε) 267 (4.30), 315 (4.37);(+NaOMe) 275 (4.35), 365 (4.34); (+AlCl3) 275 (4.31), 306 (4.34);(+AlCl3+HCl) 275 (4.33) 306 (4.35), 396 (4.00); (+NaOAc) 274(4.37), 311 (4.40).
1H NMR (500 MHz, DMSO-d6): δ 4.03 (1H, dd, J = 11.91, 6.4 Hz,H-6''a), 4.27 (1H, brd, J = 11.8 Hz, H-6''b), 5.45 (1H, d, J = 7.4 Hz,H-1''), 6.11 (1H, d, J = 15.9 Hz, H-8'''), 6.14 (1H, d, J = 2.0 Hz, H-6),6.37 (1H, d, J = 2.0 Hz, H-8), 6.78 (2H, d, J = 8.6 Hz, H-3''', 5'''),6.85 (2H, d, J = 8.9 Hz, H-3',5'), 7.34 (1H, d, J = 16.0 Hz, H-7'''),7.37 (2H, d, J = 8.9 Hz, H-2''', 6'''), 7.98 (2H, d, J = 8.8 Hz, H-2', 6'),12.57 (1H, s, 5-OH).13C NMR (125 MHz, CD3OD): δ 156.3 (C-2),133.0 (C-3), 177.3 (C-4), 161.1 (C-5), 98.7 (C-6), 164.3 (C-7), 93.6(C-8), 156.3 (C-9), 103.7 (C-10), 120.7 (C-1'), 130.8 (C-2',6'), 115.0(C-3',5'), 159.7 (C-4'), 100.9 (C-1''), 74.2 (C-2''), 76.2 (C-3''), 69.9(C-4''), 74.1 (C-5''), 62.9 (C-6''), 124.9 (C-1'''), 130.1 (C-2''',6'''),115.7 (C-3''', 5'''), 159.9 (C-4'''), 144.5 (C-7'''), 113.6 (C-8'''), 166.1(C-9''').
HRFAB-MS [M+H]+: m/z 595.5196 for C30H26O13
3.2. Antioxidant activity
The antioxidant activities of the three isolated compounds were determined using several assays: DPPH free radical scavenging activity, hydrogen peroxide radical scavenging activity, reducing power assay, and β-carotene-linoleate model assay. The results of the DPPH and hydrogen peroxide radical scavenging activity assays of the isolated compounds are presented in Table 1.
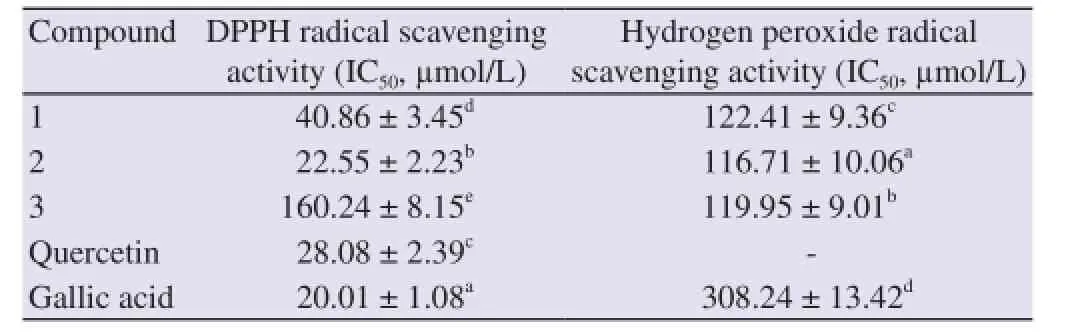
Table 1 Antioxidant activities of isolated compounds using DPPH and hydrogen peroxide radical scavenging activities.
The scavenging capacity of isolated compounds in DPPH free radical scavenging activity ranged between 22.55 and 160.24 µmol/ L. Of the isolated compounds, the highest DPPH scavenging capacity was shown by compound 2, followed by compounds 1 and 3 with IC50of 22.55, 40.86, and 160.24 µmol/L, respectively. Quercetin and gallic acid were used as positive controls in this experiment and exhibited IC50of 28.08 and 20.01 µmol/L, respectively. The values for these isolated compounds were significantly different(Table 1) at P < 0.05 using a statistical analysis with ANOVA followed by Duncan's test. Based on this statistical analysis, the antioxidant activity of compound 2 was higher than those of the other compounds including quercetin as a positive standard, but was still lower than that for gallic acid. This result showed the potential of compound 2 as a better source of an antioxidant.
The scavenging abilities of extracts on hydrogen peroxide are also shown in Table 1 and compared with gallic acid as a standard. Compound 2 exhibited the highest scavenging activity of 116.71 µmol/L, while gallic acid as a standard had scavenging activity of 308.24 µmol/L. Compounds 1 and 3 had scavenging activities of 122.41 and 119.95 µmol/L, respectively. This result is in accordance with the result for the DPPH scavenging test of compound 2, which had the highest antioxidant activity.
Overall, the results shown in Figure 2 indicated that compound 2 had the strongest reducing power among the isolated compounds investigated, with 61.47 mg/g (gallic acid equivalent) and 98.96 mg/ g (ascorbic acid equivalent).
This result is in accordance with the results of the DPPH and hydrogen peroxide radical scavenging assays.
The ability of isolated compounds to prevent the oxidation of the β-carotene-linoleate system more than ascorbic acid as the standard is shown in Figure 3.
3.3. α-Glucosidase inhibitor activities and their kinetic inhibition of α-glucosidase activity
The IC50values of α-glucosidase inhibitory activities the isolated compounds are shown in Table 2. Compound 3 had the highest α-glucosidase inhibitory activity (IC50) of 28.36 µmol/L, followed by compounds 1 and 2 with 168.60 and 920.60 µmol/L, respectively.Different letters in the same column indicate significant differences (P <0.05).

Table 2 α-Glucosidase inhibitory activities, inhibition constants (Ki value), and modes of compounds 1, 2, and 3 from Q. gilva leaves against S. cerevisiae α-glucosidase.
The inhibitory mechanisms of the isolated compounds using Lineweaver-Burk plots were shown in Figures 4 and 5.
4. Discussion
Compound 1 was isolated as a major component in the fractionation of methanol soluble using gradient hexane, ethyl acetate, and methanol as solvents. The13C-NMR spectrum indicated the presence of 15 carbons consisting of 12 aromatic and 3 aliphatic carbons, while signals that indicated the presence of a 1,3,5-trisubstituted benzene ring on the1H-NMR spectrum were observed at δ 6.71 (dd, J = 8.0, 1.9 Hz, H-6'), 6.76 (d, J = 8.0 Hz, H-5'), and 6.83 (d, J = 1.9, H-2'). Other aromatic signals were also observed at δ 5.84 (d, J = 2.2 Hz, H-8) and 5.92 (d, J = 2.3 Hz, H-6). Based on this spectroscopic information and search of the literature[14],compound 1 was identified as (2R,3S)-2-(3,4-dihydroxyphenyl)-3,4-dihydro-2H-chromene-3,5,7-triol or (+)-catechin.
Compound 2 was also isolated as a major component in the fractionation of methanol soluble using gradient hexane, ethyl acetate, and methanol as solvents. The spectra of1H and13C-NMR revealed that the signals of compound 2 were similar to those of compound 1. This suggested that compounds 1 and 2 have a similar molecular skeleton. The difference observed at δ 4.80 (d,J = 1.5 Hz, H-2) indicated a cis-conformation between H-2 and H-3[22]. Therefore, compound 2 was identified as (2R,3R)-2-(3,4-dihydroxyphenyl)-3,4-dihydro-2H-chromene-3,5,7-triol or(-)-epicatechin, the stereoisomer of compound 1.
The addition of shift reagents (NaOCH3, AlCl3, AlCl3+HCl) to compound 3 indicated the presence of free hydroxyl groups at C-5, C-7, and C-4' of the 3-hydroxyl substituted skeleton. The13CNMR of compound 3 showed 26 signals of carbon consisting of 2 carbonyl carbons, 6 oxygen-bearing aliphatic carbons and 18 sp2carbons.1H-NMR also revealed that a glucose unit was present in compound 3.1H-NMR signals at δ 6.11 (d, J = 15.9 Hz, H-8'''),6.14 (d, J = 2.0 Hz, H-6), 6.78 (d, J = 8.6 Hz, H-3''', 5'''), and 7.98 (d, J = 8.8 Hz, H-2', 6') indicated a kaempferol moiety, and this was supported by the13C-NMR signal at δ 177.3 (C-4) for a carbonyl carbon, δ 156.3 (C-2) and 133.0 (C-3) for olefin carbons,and δ 133.0 (C-3), 164.3 (C-7), 156.3 (C-9), and 159.7 (C-4') for oxygen-bearing aromatic carbons[23]. Further acid hydrolysis of compound 3 also resulted in kaempferol, glucose, and p-coumaric acid, which was confirmed by TLC and high performance liquid chromatography and compared with standard samples. Based on these results, compound 3 was identified as kaempferol-3-O-(6''-coumaroyl)glucopyranoside or tiliroside.
Overall, all compounds (1-3) exhibited significant scavenging activities against DPPH free radicals (Table 1), and may have been due to the phenolic group on these compounds. The reaction mechanism of polyphenol, which includes catechin and epicatechin,with DPPH was affected by two factors: polarity and the ratio of flavanol to DPPH radicals. Several mechanisms have been reported for this reaction. The first mechanism indicates that the DPPH radical-driven catechin oxidation product, an intermediate o-quinone, attacks the electron rich-A ring of a catechin unit and forms a hydrophilic dimer, which is further oxidized to oligomers of higher molecular weight. The second mechanism suggests that the A-ring of the o-quinone is further oxidized by a DPPH radical to form the observed adduct[22]. Evaluating antioxidant activity using a free-radical scavenging assay may provide information on the capability of an antioxidant to prevent radical species from attacking proteins, fatty acids, DNA, amino acids, and sugar in biological or food systems. DPPH is a relatively stable organic radical that hasbeen widely used to determine the antioxidant activity of natural compounds in an easy, rapid, and sensitive way[24]. The DPPH alcohol solution is deep purple with an absorption peak at 517 nm,and becomes yellow in the presence of a radical scavenger in the system and when the odd electron of nitrogen in DPPH is paired. The radical scavenging activity of DPPH stems from its ability to accept an electron or hydrogen radical and, hence, become a stable molecule.
The scavenging abilities of compounds 1-3 on hydrogen peroxide need to be evaluated (Table 1) because even though it is not very reactive in human cells, it sometimes may be toxic because it gives rise to the hydroxyl radical in cells[25]. Thus, antioxidants that remove hydrogen peroxide are important in biological and food systems.
The reducing power assay measures the ability of antioxidants to reduce ferric (Fe3+) ion to ferrous (Fe2+) ion through the donation of an electron. The ability of an antioxidant to reduce the ferric ion to ferrous ion is an indication of its ability to act as a pro-oxidant in a biological or food system. In the present study, we used gallic acid and ascorbic acid equivalents for reducing power ability. The reducing power of isolated compounds ranged between 41.60 and 61.47 µg/mL in gallic acid equivalents and between 78.77 and 98.96 µg/mL in ascorbic acid equivalents (Figure 2). The flavonoid group is well known for its ability to donate electrons[26]. Furthermore, the different substituents on the phenyl of the chalcone moiety also play an important role in the reducing power of compounds.
The β-carotene-linoleate bleaching assay was conducted because food generally consists of a lipid and water system with some emulsifier. Therefore, an aqueous emulsion system of β-carotene and linoleic acid was used to evaluate the antioxidant activities of the isolated compounds. The free peroxy radical in this system was formed when oxidized linoleic acid attacked β-carotene molecules that consequently underwent rapid decolorization. The results obtained showed that most of the investigated compounds efficiently inhibited the oxidation of emulsified linoleic acid and, as a result, inhibited β-carotene bleaching. The antioxidant activities of compounds 1 to 3 from Q. gilva at 40 µg/mL in the β-carotenelinoleate model system resulted in compound 1 having the highest ability in protecting β-carotene bleaching followed by compound 2 and compound 3, which still retained antioxidant activities of 14.93%, 10.44%, and 1.49%, respectively, after 60 min of the assay. These results were higher than that for ascorbic acid as the standard,which had an antioxidant ability of 1.47%. Anthocyanins, flavonols,and flavanols were previously reported to be active in the β-carotene bleaching test, while phenolic acids were less active[27].
By comparing antioxidant activities measured with four methods, i.e., DPPH radical scavenging, hydrogen peroxide radical scavenging, reducing power, and β-carotene-linoleate bleaching assays, all the isolated compounds showed almost similar results among the four methods. Therefore, we concluded that all these methods were consistent with each other in evaluating the antioxidant activities of isolated compounds from the leaves of Q. gilva.
α-Glucosidase inhibitors that inhibit enzymes in the intestine have been shown to effectively delay glucose absorption and prevent elevations in postprandial blood glucose levels; therefore, they play a significant role as chemotherapeutic agents for non-insulindependent diabetes mellitus. Effective and safe α-glucosidase inhibitors from nature have been sought in the development of physiological functional food or compounds for antidiabetic therapy[28]. We investigated the inhibitory activities of isolated compounds from the leaves of Q. gilva against α-glucosidase from S. cerevisiae. p-NPG was used as the substrate and the yellow color of the enzyme's degradation product, p-nitrophenol, was produced and measured using spectrometer. Quercetin was used as a positive control based on a previous study in which phenolic compounds exhibited stronger inhibitory effects on α-glucosidase than acarbose[29].
The results for α-glucosidase inhibitory activity (IC50) were consistent with previous findings on catechin and epicatechin in green tea[30], in which the α-glucosidase inhibitory activity of catechin was higher than that of epicatechin. Although compounds 1 and 2 have identical structures, their optical rotation was differed. Therefore, we assumed that these differences were affecting the inhibitory activity or recognition of the active site in α-glucosidase. The α-glucosidase inhibitory activity of compound 3 was higher than those of compounds 1 and 2 possibly because it consisted of more hydroxyl groups and the removal of hydroxyls in flavonoids has been shown to decrease α-glucosidase inhibitory activity[31]. The higher α-glucosidase inhibitory activity of compound 3 was also in accordance with tiliroside isolated from Phlomis stewartii[32]. This result showed that Q. gilva is a potential source for a supplement to replace pharmaceutical antidiabetic drugs in the future because it contains active compounds that act as α-glucosidase inhibitors.
The inhibitory mechanisms of the isolated compounds were analyzed further using Lineweaver-Burk plots (Figures 4 and 5). A substrate (p-NPG) with increasingly higher concentrations was treated with a α-glucosidase enzyme with and without the isolated compounds as inhibitors. The results obtained showed various mechanisms of action (Table 2). Compound 1 and compound 2 exhibited an uncompetitive type of inhibition (Figure 4), as shown by the straight parallel lines in the plot of 1/V versus 1/[S]. The Ki(inhibition constant) values of compounds 1 and 2 were determined to be 129.03 and 215.05 μmol/L, respectively (Table 2). Compound 3 showed non-competitive inhibition, which indicated that it bound to a site other than the active site of the α-glucosidase enzyme(Figure 5a) with a Ki value of 91.64 μmol/L, while quercetin had a mixed inhibition type (Figure 5b) with a Ki value of 34.26 μmol/L. This result indicated that the stereochemical type of the compound and the number of hydroxyl groups may have influenced the mechanism of inhibition. Furthermore, hydrogen bonding is a crucial factor in the interactions between the enzyme and its substrates and the conformation and orientations of the inhibitors at the active site[33]. However, further studies such as a molecular docking approach are required to confirm the interaction between the enzyme and the substrate. To the best of our knowledge,this study is the first to perform the bioassay guided isolation of active compounds from the leaves of Q. gilva and evaluate their antioxidant and α-glucosidase inhibitor activities.
In conclusion, three compounds were isolated from the leaves of Q. gilva. The antioxidant and α-glucosidase inhibitory activities of the isolated compounds were investigated. Four antioxidant assays were successfully conducted to evaluate the antioxidant activities of the plant extracts, giving similar results. Of the isolated compounds, catechin (1) and epicatechin (2) showed potent antioxidant activities, while tiliroside (3) and catechin (1) showed potent α-glucosidase inhibitory activities. These compounds may be employed as lead compounds for potentially new antioxidantand antidiabetic medicine derived from plants. The results of the present study showed that Q. gilva is potentially a rich source of natural antioxidants and antidiabetic medicine.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
We thank Prof. Dr. Satoshi Yamauchi and Ms. Tuti Wukirsari of the Faculty of Agriculture, Ehime University, for the measurement of optical rotation.
[1] Halliwell B. Free radicals and antioxidants: updating a personal view. Nutr Rev 2012; 70(5): 257-65.
[2] Duracková Z. Some current insights into oxidative stress. Physiol Res 2010; 59(4): 459-69.
[3] Tusevski O, Kostovska A, Iloska A, Trajkovska L, Simic SG. Phenolic production and antioxidant properties of some Macedonian medicinal plants. Cent Eur J Biol 2014; 9(9): 888-900.
[4] Lara Q, Maysam S, Zaid A. Comparative study of natural antioxidant activity for ginger-oregano and Syrian thyme. Int J Pharm Sci Rev Res 2013; 11: 56-8.
[5] Tiwari BK, Pandey KB, Abidi AB, Rizvi SI. Markers of oxidative stress during diabetes mellitus. J Biomark 2013; doi: 10.1155/2013/378790.
[6] Kumar S, Narwal S, Prakash O. α-Glucosidase inhibitor from plants: a natural approach to treat diabetes. Pharmacogn Rev 2011; 9: 19-29.
[7] Heacock PM, Hertzler SR, Williams JA, Wolf BW. Effects of a medical food containing an herbal alpha-glucosidase inhibitor on postprandial glycemia and insulinemia in healthy adults. J Am Diet Assoc 2005; 105: 65-71.
[8] Min SW, Han JS. Polyopes lancifolia extract, a potent α-glucosidase inhibitor, alleviates postprandial hyperglycemia in diabetic mice. Prev Nutr Food Sci 2014; 19(1): 5-9.
[9] Coniff RF, Shapiro JA, Seaton TB, Bray GA. Multicenter, placebocontrolled trial comparing acarbose (BAY g 5421) with placebo,tolbutamide, and tolbutamide-plus-acarbose in non-insulin-dependent diabetes mellitus. Am J Med 1995; 98: 443-51.
[10] Benalla W, Bellahcen S, Bnouham M. Antidiabetic medicinal plants as a source of alpha glucosidase inhibitors. Curr Diabetes Rev 2010;6: 247-54.
[11] Kim H, You Y. Ecophysiological responses of Quercus gilva,endangered species and Q. glauca to long-term exposure to elevated CO2concentration and temperature. J Ecol Environ 2012; 35: 203-12.
[12] Noshiro S, Sasaki Y. Identification of Japanese species of evergreen Quercus and Lithocarpus (Fagaceae). IAWA J 2011; 32: 383-93.
[13] Itokawa H, Tachi Y, Kamano Y, Iitaka Y. Structure of gilvanol, a new triterpene isolated from Quercus gilva Blume. Chem Pharm Bull 1978; 26: 331-3.
[14] Moon MY, Baik JS, Kim SS, Jang WJ, Kim MS, Lee NH. Identification of antioxidative constituents from the branches of Quercus gilva Blume. J Soc Cosmet Scientists Korea 2009; 35: 251-6.
[15] Deng J, Cheng W, Yang G. A novel antioxidant activity index (AAU)for natural products using the DPPH assay. Food Chem 2011; 125: 1430-5.
[16] Negri G, Santi D, Tabach R. Flavonol glycosides found in hydroethanolic extracts from Tilia cordata, a species utilized as anxiolytics. Rev Bras Plantas Med 2013; 15(2): 217-224.
[17] Sahu RK, Kar M, Routray R. DPPH free radical scavenging activity of some leafy vegetables used by tribals of Odisha, India. J Med Plants Stud 2013; 1(4): 21-7.
[18] Khan RA, Khan MR, Sahreen S, Ahmed M. Evaluation of phenolic contents and antioxidant activity of various solvent extracts of Sonchus asper (L.) Hill. Chem Cent J 2012; 6(1): 12.
[19] Jayanthi P, Lalitha P. Reducing power of the solvent extracts of Eichhornia crassipes (Mart.) Solms. Int J Pharm Sci 2011; 3(3): 126-8.
[20] Ramazan M, Pinar I, Havser EV, Ayse AM. Antioxidant activity and total phenolic content of Gagea fibrosa and Romulea ramiflora. Iran J Chem Chem Eng 2011; 30(3): 57-62.
[21] Moradi-Afrapoli F, Asghari B, Saeidnia S, Ajani Y, Mirjani M,Malmir M, et al. In vitro α-glucosidase inhibitory activity of phenolic constituents from aerial parts of Polygonum hyrcanicum. Daru 2012;20(1): 37.
[22] Osman AM. Multiple pathways of the reaction of 2,2-diphenyl-1-picrylhydrazyl radical (DPPH·) with (+)-catechin: evidence for the formation of a covalent adduct between DPPH· and the oxidized form of the polyphenol. Biochem Biophys Res Commun 2011; 412: 473-8.
[23] Santos-Sanchez NF, Flores-Parra A, Valadez Blanco R, Fernandes-Rojas B, Martinez-Vasquez JB, Salas-Coronado R. Polyphenolic content, free radical-scavenging activity and isolation of tiliroside from Heliocarpus terebinthinaceus (Tiliaceae) seeds. J Biol Sci 2014;14: 376-80.
[24] Kedare SB, Singh RP. Genesis and development of DPPH method of antioxidant assay. J Food Sci Technol 2011; 48(4): 412-22.
[25] Kumar RS, Rajkapoor B, Perumal P. Antioxidant activities of Indigofera cassioides Rottl. Ex. DC using various in vitro assay models. Asian Pac J Trop Biomed 2012; 2(4): 256-61.
[26] Bendary E, Francis RR, Ali HMG, Sarwat MI, Hady SE. Antioxidant and structure-activity relationships (SARs) of some phenolic and anilines compounds. Ann Agric Sci 2013; 58(2): 173-81.
[27] Singhal M, Paul A, Singh HP. Synthesis and reducing power assay of methyl semicarbazone derivatives. J Saudi Chem Soc 2014; 18(2): 121-7.
[28] Katalinić V, Možina SS, Skroza D, Generalić I, Abramovič H,Miloš M, et al. Polyphenolic profile, antioxidant properties and antimicrobial activity of grape skin extracts of 14 Vitis vinifera varieties grown in Dalmatia (Cratioa). Food Chem 2010; 119: 715-23.[29] Li YQ, Zhou FC, Gao F, Bian JS, Shan F. Comparative evaluation of quercetin, isoquercetin, and rutin as inhibitors of alpha-glucosidase. J Agric Food Chem 2009; 57: 11463-8.
[30] Yilmazer-Musa M, Griffith AM, Michels AJ, Schneider E, Frei B. Grape seed and tea extracts and catechin 3-gallates are potent inhibitors of α-amylase and α-glucosidase activity. J Agric Food Chem 2012; 60: 8924-9.
[31] Gao H, Nishioka T, Kawabata J, Kasai T. Structure-activity relationships for alpha-glucosidase inhibition of baicalein, 5,6,7-trihydroxyflavone: the effect of A-ring substitution. Biosci Biotechnol Biochem 2004; 68: 369-75.
[32] Jabeen B, Riaz N, Saleem M, Naveed MA, Ashraf M, Alam U, et al. Isolation of natural compounds from Phlomis stewartii showing α-glucosidase inhibitory activity. Phytochemistry 2013; 96: 443-8.
[33] Zechel DL, Withers SG. Glycosidase mechanisms: anatomy of a finely tuned catalyst. Acc Chem Res 2000; 33: 11-8.
14 May 2015
Sanro Tachibana, Department of Applied Biosciences,Faculty of Agriculture, Ehime University, 3-5-7 Tarumi Matsuyama, Ehime 790-8566, Japan.
Tel: +81-89-946-9864
Fax: +81-89-977-4364
E-mail: tatibana@agr.ehime-u.ac.jp
杂志排行
Asian Pacific Journal of Tropical Biomedicine的其它文章
- Leishmaniosis phytotherapy: Review of plants used in Iranian traditional medicine on leishmaniasis
- Isolation, screening and identification of Bacillus spp. as direct-fed microbial candidates for aflatoxin B1biodegradation
- Morphological and molecular characterization of fungus isolated from tropical bed bugs in Northern Peninsular Malaysia, Cimex hemipterus (Hemiptera: Cimicidae)
- Effects of filaricidal drugs on longevity and enzyme activities of the microfilariae of Setaria cervi in white rats
- Dill tablet: a potential antioxidant and anti-diabetic medicine
- Plantago major treatment enhanced innate antioxidant activity in experimental acetaminophen toxicity
