Plantago major treatment enhanced innate antioxidant activity in experimental acetaminophen toxicity
2015-10-31FaridaHussanRinaHaryaniOsmanBasahMohdRafizulMohdYusofNurAqilahKamaruddinFaizahOthman
Farida Hussan, Rina Haryani Osman Basah, Mohd Rafizul Mohd Yusof, Nur Aqilah Kamaruddin, Faizah Othman
Anatomy Department, Faculty of Medicine, Universiti Kebangsaan Malaysia, Kuala Lumpur, Malaysia
Plantago major treatment enhanced innate antioxidant activity in experimental acetaminophen toxicity
Farida Hussan*, Rina Haryani Osman Basah, Mohd Rafizul Mohd Yusof, Nur Aqilah Kamaruddin, Faizah Othman
Anatomy Department, Faculty of Medicine, Universiti Kebangsaan Malaysia, Kuala Lumpur, Malaysia
ARTICLE INFO
Article history:
in revised form 11 May,
2nd revised form 19 May 2015
Accepted 10 Jun 2015
Available online 1 Aug 2015
Plantago major
Acetaminophen
Liver injury
Oxidative stress
Antioxidants
Objective: To determine the effect of Plantago major (P. major) extract on the liver injury following acetaminophen (APAP) toxicity.
Methods: The male Sprague Dawley rats (n = 38) were randomly divided into normal control (n = 6) and experiment (n = 32) groups. The latter was subdivided into four groups and induced with APAP (1 000 mg/kg) per oral, followed by P. major extract and N-acetylcysteine orally to the respective groups for six days.
Results: On the seventh day, the serum bilirubin, liver enzymes and tissue malondialdehyde were increased in APAP groups whereas the total protein in serum, tissue superoxide dismutase and glutathione levels were reduced. The plant extract treatment reduced the histological deteriorations such as aggregation of hepatocellular cords, formation of binucleated cells and vacuolisation of the cells with scanty cytoplasm. It also revealed significant reduction of malondialdehyde and increased level of superoxide dismutase and glutathione. The findings in the extract treated groups were comparable to the group treated with N-acetylcysteine.
Conclusions: In conclusion, P. major can enhance innate antioxidant activity and ameliorate the APAP-induced liver injury.
Original article doi: 10.1016/j.apjtb.2015.06.013 ©2015 by the Asian Pacific Journal of Tropical Biomedicine. All rights reserved.
1. Introduction
Liver is susceptible to drug-induced injury owing to its major role in drug metabolism. Several drugs such as antipyretics including acetaminophen (APAP), anti-inflammatory drugs,antituberculosis drugs, antidepressants and anticancer drugs have potential hepatotoxicity. The incidence of drug-induced liver diseases is increasing day by day and unintentional drug toxicity is very common nowadays all over the world. The drug-induced liver diseases attribute more than 50% of acute liver failure[1]. The APAP overdose is associated with high tendency of development of acute liver failure[2]. The mechanism of APAP-induced hepatocyte injury is owing to accumulation of its toxic metabolites, N-acetylp-benzoquinone imine (NAPQI) which overshoots the neutralisingcapacity of the hepatic glutathione (GSH), resulting in GSH depletion[3]. Therefore, the current treatment for APAP toxicity is mainly focused to replace GSH with the precursor, N-acetylcysteine(NAC). However, oxidative stress is one of the contributing mechanisms in APAP toxicity[4]. Furthermore, the efficacy of NAC depends on timing of presentation after APAP exposure[5]. Akhtar stated that the antidote should be given as early as possible and the better prognosis would be seen when it was initiated within 8 h following the exposure[6].
The natural products with antioxidant properties showed hepatoprotective activity against APAP toxicity[7,8]. Plantago major(P. major), also known as greater plantain or broadleaf plantain,is a medicinal herb with potent antioxidant effect[9]. It belongs to the family of Plantaginaceae and is widely found in Europe and Asia in which Malaysia is one of the countries of its habitat. Its leaves are large and ovate to elliptical shaped with parallel venation(5-9 veins). It contains polysaccharides, lipids, alkaloids, caffeic acid derivatives and flavonoids[10]. It is a popular herb of wound healing[10]. Administration of this plant with the dose of 2 000 mg/ kg showed no adverse effect[11]. The extensive in vitro and in vivostudies showed its hypoglycaemic effect[12], haemopoietic activity on the bone marrow and spleen cells[13], antiulcer effect on asprininduced gastric ulcer[14], antibacterial effect on Staphylococcus aureus and Escherichia coli and anti-inflammatory properties[15,16]. Its hepatoprotective activity against carbon tetrachloride (CCl4)toxicity has been observed[17,18]. Based on the promising effect of P. major on liver injury, the present study was aimed to explore its effect on APAP toxicity and determine the potential protective mechanisms.
2. Materials and methods
2.1. Study design
Thirty-eight male Sprague Dawley rats (200-250 g) were kept at room temperature (23-32 °C) with 12 h light/dark cycle. The rats were fed with rodent pellet (Gold Coin, Kepong, Malaysia) and tap water ad libitum. The animals monitoring was done in accordance with the institutional animal ethical guideline. The approval number was PP/ANAT/2010/KHIN/25-NOVEMBER/340-NOVEMBER-2010-MAY 2012. The animals were acclimatised for one week. Then, they were randomly divided into normal control (n = 6) and experiment(n = 32) groups. The normal control (C) group was treated with 5% dimethyl sulfoxide. The experiment group was subdivided into APAP group which received 5% dimethyl sulfoxide, the extract treated groups such as P. major extract in low dose (PML) and P. major extract in high dose (PMH), and NAC treated group.
2.2. Plant extraction
P. major was collected from Cameron Highland, Malaysia and was identified by an expert in the herbal centre of Faculty of Science and Technology of the University. The leaves were cleaned thoroughly with tap water and then dried up at the room temperature. The dried leaves were weighed and processed for methanol extraction with Soxhlet apparatus (Eyela CA 1312, EM/ EME/SERIES Electromantles, Thermofisher Scientific, United States). The rotary evaporator (Eyela N-1000, Canada) was used to remove methanol. The extract was then collected and kept in a 4 °C freezer until further use.
2.3. Induction of liver toxicity and tissue collection
The animals were received oral APAP (1 000 mg/kg) except C group. The dose of APAP was selected based on Kiran et al.[19]. Twenty-four hours after the APAP ingestion, the rats in PML and PMH groups were received P. major extract 1 000 mg/kg and 1 500 mg/kg respectively, and the NAC (900 mg/kg) for the NAC group. The extract and NAC were given orally for six days consecutively. On Day 7, all the animals were sacrificed. The blood and liver tissue were collected. The serum was sent to PATHLAB to analyse liver enzymes such as aspartate aminotransferase (AST) and alanine aminotransferase (ALT), bilirubin and total protein. The liver tissue was weighed and observed for macroscopic changes. Then, the part of liver tissue was kept in -80 °C for malondialdehyde (MDA),superoxide dismutase (SOD), and GSH assays. The rest of the tissue was fixed in 10% formalin.
2.4. Determination of MDA, hepatic SOD and GSH
The MDA assay kit (Biodivision, USA), SOD determination kit(Sigma-Aldrich Chemie GmbH, Switzerland) and the GSH assay kit (Sigma-Aldrich, USA) were used and the analyses were done according to the manufacturers' protocol.
2.4.1. Tissue MDA level
The tissue was homogenised in 300 μL of the MDA lysis buffer with 3 μL butylated hydroxytoluene (100×) and subsequently centrifuged with 9 000 r/min for 10 min to remove insoluble material. The MDA in the sample was reacted with thiobarbituric acid to generate the MDA-thiobarbituric acid adduct which was detected by the microplate reader at 532 nm.
2.4.2. Tissue SOD level
For SOD determination, the tissue was homogenised and centrifuged at 8 000 r/min for 15 min and the supernatant was collected. The SOD in the sample was determined by using Dojindo's highly water soluble tetrazolium salt, WST-1 [2-(4-Iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt] that produced a water-soluble formazan dye upon reduction with a superoxide anion (Sigma Aldrich, Switzerland). The absorbance was measured at 450 nm.
2.4.3. Tissue GSH level
The sample was deproteinised with 5-sulfosalicylic acid. The GSH in liver tissue was measured by using kinetic assay in which catalytic amounts of GSH (nmol) caused continuous reduction of 5,5'-dithiobis (2-nitrobenzoic acid) to 5-thio-2-nitrobenzoic acid. The yellow product 5-thio-2-nitrobenzoic acid was measured at 412 nm. The GSH level of liver was expressed in nmol/mg.
2.5. Histological analysis
The tissue sample was fixed in 10% formalin and processed for histological analysis. The tissues were sectioned into 5 µm thickness, stained with hematoxylin and eosin and observed under light microscope.
2.6. Statistical analysis
The data were expressed as mean ± SEM and statistical analysis was performed, by using ANOVA followed by post hoc test (Tukey). The P value less than 0.05 was considered as statistically significant. The data analysis was done with SPSS version 16.0 (SPSS Inc.Chicago, IL, USA).
3. Results
The body weight, the behaviour and physical activity of the animals were monitored daily. The drastic drop of body weight was found in experiment group after 24 h of induction. However, the body weight regained back to normal in all subgroups at the end of the experiment. There was no macroscopic change in the liver.
3.1. Serum biochemical parameters
The liver enzymes such as AST, and ALT, were remained higher in APAP group compared to the PML, PMH and NAC groups; however,it was not statistically significant (P > 0.05). The total bilirubin level was significantly reduced in all the treated groups such as PML,PMH and NAC compared to the APAP group (P < 0.05). The serum protein level showed no significant change between APAP group and the treated groups (P > 0.05) (Table 1).

Table 1 Serum biochemical parameters following 7 days of treatment (mean ± SEM).
3.2. Tissue MDA, SOD and GSH levels
The results of tissue MDA, SOD and GSH levels were shown in Table 2.
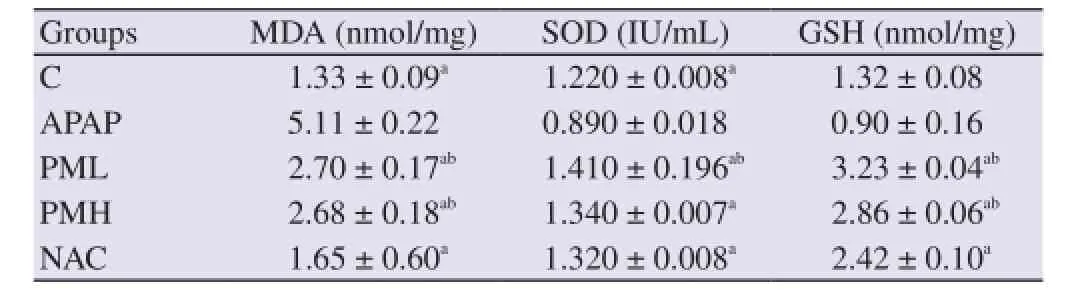
Table 2 Effect of P. major extract on the tissue MDA, SOD and GSH levels (mean ± SEM).
The tissue MDA level was significantly increased in the APAP group compared to C group (P < 0.05). The PML and PMH groups showed significant reduction in MDA level compared to the APAP group. The MDA level was significantly increased in the PML and PMH groups compared to the C and NAC groups (P < 0.05).
The tissue SOD level was significantly reduced in the APAP group compared to the C group. The P. major treated groups showed significant increased level of SOD compared to the APAP group. The PML group showed significant higher level of SOD compared to the NAC group (P < 0.05).
The GSH level was significantly higher in the PML, PMH and NAC groups compared to that of the APAP group. In the comparison among the treated groups, the plant extract treated groups showed significant higher GSH level than that of the NAC group.
3.3. Histological analysis
The histological changes were observed and shown in Figure 1. The tissue sample from the C group exhibited normal liver architecture, revealing radiating hepatocyte cords from the central vein to portal triad with sinusoidal spaces in between. In APAP group, the aggregation of hepatocytes was found around the central vein, compressing the sinusoidal spaces. The nuclei of hepatocytes became pale with fragmented nucleoli and the cytoplasm was scanty with vacuolisation, revealing the features of steatosis. The cell membrane of the hepatocytes was dissolved, resulting in fusion of two cells to form binucleated cells. There was also an infiltration of inflammatory cells around the portal triad and central vein. By treating with the P. major extract, the hepatocytes revealed less aggregation and less extent of vacuolisation. The sinusoidal spaces in between the hepatocyte cords appeared wider compared to APAP group. The PMH group showed more histological improvement which was comparable with the NAC and C groups. The evidence of histological features revealed the lesser extent of liver injury in the P. major extract treated groups compared to APAP group.
4. Discussion
Liver is a site of drug metabolism. In APAP toxicity, hepatocytes are exposed to excessive amount of toxic metabolites, NAPQI,following depletion of GSH[20]. Hepatocyte injury initiates the activation of tissue macrophages (Kupffer cells) and stimulates the release of several inflammatory cytokines and chemokines from the activated immune system. It is triggered by tyrosine nitration and oxidative stress due to the release of reactive oxygen species[4]. The reactive oxygen species especially superoxide anions are accounted for the formation of peroxynitrites and hydrogen peroxides (H2O2)[4]. The accumulation of H2O2enhances the cell injury via lipid peroxidation and the MDA is a reliable biomarker to determine the extent of lipid peroxidation[4,21]. The innate antioxidants such as SOD and the GSH, play an important role to counter-check the oxidative stress. The SOD neutralises the superoxide anions whereas the GSH is responsible to remove H2O2via glutathione peroxidase[22]. The cell injury in APAP toxicity is owing to the excess amount of unconjugated NAPQI which binds to the cellular protein of membrane-bound cytoplasmic organelles such as mitochondria, endoplasmic reticulum and the nucleus,resulting in impaired cellular function and cell death[20]. Therefore,the principle of current drug treatment following APAP toxicity is to replace GSH.
The injured hepatocytes release cytosolic enzymes such as AST and ALT, into the blood stream. The serum liver enzyme levels were markedly increased within 6 h of drug exposure and reached to the peak after 24 h[23]. The injured hepatocytes were mainly found at the perivenous area which was observed as cloudy swelling, vacuolisation, nuclear pyknosis and fragmented nucleoli[24]. Those findings were observed in the APAP group of our study. These injuries were commonly found around the central vein because the CYP2E1, a key enzyme of APAP metabolism was abundantly distributed around the perivenous area[25]. However, hepatocytes can undergo regeneration process under the circumstance of hepatotoxicity, disease or surgery[26]. The serum liver enzymes will gradually return to normal along with hepatocyte regeneration. Therefore, the serum AST and ALT levels at Day 7 showed no significant difference in the present study. However,the APAP group revealed the significant higher level of MDA with histological deterioration of liver architecture which indicated lipid peroxidation attributed to the mechanism of hepatocyte injury. The lower GSH level was also observed in this group. Therefore, lipid peroxidation in APAP toxicity was due to accumulation of H2O2following hepatic GSH depletion which leads to reduce efficacy of glutathione peroxidase[22]. Moreover, the tissue SOD level was also low in the APAP group. Our findings suggested that oxidative stress contributed to the hepatocyte injury in the late presentation of APAP toxicity.
Therefore, the natural products with high antioxidant activity might be considered as a potential alternative treatment for APAP toxicity. The P. major leaf extract was used in the present study. This plant is native to Europe and is a popular edible weed because of its wound-healing property[10]. In the United States, it is known as “white man's footprint”. P. major possesses several bioactive compounds[10]. Its antioxidant and free radical scavenging activity are contributed by its total phenol contents, flavonoids and caffeic acid derivatives such as plantamajoside and acteoside[10]. Although the hepatoprotective studies have been done previously, those were done on CCl4-induced liver injury and P. major was given prior to CCl4exposure[17,18]. Based on the promising effect of P. major on liver injury, we took up the challenge of real case scenario and P. major was given after APAP toxicity. We found that the P. major extract treated groups showed higher GSH level and increased SOD activity than the C and NAC groups. Moreover, the low MDA level was also observed in these groups. Our results proved that the P. major extract attenuated the lipid peroxidation and enhanced innate antioxidants. Thus, the P. major treatment not only prevents the GSH depletion but also reduces the oxidative stress. Among the experiment groups, the PML group showed significant lower level of ALT. It also revealed significant lower level of MDA as well as higher level of SOD and GSH compared to the experiment group. Moreover, the only mild histological changes were observed in the liver tissue of the PML group. Therefore, the low dose (1 000 mg/kg)of P. major crude extract is recommended for future application. As a conclusion, the P. major extract showed hepatoprotective effect against APAP toxicity. The potential mechanism of this effect is by enhancing the activities of innate antioxidants. The further studies are required to explore its effect on the NAPQI-protein binding status, GSH synthesis and the activities of enzymes such as CYP2E1,catalase and GSH peroxidase.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
The authors would like to express the gratitude to the research committee members of the Department of Anatomy and those of Faculty of Medicine, those of the institutional animal ethical committee, the technical staff of the Department of Anatomy and Biochemistry, the statistician, the person who introduced this medicinal plant and the herbal expert from the Faculty of Science and Technology, Univerisiti Kebangsaan Malaysia. This study was supported by a grant from the Faculty of Medicine, Universiti Kebangsaan Malaysia, Malaysia (FF-132-2011).
[1] Goldberg DS, Forde KA, Carbonari DM, Lewis JD, Leidl KB, Reddy KR, et al. Population-representative incidence of drug-induced acute liver failure based on an analysis of an integrated health care system. Gastroenterology 2015; 148(7): 1353-61.e3.
[2] Bernal W, Wendon J. Acute liver failure. N Engl J Med 2013; 369(26): 2525-34.
[3] Kaplowitz N, DeLeve LD. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier; 2013.
[4] Agarwal R, Hennings L, Rafferty TM, Letzig LG, McCullough S,James LP, et al. Acetaminophen-induced hepatotoxicity and protein nitration in neuronal nitric-oxide synthase knockout mice. J Pharmacol Exp Ther 2012; 340(1): 134-42.
[5] Green JL, Heard KJ, Reynolds KM, Albert D. Oral and intravenous acetylcysteine for treatment of acetaminophen toxicity: a systematic review and meta-analysis. West J Emerg Med 2013; 14(3): 218-26.
[6] Akhtar SD. Acetaminophen toxicity. Critical Care Alert 2010; 18: 65-8.
[7] Lee CH, Kuo CY, Wang CJ, Wang CP, Lee YR, Hung CN, et al. A polyphenol extract of Hibiscus sabdariffa L. ameliorates acetaminophen-induced hepatic steatosis by attenuating the mitochondrial dysfunction in vivo and in vitro. Biosci Biotechnol Biochem 2012; 76(4): 646-51.
[8] Sabir SM, Ahmad SD, Hamid A, Khan MQ, Athayde ML, Santos DB,et al. Antioxidant and hepatoprotective activity of ethanolic extract of leaves of Solidago microglossa containing polyphenolic compounds. Food Chem 2012; 131(3): 741-7.
[9] Beara IN, Lesjak MM, Jovin ED, Balog KJ, Anackov GT, Orcić DZ,et al. Plantain (Plantago L.) species as novel sources of flavonoid antioxidants. J Agric Food Chem 2009; 57(19): 9268-73.
[10] Haddadian K, Haddadian K, Zahmatkash M. A review of Plantago plant. Indian J Tradit Knowl 2014; 13(4): 681-5.
[11] Atta AH, Abo EL-Sooud K. The antinociceptive effect of some Egyptian medicinal plant extracts. J Ethnopharmacol 2004; 95(2-3): 235-8.
[12] Abdulghani MA, Hamid I, Al-Naggar RA, Osman MT. Potential antidiabetic activity of Plantago major leaves extract in streptozocininduced diabetic rats. Res J Pharm Biol Chem Sci 2014; 5(2): 896-902.
[13] Velasco-Lezama R, Tapia-Aguilar R, Roman-Ramos R, Vega-Avila E,Pérez-Gutiérrez MS. Effect of Plantago major on cell proliferation in vitro. J Ethnopharmacol 2006; 103(1): 36-42.
[14] Kobeasy MI, Abdel-Fatah OM, Abd El-Salam SM, Mohamed ZM. Gastroprotective effect of Plantago major L. against gastric injury induced by aspirin in rats. J Chem Acta 2013; 2(2): 86-91.
[15] Sharifa AA, Neoh YL, Iswadi MI, Khairul O, Abdul Halim M,Jamaludin M, et al. Effects of methanol, ethanol and aqueous extract of Plantago major on gram positive bacteria, gram negative bacteria and yeast. Ann Microsc 2008; 8: 42-4.
[16] Beara IN, Orcić DZ, Lesjak MM, Mimica-Dukić NM, Peković BA,Popović MR. Liquid chromatography/tandem mass spectrometry study of anti-inflammatory activity of plantain (Plantago L.) species. J Pharm Biomed Anal 2010; 52(5): 701-6.
[17] Atta AH, Nasr SM, Mouneir SM. Potential protective effect of some plant extracts against carbon tetrachloride-induced hepatotoxicity. Afr J Tradit Complement Altern Med 2006; 3(3): 1-9.
[18] Türel I, Ozbek H, Erten R, Oner AC, Cengiz N, Yilmaz O. Hepatoprotective and anti-inflammatory activities of Plantago major L. Indian J Pharmacol 2009; 41(3): 120-4.
[19] Kiran PM, Raju AV, Rao BG. Investigation of hepatoprotective activity of Cyathea gigantea (Wall. ex. Hook.) leaves against paracetamolinduced hepatotoxicity in rats. Asian Pac J Trop Biomed 2012; 2(5): 352-6.
[20] Jaeschke H, McGrill MR, Williams CD, Ramachandran A. Current issues with acetaminophen hepatotoxicity - a clinically relevant model to test the efficacy of natural products. Life Sci 2011; 88(17-18): 737-45.
[21] Spirlandeli AL, Deminice R, Jordao AA. Plasma malondialdehyde as biomarker of lipid peroxidation: effects of acute exercise. Int J Sports Med 2014; 35(1): 14-8.
[22] Dunning S, Ur Rehman A, Tiebosch MH, Hannivoort RA, Haijer FW, Woudenberg J, et al. Glutathione and antioxidant enzymes serve complementary roles in protecting activated hepatic stellate cells against hydrogen peroxide-induced cell death. Biochim Biophys Acta 2013; 1832(12): 2027-34.
[23] Jaeschke H, Williams CD, McGill MR, Xie Y, Ramachandran A. Models of drug-induced liver injury for evaluation of phytotherapeutics and other natural products. Food Chem Toxicol 2013; 55: 279-89.
[24] Hurkadale PJ, Shelar PA, Palled SG, Mandavkar YD, Khedkar AS. Hepatoprotective activity of Amorphophallus paeoniifolius tubers against paracetamol-induced liver damage in rats. Asian Pac J Trop Biomed 2012; 2(Suppl 1): S238-42.
[25] Tydén E, Tjälve H, Larsson P. Gene and protein expression and cellular localisation of cytochrome P450 enzymes of the 1A, 2A, 2C, 2D and 2E subfamilies in equine intestine and liver. Acta Vet Scand 2014; 56: 69.
[26] Ross MH, Pawlina W. Histology: a text and Atlas: with correlated cell and molecular biology. 6th ed. Philadelphia: Lippincott Williams and Wilkins; 2010.
23 Mar 2015
Farida Hussan, Anatomy Department, Faculty of Medicine, Universiti Kebangsaan Malaysia, Jalan Yaacob Latif, 56000, Cheras,Kuala Lumpur, Malaysia.
Tel: +603 91458635
Fax: +603 91458607
E-mail: khinpapah@gmail.com
Foundation Project: Supported by a grant from the Faculty of Medicine, Universiti Kebangsaan Malaysia, Malaysia (FF-132-2011).
杂志排行
Asian Pacific Journal of Tropical Biomedicine的其它文章
- Leishmaniosis phytotherapy: Review of plants used in Iranian traditional medicine on leishmaniasis
- Isolation, screening and identification of Bacillus spp. as direct-fed microbial candidates for aflatoxin B1biodegradation
- Morphological and molecular characterization of fungus isolated from tropical bed bugs in Northern Peninsular Malaysia, Cimex hemipterus (Hemiptera: Cimicidae)
- Effects of filaricidal drugs on longevity and enzyme activities of the microfilariae of Setaria cervi in white rats
- Dill tablet: a potential antioxidant and anti-diabetic medicine
- Role of secondary metabolites of wild marigold in suppression of Johnson grass and Sun spurge
