Morphological and molecular characterization of fungus isolated from tropical bed bugs in Northern Peninsular Malaysia, Cimex hemipterus (Hemiptera: Cimicidae)
2015-10-31AbdulHafizAbMajidZulaikhaZahranAbdHafisAbdRahimNorAzlizaIsmailWardahAbdulRahmanKartiekasariSyahiddaMohammadZubairiHamadyDiengTomomitsuSatho4HouseholdandStructuralUrbanEntomologyLaboratorySchoolofBiologicalSciencesUniversit
Abdul Hafiz Ab Majid, Zulaikha Zahran, Abd Hafis Abd Rahim, Nor Azliza Ismail, Wardah Abdul Rahman, Kartiekasari Syahidda Mohammad Zubairi, Hamady Dieng, Tomomitsu Satho4Household and Structural Urban Entomology Laboratory, School of Biological Sciences, Universiti Sains Malaysia, 800 Penang, Malaysia
2Plant Pathology Laboratory, School of Biological Sciences, Universiti Sains Malaysia, 11800 Penang, Malaysia
3Institute of Biodiversity and Environmental Conservation, Universiti Malaysia Sarawak, 94300 Kota Samarahan, Sarawak, Malaysia
4Department of Microbiology, Faculty of Pharmaceutical Sciences, Fukuoka University, 8-19-1 Nanakuma Johnan-ku, 814-0180 Fukuoka, Japan
Morphological and molecular characterization of fungus isolated from tropical bed bugs in Northern Peninsular Malaysia, Cimex hemipterus (Hemiptera: Cimicidae)
Abdul Hafiz Ab Majid1*, Zulaikha Zahran1, Abd Hafis Abd Rahim1, Nor Azliza Ismail2, Wardah Abdul Rahman2, Kartiekasari Syahidda Mohammad Zubairi2, Hamady Dieng3, Tomomitsu Satho41Household and Structural Urban Entomology Laboratory, School of Biological Sciences, Universiti Sains Malaysia, 11800 Penang, Malaysia
2Plant Pathology Laboratory, School of Biological Sciences, Universiti Sains Malaysia, 11800 Penang, Malaysia
3Institute of Biodiversity and Environmental Conservation, Universiti Malaysia Sarawak, 94300 Kota Samarahan, Sarawak, Malaysia
4Department of Microbiology, Faculty of Pharmaceutical Sciences, Fukuoka University, 8-19-1 Nanakuma Johnan-ku, 814-0180 Fukuoka, Japan
ARTICLE INFO
Article history:
in revised form 26 Mar 2015
Accepted 5 Apr 2015
Available online 5 Aug 2015
Cimex hemipterus
Aspergillus
Trichoderma
β-tubulin
Internal transcribed spacer
Morphological characteristics
Objective: To investigate some morphological and molecular characteristics of fungal parasites isolated from wild tropical bed bug, Cimex hemipterus.
Methods: A series of culture methods were used to obtain fungal isolates from field-collected bed bugs. Characteristics of the isolates such as colony appearance, mycelial texture and pigmentation were studied to explore their morphology. Isolates were also subjected to a PCR-based genotyping test.
Results: There were noticeable differences in morphological characteristics among the four isolates. Conidial areas of one isolate were dark green, whereas those of the remaining colonies were olive-green, black or dark brown. Conidia of the dark green isolate were globose, while those of olive-green, black and dark brown isolates were globose to subglobose, globose to spherical and globose to subglobose/finely roughened, respectively. These morphological specificities and the molecular analyses showed that the fungal internal transcribed spacer ribosomal region and β-tubulin gene sequences of the isolates shared clade with Trichoderma and Aspergillus sequences.
Conclusions: Overall, the new discovery of common pathogens in agricultural field developed in live bed bugs storage tank may initiate the use of biological agents in later years.
Original article doi: 10.1016/j.apjtb.2015.04.012 ©2015 by the Asian Pacific Journal of Tropical Biomedicine. All rights reserved.
1. Introduction
Bed bugs are considered to be household insect pests for more than 3 300 years back in those early years in ancient Egypt. They have been said to tag along with the colonists in their belongings on the ship and the dispersal occurs in America. However, the indication of bed bugs origin has not been recorded according to the interview done on Louis Sorkin, an insect expert of the American Museum of Natural History[1]. Generally, bed bugs infestation is known to be a nuisance to humans as they have been battling theinsect pests for millennia. As some factors can cause widespread population increase of bed bugs, the number of cases that related to the insect arose drastically in the late 1990s especially in United States[2].
Trichoderma and Aspergillus are very cosmopolitan sporeforming genus of fungi. The existence of these genera have been studied and reported as both have the ability to produce some of the most important mycotoxins in the world. Abbot has listed more than 15 types of mycotoxins produced by Aspergillus while three mycotoxins were produced by Trichoderma[3]. The presence of mycotoxins produced by Aspergillus such as aflatoxins, ochratoxins,citrinin and sterigmatocystin can contaminate food commodities and store products such as rice[4,5], barley[6], nuts[7], flour[8] and maize[9]. In insects, the impact or function of Aspergillus are varied,from carrier in transmitting microorganism to acting as pathogen to the host. However, reports on the occurrence of this fungi from insect are still limited and rarely reported.
About the endophytic fungus, Trichoderma can be commonly found both in cultivated and non-cultivated soils, and is used in biological control of a variety of plant-pathogenic fungi such as Fusarium verticillioides and Rhizoctonia solani[10-13]. Trichoderma species have been demonstrated to produce protease and chitinase that degrade the cell-wall during the parasitic interaction[14-17]. Protease produced by pathogenic insects possesses similar properties to those from Trichoderma, augmenting the possibility that proteinases are involved in entomopathogenicity. Previous report regarding larvicidal activity of Trichoderma harzianum (T. harzianum) against the cotton leaf worm has suggested that this species is pathogenic towards the insect[18].
However, the application of inaccurate and confusing species names has been one of the major hindrances to the study of Trichoderma and Aspergillus genera simultaneously has impeded their true potential. A meticulous study of their true diversity is mandatory since different species might produce a different array of potential metabolites to be exploited. Prior to the utilization of molecular approaches, the identification of Trichoderma and Aspergillus is historically based on the application of morphological species recognition concept[19]. Due to the few morphological differences and variations in culture, particularly those in anamorphic forms, many species including T. harzianum are inaccurately identified[20,21]. Samson et al. also suggested that misidentifications and incorrect names are always occurred in Aspergillus nomenclature[22].
Recent developments in genomics have provided better opportunities for researchers worldwide to obscure the true diversity and classification of species in the genus Trichoderma and Aspergillus. Multiple genes have been demonstrated and translation elongation factor 1-α, internal transcribed spacer (ITS) and β-tubulin have successfully delimited between the closely related species in Trichoderma[23]. In genetic characterization of Aspergillus, ITS-restriction fragment length polymorphism technique, random amplified polymorphic DNA microsatellite polymorphism analysis,random amplified polymorphic DNA and sequence analysis of intergenic spacer were used among molecular methods[24-32]. In 2007, Balajee et al.[33] proposed the use of β-tubulin gene for species identification within the section while Hong et al.[34] used phylogenetic analysis of β-tubulin sequence as one of the methods to describe new species in Aspergillus section Fumigati. Pildain et al. used a combination of phenotypic (morphology and extrolite profile)and molecular (β-tubulin and calmodulin gene sequence) to describe new species in section Flavi[35].
Thus, prior to the study of possible entomopathogenic role of Trichoderma and Aspergillus against tropical bed bugs, Cimex hemipterus, we therefore primarily examined both molecular and morphological characterizations to accurately identify the species as well as its descriptions.
2. Materials and methods
2.1. Isolation of fungal strains
Samples of eggs, nymphs and live adult bed bugs were collected and reared for chemical testing. Samples were separated and labelled according to its location. They were placed in the plastic containers(8 cm diameter × 8 cm height), which were tightly covered with net cloth (13 cm × 13 cm) and rubber band. Folded papers were provided in the container as their harborage. The containers then were kept in the plastic tank (30 cm × 18 cm × 18 cm) in the laboratory temperature and relative humidity maintained between(26 ± 2) °C and 60% ± 10% respectively. After two weeks of rearing,two bed bugs accidentally died and possible entomopathogenic fungi that caused the death were isolated.
2.2. Single spore isolation
All fungal isolates from dead bed bugs were purified through single spore isolation technique[36]. This technique was carried out by streaking the mycelia on water agar in a zigzag manner. Once germinated, a single conidium was transferred onto potato dextrose agar (PDA) or malt extract agar (MEA) plates for further studies. For preservation purpose, the single conidial isolates were maintained in distilled water with mild agar broth.
2.3. Morphological analysis of Trichoderma species
Cultural characteristics such as colony appearances, mycelial textures and pigmentations on both obverse and reverse on PDA plates were observed after 3 to 7 days of incubation under the standard incubation conditions. Growth rate via colony diameter also on PDA was measured, initially standardized at 6 mm using a cork borer and incubated for 3 days in a total darkness. Trials were repeated three times and in triplicates. For microscopic observation,mycelial plugs of Trichoderma species were transferred onto MEA plates and were incubated in a total darkness at 28 °C for 3 to 7 days. The species characteristics such as conidia, conidiophores, branching patterns and chlamydospores were observed. Morphological species characterization was determined following the key characters by Samuels et al.[23].
2.4. Morphological analysis of Aspergillus species
For morphological observations, Czapek yeast extract agar (CYA),oatmeal agar (OA), creatine sucrose agar (CREA) and MEA were used. The isolates were inoculated at three points on each plate of each medium and incubated at 25 °C in the dark for 7 days. For microscopic observations, microscopic mounts were made in sterile distilled water from PDA or MEA colonies. The species characteristics such as colony colours, the structure of conidial heads and the shapes of conidia were observed. The fungi were used to identify the species levels[37].
2.5. DNA extraction
Conidia of fungal isolates were inoculated in MEA broth and incubated at 25 °C in the dark. The fungal mycelium were harvested after 36 h of incubation and transferred into sterile freeze dry bottles. After 2 days of freeze dried, the dried mycelium was ground using liquid nitrogen into fine powders. The genomic DNA was extracted by using DNA extraction kit and DNeasy plant mini kit (Qiagen)according to the manufacturer's instructions.
2.6. PCR amplification
Primer pairs ITS1 and ITS4 and Bt2a and Bt2b were used to amplify ribosomal ITS regions and β-tubulin genes, respectively[38].The 25 µL PCR mix contained 6 ng of genomic DNA, 25 mmol/ L MgCl2, 40 mmol/L of dNTP mix, 5 IU of Taq DNA polymerase and 1 µmol/L concentrations of each primer. Amplification with the primers pairs ITS1 and ITS4 was performed using a denaturation step of 95 °C for 5 min, followed by 30 cycles of 95 °C for 30 s, 58 °C for 30 s and 72 °C for 1 min. A final extension step at 72 °C for 5 min was then employed. Amplification with primer pairs Bt2a and Bt2b included denaturation at 94 °C for 5 min, followed by 35 cycles of 94 °C for 1 min, 58.6 °C for 30 s and 72 °C for 1 min. A final extension step at 72 °C for 5 min was then used.
2.7. DNA sequencing
2.7.1. Analysis of sequence data
The alignment of ITS region and β-tubulin gene sequence data were performed using the MEGA 4 software. The consensus sequences were basic local alignment search tool (BLAST) against Genbank database (http: //www.ncbi.nlm.nih.gov) to infer the identification of the isolates.
2.7.2. Nucleotide sequence accession numbers
The fungal ITS ribosomal region and β-tubulin gene sequences determined in this study were deposited in GenBank. The BLAST search result and sequence similarities were listed in Table 1 using the GenBank database along with assigned accession numbers.
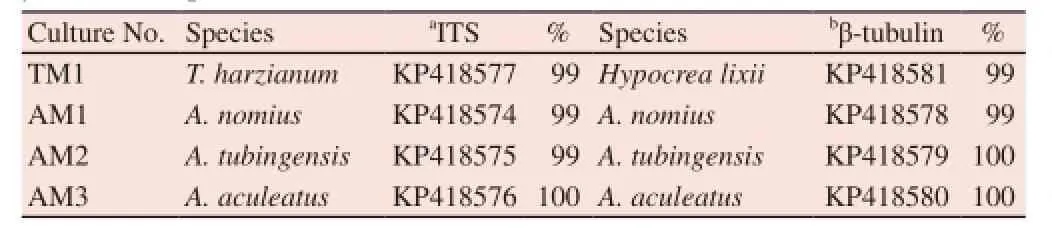
Table 1 Identification of fungal isolates from bed bug based on ITS region and β-tubulin sequences.
3. Results
3.1. Morphological analysis of isolate TM1
Dark green conidia were densely formed in concentric rings toward the edge of Petri dish after 7 days incubation (Figures 1A-1B). Mycelia were scarcely found in the culture due to abundance formation of conidia, hence, suppressing the mycelial growth. While,the growth rate of T. harzianum was measured on PDA at 28 °C after3 days incubation in a total darkness. The mean value for growth rate was 21 to 25 mm/day. Primary branches of conidiophores were in paired and held in whorls of 2 to 3 phialides (Figures 1C-1D). Phialides were typically flask-shaped, swollen in the middle with pointed tip and slightly narrowed base. Green conidia were profusely produced, one-celled, smooth surface and mostly globose. Only a few conidia were found in slightly ovoidal shaped (Figures 1E-1F). Chlamydospores were rarely found in the culture, thick and rough-walled, globose to subglobose and formed at the terminal or intercalary in hyphae (Figures 1G-1H). Macroscopic and microscopic characters of T. harzianum were presented in Figure 1.
3.2. Morphological analysis of isolate AM1
Colony colours on MEA were olive green and sclerotia when presented were brown to black. On CYA, conidial area was yellow and sometimes overlaid with white to olive yellow areas. Mycelium on both MEA and CYA was white. Reverse colonies were orangish to light yellow. A. nomius produced uniseriate phialides, radiate to columnar conidial heads, smooth-walled stipes and globose to subglobose vesicles. The stipes average length was 200 to 500 µm while the width ranged between 5 to 9 µm. The vesicle diameter was 20 to 40 µm in range. Conidia diameter was 3.5 to 4.5 µm in range with very rough to finely roughened surface. Macroscopic and microscopic characters of A. nomius are presented in Figure 2.
3.3. Morphological analysis of isolate AM2
Colony colours on MEA and CYA were black to dark black and the colony was densely packed. Reverse colonies were colourless to light yellow. A. tubingensis produced uniseriate and biseriate phialides, radiate conidial heads, smooth-walled stipes and globose to spherical vesicles. The stipes average length was 500 to 550 µm while the width ranged between 12 to 15 µm. The vesicle diameter was 50 to 70 µm in range. Conidia diameter was 3.0 to 4.5 µm in range with finely roughened surface. Macroscopic and microscopic characters of A. tubingensis are presented in Figure 3.
3.4. Morphological analysis of isolate AM3
Colony colours on MEA and CYA were black to dark brown and the colony was densely packed. Reverse colonies were brownish to light yellow. A. aculeatus produced uniseriate phialides, radiate conidial heads, smooth-walled stipes and globose to subglobose vesicles. The stipes average length was 500 to 700 µm while the width ranged between 10 to 16 µm. The vesicle diameter was 55 to 70 µm in range. Conidia diameter was 2.0 to 3.5 µm in range with finely roughened surface. Macroscopic and microscopic characters of A. aculeatus are presented in Figure 4.
3.5. Molecular analysis and DNA sequencing
From PCR amplification of ITS region and β-tubulin gene, four fungal isolates obtained from the bed bug yielded a single fragment of an approximately 500 and 600 bp, respectively. BLAST search using Genbank database showed that isolate TM1 shared 99% similarities with T. harzianum, isolate AM1 shared 99% similarity with A. nomius and isolate AM2 shared 99%-100% similarity with A. tubingensis. Meanwhile, sequence similarity of isolate AM3 showed 100% similarity to A. aculeatus. Sequence results of all four fungal isolates are summarized in Table 1.
4. Discussion
Fungus is an opportunistic organism that is able to develop rapidly depending on favorable temperature and moisture[39]. Despite of their main roles in contaminating large amount of agricultural crops in storage or field, they also had potential in becoming natural enemy of insect[40,41]. A form of defense mechanism produced by fungus is known as mycotoxin, secondary metabolites that may also affect human health and insect which however, relied on the amount of toxicity produced[42].
In this study, bed bugs (Cimex hemipterus) are dominantly affected by T. harzianum as well as Aspergillus sp. namely A. nomius, A. tubingensis and A. aculeatus. Trichoderma sp. are commonly found in enclosed environment such as in dust and water-damaged buildings, while a study conducted by Docampo et al. showed that spores of Aspergillus are predominantly found in both indoor and outdoor environment[43-45]. A ten years study performed by Falvey and Streifel also reported that it is almost impossible that any environment is completely devoid of Aspergillus spores thus making the exposure to Aspergillus is difficult to avoid[46]. In Malaysia's warm climate, Aspergillus sp. are proven to be widespread in indoor environments[47]. However, the occurrence of Trichoderma sp. in enclosed building has never been reported thus far. Apart of being mycoparasitic towards other fungi, Trichoderma sp. also can be pathogenic towards insects[48]. Production of enzymes and antibiotics in Trichoderma sp. causes a decrease in other plant pathogens as well as insect cuticle when they are exposed to the fungal strain. This includes studies revolving peptaibols production in T. harzianum strain that represent insecticidal activity against plant pathogens and insect[49]. T. harzianum, for instance, has been tested in previous studies in controlling termites through their feeding behavior by growing fungal strain along with the bait but the outcomes showed fluctuated responses[31]. Aspergillus sp. on the other hand, has been discovered majorly contaminating early harvest crops including corn,barley, wheat and many others. Some of the species in the genus Aspergillus are also known to produce secondary metabolites called mycotoxins which have been reported to be carcinogenic, teratogenic,tremorgenic, haemorrhagic, nephrogenic, hepatogenic and dermatitis to both humans and animals[50-52].
Aflatoxins which is mass-produced of some Aspergillus sp., is the most carcinogenic mycotoxin that may cause aflatoxicosis,an infection involving vital organ like liver wihch could be useful in biological studies for further discovery in biological control[2]. A. nomius retrieved from this study is classified as the strain predominantly formed uniseriate conidial heads. This species has the ability to produce aflatoxin B1and G1[53]. Commonly, aflatoxin is classified as Group 1 carcinogens by the International Agency for Research on Cancer but aflatoxin B1has been proved to have the highest toxicity. In the meantime, ochratoxins A which is mostly found in A. tubingensis and A. aculeatus is classified as Group 2B that has less toxic effects. To this date, researches regarding their toxins were continuously carried out despite their toxicity uses. Reports using entomopathogenic fungi such as Beauveria bassiana and Metarhizium anisopliae in controlling hematophagous insect including biting midges, mosquitoes and even bed bugs have been recently updated[54,55]. This is due to their ability in probing throughout the cuticles of the host and eventually kills the entire population when the infected insect came in contact with other colony members. Nevertheless, investigations on developing fungal strains as biological agents especially from these two species in this research against household insect pest should be further studied to prevent harmful effects in the surrounding.
Morphological and molecular identification using ITS region and β-tubulin gene confirmed the identity of non-aggressive type 1 (Th 1)T. harzianum. Published works on morphological characteristics and phylogenetic analysis of Trichoderma species suggest that this genus is a species complex[23,56]. In case of T. harzianum, four genetically distinct biotypes, Th 1 to Th 4 have been recognized in which Th 1 and Th 3 are non-aggressive and Th 2 and Th 4 are aggressive types. Molecular data has shown that these four biotypes are in fact different species. For instance, Th 1 is T. harzianum, Th 2 is Trichoderma aggressivum, Th 3 is Trichoderma atroviride and Th 4 is Trichoderma aggressivum f. europaeum. Identifying all these species is difficult given that they are morphologically indistinguishable and their complexity has long been discussed by numerous authors[57-59]. According to Samuels et al., several morphological characteristics of T. harzianum differed from others such as the fast growth rateon media cultures, smaller conidia, less chlamydospore formation and shorter phialides[23]. Those are quantifiable characters that separate this species from other biotypes. However, only one strain of T. harzianum was isolated from bed bug sample, thus, characters comparison with other biotypes was not possible in this study. It is now widely acknowledged that morphological and colony appearance alone is insufficient to accurately identify this species. For that reason,DNA sequencing using β-tubulin gene and ITS region was used to support morphological identification of this species.
With respect to species identification in the genus Aspergillus, three species belonged to the different sections were identified, namely,A. nomius from section Flavi, A. tubingensis and A. aculeatus from section Nigri. The typically yellow to green conidial heads of A. nomius as well as brown to black colour of A. tubingensis and A. Aculeatus colonies were in agreement with the descriptions by Raper and Fennell[60] and Klich[36] who described colour of the colonies as the distinguishable characters of section Flavi and Nigri. Balajee et al. suggested that species in section Nigri, Flavi and Fumigati are referred as species complex due to the complexity and variability of phylogenetic relationship within Aspergillus section[33].
Additional works on possible metabolite compounds including mycotoxins produced by each of these fungi, however, should be done prior to select a potential candidate as a bed bug controlling agent.
Overall, the new discovery of common pathogens in agricultural field developed in live bed bugs storage tank may initiate the use of biological agents in later years. This however includes further researches by inventing such formulations and upgrading existing biopesticide so that it is well-adapted to the environment while effectively reduce the pest population.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
The authors would like to acknowledge Universiti Sains Malaysia for funding the research (Short Term Grant: 304/PBIOLOGI/6313030). We sincere thank to Malaysian Pest Control Companies from all states in participating bed bugs sample collection.
[1] Cohen J. They're back: a bed bug history. History in the Headlines; 2010.[Online] Available from: http://www.history.com/news/theyre-back-abed-bug-history [Accessed on 1st March, 2015]
[2] Boase CJ. Bedbugs-back from the brink. Pestic Outlook 2001; 12: 159-62.
[3] Abbott SP. Mycotoxins and indoor molds. Indoor Environ Connect 2002;3: 14-4.
[4] Almeida MI, Almeida NG, Carvalho KL, Gonçalves GA, Silva CN,Santos EA, et al. Co-occurrence of aflatoxins B1, B2, G1and G2,ochratoxin A, zearalenone, deoxynivalenol, and citreoviridin in rice in Brazil. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 2012; 29: 694-703.
[5] Fushimi Y, Takagi M, Uno S, Kokushi E, Nakamura M, Hasunuma H, et al. Measurement of sterigmatocystin concentrations in urine for monitoring the contamination of cattle feed. Toxins (Basel) 2014; 6: 3117-28.
[6] Kuruc JA, Schwarz P, Wolf-Hall C. Ochratoxin A in stored U.S. barley and wheat. J Food Prot 2015; 78: 597-601.
[7] Adaya-González J, Carvajal-Moreno M, Rojo-Callejas F, Ruiz-Velasco S. Aflatoxins in walnut (Juglans regia L.), pecan (Carya illinoinensis(Wangenh.) K. Koch) and cashew (Anacardium occidentale L.) nuts in Mexico. Pharm Anal Acta 2015; doi: 10.4172/2153-2435.1000338.
[8] Kara GN, Ozbey F, Kabak B. Co-occurrence of aflatoxins and ochratoxin A in cereal flours commercialised in Turkey. Food Control 2015; 54: 275-81.
[9] Perrone G, Haidukowski M, Stea G, Epifani F, Bandyopadhyay R, Leslie JF, et al. Population structure and aflatoxin production by Aspergillus Sect. Flavi from maize in Nigeria and Ghana. Food Microbiol 2014; 41: 52-9.
[10] Błaszczyk L, Popiel D, Chełkowski J, Koczyk G, Samuels GJ, Sobieralski K, et al. Species diversity of Trichoderma in Poland. J Appl Genet 2011;52: 233-43.
[11] Gherbawy YA, Hussein NA, Al-Quashi AA. Molecular characterization of Trichoderma populations isolated from soil of Taif City, Saudi Arabia. Int J Curr Microbiol Appl Sci 2014; 3: 1059-71.
[12] Suhaida S, NurAinIzzati MZ. The efficacy of Trichoderma harzianum T73s as a biocontrol agent of Fusarium ear rot disease of maize. Int J Agric Biol 2013; 15: 1175-80.
[13] Asad SA, Ali N, Hameed A, Khan SA, Ahmad R, Bilal M, et al. Biocontrol efficacy of different isolates of Trichoderma against soil borne pathogen Rhizoctonia solani. Pol J Microbiol 2014; 63: 95-103.
[14] López-Mondéjar R, Ros M, Pascual JA. Mycoparasitism-related genes expression of Trichoderma harzianum isolates to evaluate their efficacy as biological control agent. Biol Control 2011; 56: 59-66.
[15] Qualhato TF, Lopes FAC, Steindorff AS, Brandão RS, Jesuino RSA,Ulhoa C. Mycoparasitism studies of Trichoderma species against three phytopathogenic fungi: evaluation of antagonism and hydrolytic enzyme production. Biotechnol Lett 2013; 35: 1461-8.
[16] Parmar HJ, Bodar NP, Lakhani HN, Patel SV, Umrania VV, Hassan MM. Production of lytic enzymes by Trichoderma strains during in vitro antagonism with Sclerotium rolfsii, the causal agent of stem rot of groundnut. Afr J Microbiol Res 2015; 9: 365-72.
[17] Kubicek CP, Starr TL, Glass NL. Plant cell wall-degrading enzymes and their secretion in plant-pathogenic fungi. Annu Rev Phytopathol 2014; 52: 427-51.
[18] El-Katatny MH. Virulence potential of some fungal isolates and their control-promise against the Egyptian cotton leaf worm, Spodoptera littoralis. Arch Phytopathol Plant Prot 2010; 43: 332-56.
[19] Gams W, Bissett J. Morphology and identification of Trichoderma. In: Kubicek CP, Harman GE, editors. Trichoderma and Gliocladium. Vol 1. Basic biology, taxonomy and genetics. London: Taylor and Francis Ltd.;1998, p. 3-34.
[20] Chaverri P, Samuels GJ. Hypocrea/Trichoderma (Ascomycota,Hypocreales, Hypocreaceae): species with green ascospores. Stud Mycol 2003; 48: 1-119.
[21] Druzhinina IS, Kopchinskiy AG, Kubicek CP. The first 100 Trichoderma species characterized by molecular data. Mycoscience 2006; 47: 55-64.
[22] Samson RA, Hong SB, Frisvad JC. Old and new concepts of species differentiation in Aspergillus. Med Mycol 2006; 44: 133-48.
[23] Samuels GJ, Dodd SL, Gams W, Castlebury LA, Petrini O. Trichoderma species associated with the green mold epidemic of commercially grown Agaricus bisporus. Mycologia 2002; 94: 146-70.
[24] Spadaro D, Patharajan S, Lorè A, Garibaldi A, Gullino ML. Ochratoxigenic black species of Aspergilli in grape fruits of northern Italyidentified by an improved PCR-RFLP procedure. Toxins (Basel) 2012; 4: 42-54.
[25] Kizis D, Natskoulis P, Nychas GJ, Panagou EZ. Biodiversity and ITSRFLP characterisation of Aspergillus section Nigri isolates in grapes from four traditional grape-producing areas in Greece. PLoS One 2014;9: e93923.
[26] Gautam AK, Bhadauria R. Characterization of Aspergillus species associated with commercially stored triphala powder. Afr J Biotech 2012;11: 16814-23.
[27] Al-Wadai AS, Al-Othman MR, Mahmoud MA, Abd El-Aziz AR. Molecular characterization of Aspergillus flavus and aflatoxin contamination of wheat grains from Saudi Arabia. Genet Mol Res 2013;12: 3335-52.
[28] Karthikeyan M, Karthikeyan A, Velazhahan R, Madhavan S, Jayaraj T. Occurrence of aflatoxin contamination in maize kernels and molecular characterization of the producing organism, Aspergillus. Afr J Biotech 2013; 12: 5839-44.
[29] Hadrich I, Amouri I, Neji S, Mahfoud N, Ranque S, Makni F, et al. Genetic structure of Aspergillus flavus populations in human and avian isolates. Eur J Clin Microbiol Infect Dis 2013; 32: 277-82.
[30] Chiotta ML, Reynoso MM, Torres AM, Combina M, Chulze SN. Molecular characterization and toxigenic profile of Aspergillus section Nigri populations isolated from the main grape-growing regions in Argentina. J Appl Microbiol 2011; 110: 445-54.
[31] de Oliveira Rocha L, Reis GM, Braghini R, Kobashigawa E, de Araújo J, Corrêa B. Characterization of aflatoxigenic and non-aflatoxigenic strains of Aspergillus section Flavi isolated from corn grains of different geographic origins in Brazil. Eur J Plant Pathol 2012; 132: 353-66.
[32] Kana JR, Gbemenou B, Gnonlonfin J, Harvey J, Wainaina J, Wanjuki I,et al. Mycobiota and toxigenecity profile of Aspergillus flavus recovered from food and poultry feed mixtures in Cameroon. J Anim Poult Sci 2013; 2: 98-107.
[33] Balajee SA, Houbraken J, Verweij PE, Hong SB, Yaghuchi T, Varga J, et al. Aspergillus species identification in the clinical setting. Stud Mycol 2007; 59: 39-46.
[34] Hong SB, Shin HD, Hong JB, Frisvad JC, Nielsen PV, Varga J, et al. New taxa of Neosartorya and Aspergillus in Aspergillus section Fumigati. Antonie Van Leeuwenhoek 2008; 93: 87-98.
[35] Pildain MB, Frisvad JC, Vaamonde G, Cabral D, Varga J, Samson RA. Two novel aflatoxin-producing Aspergillus species from Argentinean peanuts. Int J Syst Evol Microbiol 2008; 58: 725-35.
[36] Klich MA. Identification of common Aspergillus species, Centraalbureau voor Schimmelcultures. Utrecht: The Netherlands; 2002.
[37] Hansen HN, Smith RE. The mechanism of variation in imperfect fungi: Botrylis cinerea. Phytopathology 1932; 22: 953-64.
[38] Glass NL, Donaldson GC. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl Environ Microbiol 1995; 61: 1323-30.
[39] Abdin MZ, Ahmad MM, Javed S. Advances in molecular detection of Aspergillus: an update. Arch Microbiol 2010; 192: 409-25.
[40] Trienens M, Rohlfs M. Insect-fungus interference competition-the potential role of global secondary metabolite regulation, pathway-specific mycotoxin expression and formation of oxylipins. Fungal Ecol 2012; 5: 191-9.
[41] Batta YA. The first report on entomopathogenic effect of Fusarium avenaceum (Fries) Saccardo (Hypocreales, Ascomycota) against rice weevil (Sitophilus oryzae L.: Curculionidae, Coleoptera). J Entomol Acarol Res 2012; 44: e11.
[42] Guo Z, Döll K, Dastjerdi R, Karlovsky P, Dehne HW, Altincicek B. Effect of fungal colonization of wheat grains with Fusarium spp. on food choice, weight gain and mortality of meal beetle larvae (Tenebrio molitor). PloS One 2014; 9: e100112.
[43] Rahman A, Begum MF, Rahman M, Bari MA. Isolation and identification of Trichoderma species from different habitats and their use for bioconversion of solid waste. Turk J Biol 2010; 34: 1-12.
[44] Thrane U, Poulsen SB, Nirenberg HI, Lieckfeldt E. Identification of Trichoderma strains by image analysis of HPLC chromatograms. FEMS Microbiol Lett 2001; 203: 249-55.
[45] Docampo S, Trigo MM, Recio M, Melgar M, García-Sánchez J,Cabezudo B. Fungal spore content of the atmosphere of the Cave of Nerja(Southern Spain): diversity and origin. Sci Total Environ 2011; 409: 835-43.
[46] Falvey DG, Streifel AJ. Ten-year air sample analysis of Aspergillus prevalence in a university hospital. J Hosp Infect 2007; 67: 35-41.
[47] Rahman WA, Rosli H, Baharuddin SN, Salleh B. Incidence and remediation of fungi in a sick building in Malaysia: a case study. Aerobiologia 2012; 28: 275-83.
[48] Clarkson JM, Charnley AK. New insights into the mechanisms of fungal pathogenesis in insects. Trends Microbiol 1996; 4: 197-203.
[49] Ownley BH, Gwinn KD, Vega FE. Endophytic fungal entomopathogens with activity against plant pathogens: ecology and evolution. BioControl 2010; 55: 113-28.
[50] Cafarchia C, Camarda A, Iatta R, Danesi P, Favuzzi V, Di Paola G, et al. Environmental contamination by Aspergillus spp. in laying hen farms and associated health risks for farm workers. J Med Microbiol 2014; 63: 464-70.
[51] Copetti MV, Barcelos Ada S, Kommers GD, Santurio JM, Oliveira FN,Lovato M. Cutaneous, respiratory and hepatic aspergillosis in Brazilian white Pekin mallards (Anas platyrhynchos). Mycopathologia 2015; 179: 321-5.
[52] Afsah-Hejri L, Jinap S, Hajeb P, Radu S, Shakibazadeh S. A review on mycotoxins in food and feed: Malaysia case study. Compr Rev Food Sci Food Saf 2013; 12: 629-51.
[53] Reis TA, Baquião AC, Atayde DD, Grabarz F, Corrêa B. Characterization of Aspergillus section Flavi isolated from organic Brazil nuts using a polyphasic approach. Food Microbiol 2014; 42: 34-9.
[54] Barbarin AM, Jenkins NE, Rajotte EG, Thomas MB. A preliminary evaluation of the potential of Beauveria bassiana for bed bug control. J Invertebr Pathol 2012; 111: 82-5.
[55] Ansari MA, Pope EC, Carpenter S, Scholte EJ, Butt TM. Entomopathogenic fungus as a biological control for an important vector of livestock disease: the Culicoides biting midge. PLoS One 2011; 6: e16108.
[56] Jaklitsch WM, Voglmayr H. Biodiversity of Trichoderma (Hypocreaceae)in Southern Europe and Macaronesia. Stud Mycol 2015; 80: 1-87.
[57] Harman GE, Herrera-Estrella AH, Horwitz BA, Lorito M. Special issue: Trichoderma-from basic biology to biotechnology. Microbiology 2012;158: 1-2.
[58] Mukherjee PK, Horwitz BA, Herrera-Estrella A, Schmoll M, Kenerley CM. Trichoderma research in the genome era. Annu Rev Phytopathol 2013; 51: 105-29.
[59] Atanasova L, Druzhinina IS, Jaklitsch WM. Two hundred Trichoderma species recognized on the basis of molecular phylogeny. In: Mukherjee PK, Horwitz BA, Singh US, Mukherjee M, Schmoll M, editors. Trichoderma: biology and applications. Nosworthy: CABI; 2013, p. 10-42.
[60] Raper KB, Fennell DI. The genus Aspergillus. Baltimore: Williams & Wilkins; 1965, p. 1-686.
9 Mar 2015
Abdul Hafiz Ab Majid, Household and Structural Urban Entomology Laboratory, School of Biological Sciences, Universiti Sains Malaysia,11800 Penang, Malaysia.
Tel: +604-6534893
Fax: +604-6565125
E-mail: abdhafiz@usm.my
Foundation Project: Supported by Universiti Sains Malaysia (Short Term Grant: 304/PBIOLOGI/6313030).
杂志排行
Asian Pacific Journal of Tropical Biomedicine的其它文章
- Leishmaniosis phytotherapy: Review of plants used in Iranian traditional medicine on leishmaniasis
- Isolation, screening and identification of Bacillus spp. as direct-fed microbial candidates for aflatoxin B1biodegradation
- Effects of filaricidal drugs on longevity and enzyme activities of the microfilariae of Setaria cervi in white rats
- Dill tablet: a potential antioxidant and anti-diabetic medicine
- Plantago major treatment enhanced innate antioxidant activity in experimental acetaminophen toxicity
- Role of secondary metabolites of wild marigold in suppression of Johnson grass and Sun spurge
